Abstract
Dipicolinic acid (DPA) is employed as a significant biomarker to detect Bacillus anthracis, which can do serious damages to the health of human beings. Hence, it is crucial to develop a fast and highly efficient strategy for DPA monitoring. In this work, based on silicon nanoparticles (Si NPs) and terbium metal-organic frameworks (Tb-MOFs), a hybrid structure (Si NPs/Tb-MOFs) as a novel dual-emitting fluorescence probe was fabricated for ratiometric detection of DPA, where blue light-emitting Si NPs (Ex: 280 nm; Em: 422 nm) are encapsulated into green light-emitting Tb-MOFs (Ex: 280 nm; Em: 547 nm). The optical properties and chemical composition of the as-obtained Si NPs/Tb-MOFs were characterized in detail. The Si NPs/Tb-MOFs probe not merely possesses the merits of a facile synthesis method but also is an excellent fluorescence probe. The response time towards DPA is less than 30 s, revealing that the process of detecting DPA can be completed in such a short time. The limit of detection for DPA is 5.3 nM, which is four orders of magnitude lower than an infectious dosage of anthrax spores for human beings (60 μM). This dual-emitting Si NPs/Tb-MOFs probe with interference-free and self-calibrating properties may be a potential candidate for further development in medical diagnosis.

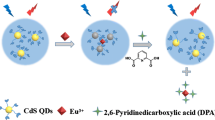
Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthrax is a serious disease caused by Bacillus anthracis infection. Fast detection methods towards anthrax spores are crucial to minimize their damage to human beings. Dipicolinic acid (DPA), one of the main ingredients of anthrax spores, is considered as a significant biomarker in detection of anthracis diseases [1]. Various DPA detection methods have been reported in the past decades, including molecular imprinting [2], polymerase chain reaction (PCR) [3], surface plasmon resonance (SPR) [4], and surface enhanced Raman spectroscopy (SERS) [5]. Compared with other methods, fluorescence detection is more suitable for DPA detection due to its merits of excellent selectivity, high sensitivity, and fast response ability [6].
Luminescent metal-organic frameworks (MOFs), particularly lanthanide-based MOFs, have had a wide range of applications in sensing cations [7], anions [8], molecules [9, 10], and so on. Luminescent lanthanide-MOFs (Ln-MOFs) can be synthesized by taking lanthanide ions as coordination centers. Through f-f or f-d energy transfer from ultraviolet to visible and near-infrared, most Ln3+ ions can emit visible or near-infrared luminescence [11]. Typically, europium ion (Eu3+) and terbium ion (Tb3+), which can emit red and green light, respectively, are applied widely as chemical sensors. In the past few years, some studies have reported encapsulating functional species (e.g., luminescent nanoparticles) into nonluminous microporous MOFs to obtain luminescent MOFs [12]. For example, carbon quantum dots encapsulated zeolitic imidazolate framework materials (ZIF-8) with high quantum yields could be used in enhancing chemical sensing [13].
Many fluorescence probes are based on a single-switch mechanism of fluorescence enhancement (turn-on mode) or fluorescence quenching (turn-off mode) [14,15,16,17]. Some luminescent anthrax spores sensors, including polymer nanoparticles, polymer microspheres, silica, and quantum dots, have also been studied based on these mechanisms. For example, Luo et al. proposed using multiporous terbium phosphonate coordination polymer microspheres as a fluorescence probe to detect anthrax spores [18]. This fluorescence probe is based on the coordination of terbium ions which improve the sensitivity of DPA detection greatly. However, the results based on these single-switch mechanisms are easy to be influenced by background light, temperature, and other environment interference, which will reduce the accuracy of measurements. Thus, ratiometric fluorescence probes, which can solve the above problems effectively, are considered as ideal tools to build sensing platforms [19,20,21]. By measuring the fluctuation of fluorescence intensity ratio of two wavelengths, ratiometric fluorescence probes could eliminate errors caused by surroundings or instruments. However, it is difficult to find an appropriate material which has two clear signals and can be excited under one excitation simultaneously.
