Abstract
A novel ratiometric fluorescent probe for the determination of oxytetracycline (OTC) was developed. The method is based on the use of adenosine monophosphate/Eu(III) nanoscale coordination polymers doped with carbon dots (CDs) (CD@AMP/Eu NCPs). These were fabricated by self-assembly of Eu3+ and AMP on the surface of CDs containing large amounts of hydroxyl and carbonyl groups. Under the excitation at 310 nm wavelength, the doped NCPs display strong pink emission of Eu3+ at 615 nm and blue emission of the CDs at 430 nm on exposure to OTC. The ratio of fluorescence intensity (F615/F430) of such NCPs displays excellent linear relationship with OTC concentration ranging from 0.2 to 60 μM and the limit of detection (LOD) is 25 nM (3σ). The doped NCPs were evenly immobilized on common filter paper to prepare a visual ratiometric probe for the determination of OTC. Assisted by a digital camera with an APP color detector, the paper-based test strip was applied for the quantitative determination of OTC with a LOD of 0.5 μM and a wide linear scope of 1–100 μM. The method was applied to the determination of OTC in milk samples.

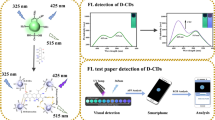
Schematic representation of the principle for oxytetracycline (OTC) determination using carbon dots (CD)@adenosine monophosphate (AMP)/Europium (Eu3+) paper-based ratiometic probe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The antibiotic oxytetracycline (OTC) is widely used as food additives on cows feeding and then may be found as a residue in the dairy products [1]. In view of this, relative regulations regarding the control of OTC residues in live animal and animal products have been established by the World Health Organization to protect public health. The daily OTC intake should be strictly in the range of 0–30 μg·kg−1 body weight. And also the Environmental Protection Agency (EPA) stipulates that the maximum allowable amount of OTC in some fruits such as apple, pear and so on is 350 ng·g−1 [2]. These strict regulations have increased demand for accurate, sensitive, selective and high throughput determination methods for OTC in milk.
Nowadays, several traditional methods for OTC residues determination are currently available, such as microbial inhibition screening methods [3], immunoassay methods [4], surface enhanced raman scattering (SERS) [5] and chromatography-mass spectrometry (LC–MS, HPLC–MS etc.) determination methods [6]. However, the microbial inhibition and enzyme-linked immunosorbent assay (ELISA) methods display low sensitivity, poor selectivity and time-consuming. Although these instrumental analytical methods are sensitive, few of them can be used for portable real time on-site determination. Therefore, the development of new methods for OTC determination with convenience, rapidity and real time on-site remain an important need in food safety monitoring.
Optical methods, especially fluorometry, have received extensive attention in analytical fields due to their advantages of simple operation, excellent sensitivity, real-time determination and low cost [7, 8]. Several fluorescent sensors such as metal-organic coordination polymers [9, 10] and carbon dots [11, 12] have been designed to detect OTC. However, most of these reported fluorescent sensors are easily to be suffered from the external factors, such as probe concentration, excitation intensity etc. due to their single emission spectral properties, thus their determination sensitivity and selectivity are greatly restricted. To overcome this disadvantage, the ratiometric fluorescent sensors based on dual emissions may be the superior candidates [13, 14]. Ratiometric fluorescent sensors are usually constructed by dual emission fluorophores which function as reference unit or response moiety. Many fluorescent materials, especially semiconductor quantum dots have selected as the reference or response signal. Whereas semiconductor quantum dots with high and stable fluorescence yields would leak the toxic compounds (such as CdSe, CdTe) thus induce the severe toxicity to the outer environment [15, 16]. As a most popular new luminescent nanomaterials, CD exhibit high water solubility, superior photostability, low toxicity, high stability and size tunable emission spectral properties. The unique properties of CD lead to their promising applications for ratiometric fluorescent sensor construction so as for environmental analysis and other areas [17, 18]. However, ultrasensitive ratiometric fluorescent probes for OTC determination have rarely been reported based on CD.
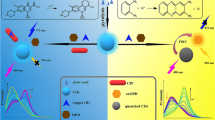
With the above considerations, a portable paper-based ratiometric probe based on CD doped 5′-adenosine monophosphate (AMP)/Eu nanoscale coordination polymers (CD@AMP/Eu NCPs) was developed for rapid and visual determination of OTC (Scheme 1). AMP has good water solubility and shows low coordination ability to Eu3+. The coordination of water molecules results in fluorescence quenching of Eu3+ in AMP/Eu NCPs [19]. CD@AMP/Eu NCPs show a strong characteristic emission peak of CD at 430 nm and no fluorescence of Eu3+can be observed. OTC is an ideal ligand and can sensitize the fluorescence of Eu3+ [20]. As predicted, upon addition of OTC, the fluorescence of Eu3+ enhanced, while that of the CD decreased. Therefore, CD@AMP/Eu NCPs can be used as a ratiometric fluorescent probe for OTC.