Blue-emitting silicon nanoparticles (Si NPs) embedded in green-emitting terbium-based MOFs (Tb-MOFs) are proposed in this work. Two different fluorescence signals can be excited under the same excitation and have certain separation. After encapsulating Si NPs into Tb-MOFs, the as-obtained hybrids comprise the merits of lanthanide fluorescence of MOFs, together with the advantage of Si NPs. The fluorescence detection of DPA has been studied for a few decades; however, to our knowledge, it is the first time that DPA has been detected using a ratiometric fluorescence probe based on a hybrid of Ln-MOFs and Si NPs.
Materials and methods
Materials
Trisodium citrate dehydrate (Na3C6H5O7·2H2O), barium chloride dihydrate (BaCl2·2H2O), cadmium chloride (CdCl2·2.5H2O), iron (III) chloride hexahydrate (FeCl3·6H2O), manganese (II) chloride tetrahydrate (MnCl2·4H2O), DL-methionine (Met), L-histidine (His), L-glutamic acid (Glu), L-alanine (Ala), L-arginine (Arg), and L-tyrosine (Tyr) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). D-Aspartic acid (Asp), 2,6-pyridinedicarboxylic acid (DPA), 2-picolinic acid (PA), 2-hydroxypyridine (HP), and 3,5-pyridinedicarboxylic acid (PDA) were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). Terbium (III) chloride hexahydrate (TbCl3·6H2O), 3,5-dicarboxybenzeneboronic acid, and (3-aminopropyl) triethoxysilane (APTES) were obtained from Saan Chemical Technology (Shanghai) Co., Ltd. (Shanghai, China). Anhydrous magnesium sulfate (MgSO4) was purchased from Shanghai Dahe Chemical Co., Ltd. (Shanghai, China). Isophthalic acid was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Phosphate buffer saline (PBS) was purchased from Wisent Biotechnology Co. Ltd. (Nanjing, China). Fetal Bovine Serum was purchased from Gibco Life Technologies (New York, USA). 4-Aminobenzenesulfonic acid (Ami) was purchased from J&K Chemical Ltd. (Beijing, China).
Preparation of Tb-MOFs
Tb-MOFs were prepared with reference to a facile solvothermal method reported in a previous work [22] with a minor modification. Details could be found in the Electronic Supplementary Material.
Preparation of Si NPs/Tb-MOFs
At first, Tb-MOFs powder (0.2 g) and trisodium citrate dehydrate (0.4 g) were dissolved in 13 mL DI water under continuous stirring for 10 min; then, 2 mL of APTES was added to this mixture and stirred for another 10 min. The mixture was treated by a one-step hydrothermal method in a Teflon-lined stainless steel autoclave and heated at 200 °C for 3 h to get Si NPs/Tb-MOFs samples. The obtained samples of Si NPs/Tb-MOFs were stored at 4 °C.
Apparatus and characterization
The obtained Si NPs/Tb-MOFs were characterized by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI, USA) and Fourier transform-infrared spectroscopy (FTIR, Nicolet 6700, ThermoFisher). Scanning electron microscopy (SEM) images were obtained by field emission scanning electron microscope (MIRA3, TESCAN). UV-vis absorption spectra and PL spectra were recorded using UV-vis spectrophotometer (759S, Shanghai Lengguang, China) and fluorescence spectrophotometer (F97XP, Shanghai Lengguang, China), respectively.
Procedures for DPA detection
Thirty microliter of the as-prepared Si NPs/Tb-MOFs were dissolved in 2.94 mL PBS buffer, and then, 30 μL DPA solution with different concentration was added to the above solution to make final concentration range from 0 to 4.0 μM. To investigate the selectivity property of Si NPs/Tb-MOFs, the fluorescence spectra of different interferences were detected, including Ba2+, Fe3+, Mg2+, Mn2+, Cd2+, HP, PA, PDA, Ami, Met, His, Asp, Glu, Tyr, Arg, and Ala. The final concentrations of these interferences were 10 μM. All the fluorescence spectra were obtained at 280 nm excitation.
Results and discussions
Preparation and characterization of Si NPs/Tb-MOFs probe
As shown in Scheme 1, the as-prepared Tb-MOFs are firstly mixed with APTES and trisodium citrate dihydrate. The mixture was then treated by hydrothermal method, and finally the Si NPs encapsulated in Tb-MOFs were obtained. Interestingly, Tb-MOFs emitted green light under the excitation of 280 nm; however, the green light could hardly be observed after Tb-MOFs being hybridized with Si NPs. This phenomenon might be attributed to the energy transfer between Tb-MOFs and Si NPs. After adding DPA into Si NPs/Tb-MOFs, DPA can sensitize Tb3+ ions and make the green light recovered. In the whole process, the fluorescence intensity at 422 nm almost stayed the same. Therefore, the Si NPs/Tb-MOFs probe has the potential to be applied as a dual-emission sensing platform for DPA detection.
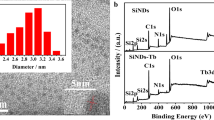
XPS spectra were obtained to characterize the surface composition of Si NPs, Tb-MOFs, and Si NPs/Tb-MOFs. As shown in Fig. 1a, the full range XPS analysis presents five major peaks at 102.33, 153.28, 154.08, 284.73, and 532.08 eV, which represents Si 2p, Tb 4d, Si 2s, C 1s, and O 1s, respectively. It is clear that Tb and Si element existed in Si NPs/Tb-MOFs, which could prove that the hybrid structure was synthesized successfully. The high-resolution C 1 s XPS spectra of Si NPs/Tb-MOFs (Fig. S1a) indicates the presence of C–Si (284.26 eV), C–C (284.67 eV), C–N (285.33 eV), C–O (286.26 eV), and C=O (288.1 eV) groups, respectively. The C 1s XPS spectrum of Si NPs (Fig. S1b) is similar to Si NPs/Tb-MOFs, except for lower intensity of C–O peak than that of Si NPs/Tb-MOFs. However, in the C 1s XPS spectrum of Tb-MOFs (Fig. S1c), there exists a new O–C=O group located at 289.58 eV due to the carboxyl introduced by isophthalic acid and 3,5-dicarboxybenzeneboronic acid. When Si NPs were hybridized with Tb-MOFs, the C=O in carboxyl of Tb-MOFs were transferred to Si–O–C groups. Moreover, the two peaks of Si NPs/Tb-MOFs fitted at 102.28 eV and 103.13 eV are attributed to Si–N and Si–O, respectively (Fig. S1d). The Si 2p spectrum of Si NPs only has a Si–N peak located at 102.38 eV (Fig. S1e). The Tb 4d peak of Si NPs/Tb-MOFs is distinctly different from that of Tb-MOFs (Fig. S1f), which illustrated that the hybrid structure was obtained.
The chemical composition of Si NPs/Tb-MOFs was also studied by FTIR spectroscopy. As shown in Fig. 1b, the surface groups could be demonstrated, and the structure of Si NPs/Tb-MOFs could be confirmed through FTIR spectra of Si NPs, Tb-MOFs, and Si NPs/Tb-MOFs. For these three lines, strong peaks at 1400 cm−1 and 1570 cm−1 were assigned to C–O and N–H bending vibrations, respectively. However, the stretching vibration of C=O in carboxyl at 1650 cm−1 is observed in Tb-MOFs but not observed in Si NPs/Tb-MOFs, which might be attributed to the conjunction between Si NPs and C=O in carboxyl that changed previous electron-conjugated system [23].
The shapes of the Tb-MOFs and Si NPs/Tb-MOFs are characterized by SEM, revealing that both Tb-MOFs and Si NPs/Tb-MOFs are spheroidal (Fig. 2a, b). In general, the morphology of the Si NPs/Tb-MOFs has no obvious difference from Tb-MOFs. As shown in Fig. 2c, the EDX elemental mapping analysis results illustrate that O, Si, and Tb are uniformly distributed.
As shown in Fig. S2, the as-obtained Tb-MOFs show four emission peaks at 491, 547, 586, and 622 nm, respectively. Si NPs exhibit only one emission peak at 448 nm, while this emission peak of Si NPs/Tb-MOFs has a blue shift after encapsulating Si NPs into Tb-MOFs. This result might be owing to that the size of Si NPs decreases after Si NPs growing in the framework of Tb-MOFs.