Experimental
Materials
Europium nitrate (Eu(NO3)3·6H2O, 99.99%), tetracycline (TC) and chlorotetracycline (CTC), citrate (CA), cysteine (Cys), phenylalanine (Phe), valine (Val), lysine (Lys), proline (Pro), tyrosine (Tyr), glutamic acid (Glu), alanine (Ala), glutathione (GSH), glucose, ascorbic acid (Aa), N-2-hydroxyethyl piperazine-N′-2-ethanesulfonic acid (HEPES), OTC and AMP, chloramphenicol (CAP), sulfamonomethoxine (SMM), trimethoprim (Trim) and ceftriaxone (Cef) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China) (www.aladdine.com). All reagents were analytical grade and used without further purifications. Different pH solutions were obtained by mixing 50 mM HEPES and appropriate amount of 1 M NaOH. Ultrapure water from a Milli-Q equipment was used to prepare throughout this work.
Instrument
The morphologies of CD and CD@Eu/AMP nanocomposite materials were measured by transmission electron microscopy (TEM, JEM-2100, Japan). UV–visible absorption spectra were obtained on Lambda 35 spectrophotometer (Perkin Elmer, U.K.). An Avatar 360 FTIR spectrometer (Nicolet, USA) was used to record Fourier transform infrared spectra (FTIR). The X-ray diffraction (XRD) spectrum was performed with an AXS-D8 X-Ray diffractometer (Bruker, Germany). The measurements of fluorescence spectra were monitored on an LS-55 fluorescence spectrometer (PerkinElmer, UK). All the measurements were repeated for 3 times.
Synthesis of CD@AMP/Eu nanocomposites
The water-soluble CD was synthesized based on the previous report [21]. To prepare CD@AMP/Eu nanocomposites, 4.0 mL of 10 mM AMP stock solution and 500 μL CD solution (400 mg/L) were mixed together under magnetic stirring, 30 min later, followed by addition of 4.0 mL Eu(NO3)3·6H2O solution (10 mM) and the stirring maintained for 2 h at ambient temperature. Then the precipitate was separated by centrifuging and washing with massive ultrapure water. Finally, the solid products obtained were dried at 60 °C under vacuum for 24 h. To prepare CD@AMP/Eu suspension, 10.0 mg of CD@AMP/Eu powder was dissolved in 5.0 mL ultrapure water and sonicated for 10 min.
Procedures for quantitative determination of OTC in water
For the quantitative determination of OTC, various amounts of OTC were first added to 1.5 mL HEPES buffer containing 200 μL of CD@AMP/Eu (2.0 mg·mL−1) suspension, the final OTC concentrations were in the range of 0–100 μM. 3 min later, the emission spectra of the solutions were tested under 310 nm excitation. To test the effects of coexistent substances on the fluorescence properties of CD@AMP/Eu solution, 9 μL of 10 mM amino acids (Val, Pro, Gly, Thr, Glu, Ala, Lys, Phe, Tyr and Cys), GSH, glucose, Aa, metal ions (including Ca2+, Mg2+, Al3+, Fe3+, Zn2+, K+ and Cu2+) or Cl− was added into 1.5 mL HEPES buffer containing 200 μL of CD@AMP/Eu solution, respectively. The identification experiments of different antibiotics (TC, CTC, CAP, SMM, Trim and Cef) were carried out. The test procedure is as metioned above. The competition assays were also performed by introducing these coexisting substances into CD@AMP/Eu-OTC solution, respectively. After 3 min, fluorescence intensities at 430 and 615 nm were monitored to evaluate the influence of coexisting substances on the OTC selective determination ability.
Determination of OTC in milk samples
The milk samples used in this assay were Mengniu milk purchased from Nanchang Walmart Supercenter and were treated according to the previous report with minor modifications [9]. Briefly, 2 mL of trichloroacetic acid solution (0.61 M) was introduced to 10 mL of liquid milk to remove proteins. After sonicated for 20 min, the mixture solution was then centrifuged and filtered to eliminate lipids by using a 0.22 μm membrane and adjusted to pH 7.0 with NaOH solution. Milk samples with appropriated amount of OTC were obtained by diluting a stock solution of OTC (10 mM). Then, 200 μL of the spiked milk samples were added to 1.3 mL HEPES buffer containing 200 μL of CD@AMP/Eu (2.0 mg·mL−1) suspension. The fluorescence intensities at 430 and 615 nm were monitored under 310 nm excitation.