Sensing mechanism of the Si NPs/Tb-MOFs probe
The fluorescence measurement of the probe towards DPA was taken in the PBS buffer (pH = 7.4). The Si NPs/Tb-MOFs without DPA only show a blue fluorescence with an emission peak at 422 nm under the excitation of 280 nm. After adding 30 μM DPA, new emission bands are observed at 491 nm (5D4→7F6), 547 nm (5D4→7F5), 586 nm (5D4→7F4), and 622 nm (5D4→7F4) [24] under 280 nm excitation, which were instinct bands of Tb3+ ions (Fig. 3a). Moreover, Fig. 3a also indicates that the absorption increases slightly after adding DPA. As presented in Fig. 3b, the fluorescence lifetime transfers from 336.6 to 354.8 μs after adding 10 μM DPA. This is because Si NPs/Tb-MOFs-DPA complex has a stronger fluorescence in 547 nm.
a UV-vis absorption and emission spectra of the Si NPs/Tb-MOFs probe before and after adding DPA (30 μM) at room temperature. b Time-resolved decay curves of Si NPs/Tb-MOFs before and after adding DPA (10 μM), λex = 280 nm and λem = 547 nm. c Simplified schematic diagrams of the ligands-to-Tb energy transfer process in Tb-MOFs. d The schematic illustration of the mechanism of DPA sensing of the Si NPs/Tb-MOFs probe
The emission of the Tb-MOFs is governed by the antenna effect [25]. As shown in the Fig. 3c, ligands of Tb-MOFs absorb energy and are excited to their singlet state (S1). Then, they transfer to triplet state (T1) through intersystem crossing process. An energy transfer occurs from T1 to Tb3+ ions, resulting in the emission of Tb3+ ions. The energy gaps between S1 and T1 of both 3,5-dicarboxybenzeneboronic acid and isophthalic acid are larger than 5000 cm−1 (Table S1), which is favorable for the intersystem crossing process according to Reinhoudt’s empirical rule [26]. The energy difference of the ligands’ T1 and the excited state of Tb3+ ions is calculated to be around the optimal ΔE (2500–3500 cm−1) [27]. Therefore, Tb3+ ions were sensitized efficiently for green emission. In order to explain the decrease of Tb3+ ions’ emission when the Si NPs were present, possible mechanism was discussed and verified. There is a spectrum overlap between the absorption spectrum of Si NPs and the excitation spectrum of Tb-MOFs (Fig. S3), which makes the fluorescence of Tb-MOFs greatly quenched after the Si NPs/Tb-MOFs formed [28]. To illustrate whether the spectrum overlap comes from fluorescence resonance energy transfer (FRET) or inner filter effect (IFE), the related fluorescence lifetimes were measured. Generally, the fluorescence lifetime will be shortened during FRET because energy transfers from donor to acceptor, while the fluorescence lifetime remains unchanged for IFE [29]. As shown in Table S2, the fluorescence lifetimes of Tb-MOFs and Si NPs/Tb-MOFs have a significant difference, which indicates that the quenching of Tb3+ ions’ emission is mainly due to FRET rather than IFE. After adding DPA, DPA becomes an antenna chelated with Tb3+ [30], and the energy transfer from the DPA to Tb3+ becomes the dominating process (Fig. 3d). Thus, the energy could be transferred from DPA to Tb3+ ions efficiently, and the characteristic emission of Tb3+ ions is enhanced significantly. With adding 30 μM DPA, the intensity of emission peak at 422 nm is almost unchanged, while the intensity of Tb3+ ions increases significantly (Fig. 3a). In addition, the morphology of the Si NPs/Tb-MOFs is still maintained after adding DPA (Fig. S4).Therefore, Si NPs/Tb-MOFs can be considered as an ideal ratiometric fluorescence probe of DPA concentration, which could reduce the interference of environmental and instrumental deviation due to that fluorescence reference has a resultful effect in correction.
The pH and time response of Si NPs/Tb-MOFs probe
The pH value is one of the most important factors which may influence fluorescence intensity. Therefore, Si NPs/Tb-MOFs solutions were prepared with different pH values, ranging from 2.07 to 12.03. As shown in Fig. S5, even in acidic or alkaline environment, the fluorescence intensity ratios F547/F422 are almost unchanged. This result demonstrated that our Si NPs/Tb-MOFs solutions would be hardly influenced by pH values of the environment.
To evaluate the response time of the probe towards DPA, the fluorescence intensity ratios I547/I422 under 280 nm excitation were monitored. As shown in Fig. S6, after adding 10 μM DPA into Si NPs/Tb-MOFs solutions, the F547/F422 ratios increased in the beginning and stayed stable after 30 s. However, the F547/F422 ratios in the control group without DPA remain not only weak but stable in the whole time. This result proves that the as-prepared probe can monitor DPA in a very short time.
Sensing property of the Si NPs/Tb-MOFs probe
For the purpose of investigating the sensing property of Si NPs/Tb-MOFs probe towards DPA, this probe was applied to detect various DPA concentrations in PBS buffer. As illustrated in Fig. 4a, with DPA concentration increasing, the fluorescence intensity at 547 nm increases but almost remains unchanged at 422 nm. Moreover, the fluorescence intensity ratios F547/F422 increased exponentially when DPA concentrations ranged from 0.025 to 3 μM. As illustrated in Fig. 4b, the fluorescence intensity ratios F547/F422 are fitted well with single exponential.
a Fluorescence emission spectra of Si NPs/Tb-MOFs probe in the presence of DPA with different concentrations: 0, 0.1, 0.25, 0.5, 0.75, 1, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, and 30 μM (under the excitation of 280 nm). Inset: Photographs of Si NPs/Tb-MOFs taken under a UV lamp at 254 nm before (left) and after (right) adding DPA. b Fluorescence intensity ratios F547/F422 of Si NPs/Tb-MOFs probe towards various DPA concentrations in PBS buffer (pH = 7.4). c CIE chromaticity diagram of Si NPs/Tb-MOFs probe after adding different concentrations of DPA (ranging from 0 to 30 μM). d Linear relationship between the color coordinates x and y of CIE chromaticity diagram
As signal-to-noise (S/N) ratio is 3, limit of detection (LOD) was calculated as 5.3 nM, which was much lower than an infectious dosage of Bacillus anthrax spores (60 μM) [31]. Moreover, when DPA concentration increased from 0 to 30 μM, the fluorescence of the Si NPs/Tb-MOFs probe turns from blue to green (Fig. 4c). In addition, the fluorescent color coordinates of different concentration fit a linear relationship in the CIE chromaticity diagram (Fig. 4d), which is y = − 0.580 + 3.793 * x (R2 = 0.995). Furthermore, the sensing property of Si NPs/Tb-MOFs towards DPA is compared with other fluorescence probes in previous work, indicating that the LOD in this work is quite competitive (Table 1). The sensing ability of pure Tb-MOFs for DPA is shown in Fig. S7, which proves that Si NPs/Tb-MOFs are superior. The spectra of Si NPs/Eu-MOFs towards DPA are also detected (Fig. S8). To some extent, it proves that Si NPs/Ln-MOFs could be a class of universal fluorescence probes which can be further explored in the future.
Selectivity and analytical performance of Si NPs/Tb-MOFs probe
Selectivity is crucial for fluorescence probes. Hence, the selectivity of Si NPs/Tb-MOFs probe was investigated with the presence of potential interferences including metal ions and amino acids. As shown in Fig. 5a, after adding Ba2+, Fe3+, Mg2+, Mn2+, Cd2+, HP, PA, PDA, Ami, Met, His, Asp, Glu, Tyr, Arg, and Ala, the detected intensity ratio F547/F422 can only increase significantly with the presence of DPA (10 μM), and there were negligible changes with other interfering substances (10 μM). This result indicates that Si NPs/Tb-MOFs probe can detect DPA selectively. To further confirm the selectivity performance of the probe, competition experiments were performed in the presence of DPA (10 μM) mixed with several interfering substances (10 μM). Figure 5b shows that the signals towards DPA are not changed obviously at the presence of these interferences. The results are attributed to that Tb3+ ions can chelate with the carboxylate groups and nitrogen atoms of DPA [8], but could not interact with other interferences sufficiently. The high selectivity guarantees that the detection property of DPA with Si NPs/Tb-MOFs probe cannot be influenced by other coexistent interferences. When in high concentration (100 μM) of DPA and other interferences, the intensity ratios F547/F422 of the probe are detected (Fig. S9). The results show that the selectivity of the probe is not influenced by interferences even when interferences concentration is up to 100 μM.
To investigate the practicability of Si NPs/Tb-MOFs probe towards DPA, bovine serum was selected as real sample for DPA detection of our probe. Bovine serum samples were achieved by diluting bovine serum to 1000-fold with PBS buffer. Through adding different concentrations of DPA to bovine serum samples, recovery values can be calculated from 88.2 to 94.8% (Table S3). The results indicated that Si NPs/Tb-MOFs have potential to detect anthrax biomarker DPA for low concentrations of analyte. In more complex analyte, background fluorescence of some biological substances may affect the sensing performance of the presented probe.
Conclusion
In this study, a ratiometric fluorescence probe (Si NPs/Tb-MOFs) was developed via a simple hydrothermal synthesis for DPA detection. This ratiometric fluorescence probe uses Si NPs as a stable reference and Tb-MOFs as a sensitive response signal. Increasing concentration of DPA can lead to an enhancement of Tb-MOFs’ green light, but the intensity of blue fluorescence of Si NPs is almost unchanged. The fluorescence intensity ratios F547/F422 can be fitted well with single exponential when DPA concentrations range from 0.025 to 3 μM. The selectivity of the probe is not influenced by interferences (10 μM). Furthermore, the ratiometric fluorescence probe can detect DPA for low concentrations of analyte. The experiment results prove that Si NPs/Tb-MOFs probe is rapid, sensitive, selective, and self-calibrating for DPA detection. We anticipate that this report will open ways for (i) detecting DPA for more complex analyte and (ii) using other Ln-MOFs as probes to sensing and achieving enhanced performance.
References
Ai K, Zhang B, Lu L (2009) Europium-based fluorescence nanoparticle sensor for rapid and ultrasensitive detection of an anthrax biomarker. Angew Chem Int Ed 48:304–308. https://doi.org/10.1002/anie.200804231
Rizk M, Toubar SS, Sayour HEE-D, Mohamed D, Touny RM (2014) A new potentiometric sensor based on molecularly imprinted polymer for analysis of a veterinary drug imidocarb dipropionate. Eur J Chem 5:18–23. https://doi.org/10.5155/eurjchem.5.1.18-23.876
Das R, Goel AK, Sharma MK, Upadhyay S (2015) Electrochemical DNA sensor for anthrax toxin activator gene atxA-detection of PCR amplicons. Biosens Bioelectron 74:939–946. https://doi.org/10.1016/j.bios.2015.07.066
Lepage D, Jiménez A, Beauvais J, Dubowski JJ (2013) Real-time detection of influenza A virus using semiconductor nanophotonics. Light: Science and Applications 2:e62. https://doi.org/10.1038/lsa.2013.18
Sajanlal PR, Pradeep T (2012) Functional hybrid nickel nanostructures as recyclable SERS substrates: detection of explosives and biowarfare agents. Nanoscale 4:3427–3437. https://doi.org/10.1039/c2nr30557g
Luan K, Meng R, Shan C, Cao J, Jia J, Liu W, Tang Y (2018) Terbium functionalized micelle nanoprobe for ratiometric fluorescence detection of anthrax spore biomarker. Anal Chem 90:3600–3607. https://doi.org/10.1021/acs.analchem.8b00050
Samanta P, Desai AV, Sharma S, Chandra P, Ghosh SK (2018) Selective recognition of Hg2+ ion in water by a functionalized metal-organic framework (MOF) based chemodosimeter. Inorg Chem 57:2360–2364. https://doi.org/10.1021/acs.inorgchem.7b02426
Yao ZQ, Li GY, Xu J, Hu TL, Bu XH (2018) A water-stable luminescent ZnII metal-organic framework as chemosensor for high-efficiency detection of CrVI-anions (Cr2O72− and CrO42−) in aqueous solution. Chem Eur J 24:3192–3198. https://doi.org/10.1002/chem.201705328
Zhu XD, Zhang K, Wang Y, Long WW, Sa RJ, Liu TF, Lü J (2018) Fluorescent metal-organic framework (MOF) as a highly sensitive and quickly responsive chemical sensor for the detection of antibiotics in simulated wastewater. Inorg Chem 57:1060–1065. https://doi.org/10.1021/acs.inorgchem.7b02471
Qu F, Sun C, Lv X, You J (2018) A terbium-based metal-organic framework@gold nanoparticle system as a fluorometric probe for aptamer based determination of adenosine triphosphate. Microchim Acta 185:359. https://doi.org/10.1007/s00604-018-2888-1
Rocha J, Carlos LD, Paz FAA, Ananias D (2011) Luminescent multifunctional lanthanides-based metal-organic frameworks. Chem Soc Rev 40:926–940. https://doi.org/10.1039/c0cs00130a
Cui Y, Yue Y, Qian G, Chen B (2012) Luminescent functional metal-organic frameworks. Chem Rev 112:1126–1162. https://doi.org/10.1021/cr200101d
Dong Y, Cai J, Fang Q, You X, Chi Y (2016) Dual-emission of lanthanide metal-organic frameworks encapsulating carbon-based dots for ratiometric detection of water in organic solvents. Anal Chem 88:1748–1752. https://doi.org/10.1021/acs.analchem.5b03974
Sun C, Zhao S, Qu F, Han W, You J (2020) Determination of adenosine triphosphate based on the use of fluorescent terbium(III) organic frameworks and aptamer modified gold nanoparticles. Microchim Acta 187:34. https://doi.org/10.1007/s00604-019-4019-z
Wei X, Mei S, Yang D, Zhang G, Xie F, Zhang W, Guo R (2019) Surface states induced photoluminescence enhancement of nitrogen-doped carbon dots via post-treatments. Nanoscale Res Lett 14:172–181. https://doi.org/10.1186/s11671-019-3008-9
Li Y, Wen QL, Liu AY, Long Y, Liu P, Ling J, Ding ZT, Cao QE (2020) One-pot synthesis of green-emitting gold nanoclusters as a fluorescent probe for determination of 4-nitrophenol. Microchim Acta 187:106. https://doi.org/10.1007/s00604-019-4090-5
Mei S, Wei X, Hu Z, Wei C, Su D, Yang D, Zhang G, Zhang W, Guo R (2019) Amphipathic carbon dots with solvent-dependent optical properties and sensing application. Opt Mater 89:224–230. https://doi.org/10.1016/j.optmat.2019.01.021
Luo Y, Zhang L, Zhang L, Yu B, Wang Y, Zhang W (2019) Multiporous terbium phosphonate coordination polymer microspheres as fluorescent probes for trace anthrax biomarker detection. ACS Appl Mater Interfaces 11:15998–16005. https://doi.org/10.1021/acsami.9b01123
Chen L, Xu H, Wang L, Li Y, Tian X (2020) Portable ratiometric probe based on the use of europium(III) coordination polymers doped with carbon dots for visual fluorometric determination of oxytetracycline. Microchim Acta 187:125. https://doi.org/10.1007/s00604-019-4104-3
Xu J, Shen X, Jia L, Zhang M, Zhou T, Wei Y (2017) Facile ratiometric fluorapatite nanoprobes for rapid and sensitive bacterial spore biomarker detection. Biosens Bioelectron 87:991–997. https://doi.org/10.1016/j.bios.2016.09.070
Bu X, Fu Y, Jiang X, Jin H, Gui R (2020) Self-assembly of DNA-templated copper nanoclusters and carbon dots for ratiometric fluorometric and visual determination of arginine and acetaminophen with a logic-gate operation. Microchim Acta 187:154. https://doi.org/10.1007/s00604-020-4146-6
Wang YM, Tian XT, Zhang H, Yang ZR, Yin XB (2018) Anticounterfeiting quick response code with emission color of invisible metal-organic frameworks as encoding information. ACS Appl Mater Interfaces 10:22445–22452. https://doi.org/10.1021/acsami.8b06901
Li S, Wang F, He XW, Li WY, Zhang YK (2018) One-pot hydrothermal preparation of gadolinium-doped silicon nanoparticles as a dual-modal probe for multicolor fluorescence and magnetic resonance imaging. J Mater Chem B 6:3358–3365. https://doi.org/10.1039/c8tb00415c
Li G, Zhang Y, Geng D, Shang M, Peng C, Cheng Z, Lin J (2012) Single-composition trichromatic white-emitting Ca4Y6(SiO4)6O: Ce3+/Mn2+/Tb3+ phosphor: luminescence and energy transfer. ACS Appl Mater Interfaces 4:296–305. https://doi.org/10.1021/am201335d
Wang QX, Xue SF, Chen ZH, Ma SH, Zhang S, Shi G, Zhang M (2017) Dual lanthanide-doped complexes: the development of a time-resolved ratiometric fluorescent probe for anthrax biomarker and a paper-based visual sensor. Biosens Bioelectron 94:388–393. https://doi.org/10.1016/j.bios.2017.03.027
Steemers FJ, Verboom W, Reinhoudt DN, van der Tol EB, Verhoeven JW (1995) New sensitizer-modified Calixarenes enabling near-UV excitation of complexed luminescent lanthanide ions. J Am Chem Soc 117:9408–9414. https://doi.org/10.1021/ja00142a004
Heine J, Müller-Buschbaum K (2013) Engineering metal-based luminescence in coordination polymers and metal-organic frameworks. Chem Soc Rev 42:9232–9242. https://doi.org/10.1039/c3cs60232j
Zhang J, Zhou R, Tang D, Hou X, Wu P (2019) Optically-active nanocrystals for inner filter effect-based fluorescence sensing: achieving better spectral overlap. TrAC Trends Anal Chem 110:183–190. https://doi.org/10.1016/j.trac.2018.11.002
Han L, Fan YZ, Qing M, Liu SG, Yang YZ, Li NB, Luo HQ (2020) Smartphones and test paper-assisted ratiometric fluorescent sensors for semi-quantitative and visual assay of tetracycline based on the target-induced synergistic effect of antenna effect and inner filter effect. ACS Appl Mater Interfaces 12:47099–47107. https://doi.org/10.1021/acsami.0c15482
Qu S, Song N, Xu G, Jia Q (2019) A ratiometric fluorescent probe for sensitive detection of anthrax biomarker based on terbium-covalent organic polymer systems. Sensors Actuators B Chem 290:9–14. https://doi.org/10.1016/j.snb.2019.03.110
Gao N, Zhang Y, Huang P, Xiang Z, Wu FY, Mao L (2018) Perturbing tandem energy transfer in luminescent heterobinuclear lanthanide coordination polymer nanoparticles enables real-time monitoring of release of the anthrax biomarker from bacterial spores. Anal Chem 90:7004–7011. https://doi.org/10.1021/acs.analchem.8b01365
Zhang Y, Li B, Ma H, Zhang L, Zheng Y (2016) Rapid and facile ratiometric detection of an anthrax biomarker by regulating energy transfer process in bio-metal-organic framework. Biosens Bioelectron 85:287–293. https://doi.org/10.1016/j.bios.2016.05.020
Shi K, Yang Z, Dong L, Yu B (2018) Dual channel detection for anthrax biomarker dipicolinic acid: the combination of an emission turn on probe and luminescent metal-organic frameworks. Sensors Actuators B Chem 266:263–269. https://doi.org/10.1016/j.snb.2018.03.128
Chen H, Xie Y, Kirillov AM, Liu L, Yu M, Liu W, Tang Y (2015) A ratiometric fluorescent nanoprobe based on terbium functionalized carbon dots for highly sensitive detection of an anthrax biomarker. Chem Commun 51:5036–5039. https://doi.org/10.1039/c5cc00757g
Funding
The authors gratefully thank the supports of the National Natural Science Foundation of China (NSFC, No. 61675049, NSFC, No. 61377046, and NSFC, No. 61177021), Fudan University-CIOMP Joint Fund (FC2017-004) and Jihua Laboratory Projects of Guangdong Province (X190111UZ190).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 37437 kb).
Rights and permissions
About this article
Cite this article
Yang, D., Mei, S., Wen, Z. et al. Dual-emission of silicon nanoparticles encapsulated lanthanide-based metal-organic frameworks for ratiometric fluorescence detection of bacterial spores. Microchim Acta 187, 666 (2020). https://doi.org/10.1007/s00604-020-04643-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04643-7