Fabrication of the paper-based ratiometric probe for OTC determination
Paper-based ratiometric probe was prepared by using common fiber filter paper as carrier, which was cut into 6-mm-diameter circular strips by a common hole puncher. The paper strips were then immersed into CD@AMP/Eu aqueous solution (2 mg·mL−1). After 20 s, the paper strips were taken out and dried at 40 °C under vacuum for 4 h. The dried paper-based ratiometric probes are sealed and preserved in the dark. For quantitative determination of OTC concentration, 10 μL of OTC solution with different concentrations (1–100 μM) was dropped on the prepared paper strips. Under irradiation of 302 nm UV lamp, the paper-based ratiometric probe displayed a visible fluorescence color switching and a smartphone was used to take photos of the fluorescence colors. The color parameters such as red (R), green (G) and blue (B) of the photos were quickly obtained by a color detector APP (Maarten Zonneveld) equipped in the smartphone (Huawei Glory 8). The OTC concentrations were calculated according to linear equation between the ratio value of R/B and the OTC concentration. The milk samples used in the paper-based assay were pretreated as the above mentioned. Standard addition method was used by the paper-based assay for the milk samples.
Results and discussion
Characterization of carbon dots (CDs) and CD@Eu/AMP nanocomposites
In this study, the structures of CD and CD@Eu/AMP were characterized systematically. TEM images (in Fig. 1a) clearly reveal that the average particle size of 20 CD is 5 nm. The HRTEM image shows that the lattice spacing of CD is 0.20 nm (inset of Fig. 1a), which is consistent with that of graphene carbon (100 facet) [22]. SEM image (Fig. 1b) reveals that the CD@AMP/Eu forms a 3D nanoparticle network structure. XRD pattern of CD (Fig. S1(A)) displays the presence of a strong peak at 21.5° attributed to the highly disordered C species [23]. XRD pattern in Fig. S1(B) demonstrates that CD@AMP/Eu nanocomposites are amorphous. EDS spectra of CD@AMP/Eu in Fig. S2 reveal the presence of C, N, O, P and Eu elements in the CD@AMP/Eu nanocomposites.
The FTIR spectra of CD, CD@AMP/Eu and CD@AMP/Eu -OTC nanocomposites are displayed in Fig. 2. Three characteristic peaks at 3422, 1722 and 1632 cm−1 are presented in CD spectra which are ascribed to the stretching vibration modes of OH, C=O and C=C groups, respectively [24], suggesting that abundant OH and C=O groups are distributed on the surface of CD. Compared with the FTIR spectra of CD, the peak of C=O of CD@AMP/Eu shifts from 1722 to 1710 cm−1, indicating that Eu/AMP combine with CD through C=O groups. The vibration peaks of P-O and C-N of AMP appear at 1080 and 1384 cm−1, respectively, which suggest that Eu3+ has coordinated with AMP and the CD@AMP/Eu nanocomposites have synthesized successfully [25].
Optical properties of CD and of CD@Eu/AMP nanocomposites
UV-Vis absorption spectrum and fluorescence spectrum of CD solution are depicted in Fig. S3. It is noteworthy that the CD presents two strong absorption peaks at 220 and 290 nm. The CD aqueous solution emitts strong blue fluorescence after 302 nm UV irradiation (inset in Fig. S3). The position of the CD maximum emission maintains constant when the excitation wavelength varies from 260 to 320 nm (Fig. S4), which demonstrates that the emission property of CD is excitation independence as a result of uniform particle size and surface states, which is similar to other luminescent CD derived from CA [26]. As shown in Fig. 3a, the UV–vis absorption peaks of OTC and CD@AMP/Eu appear at 369, 276 and 263 nm, respectively. While the peak at 369 nm red-shifts about 20 nm to 389 nm with the addition of OTC. The great red-shift for the absorption peak of OTC indicates the strong affinity between OTC and CD@AMP/Eu. Fluorescence spectra of CD@Eu/AMP are illustrated in Fig. 3b, which appear a strong fluorescence peak at 430 nm assigned to the encapsulated CD. Upon addition of OTC, the emission of CD@Eu/AMP nanocomposites presents two new peaks at 590 and 615 nm which are originated from 5D0 to 7F1 and 5D0 to 7F2 transitions of Eu3+, respectively, and the characteristic peaks of Eu3+ can discriminate from the blue emission of CD at 430 nm. Upon addition of 60 μM OTC, the fluorescence intensity of Eu3+ in CD@AMP/Eu solution shows 7-folds higher than that of Eu3+in AMP/Eu solution. The result attributes to the fact that CD has incorporated into AMP/Eu coordination polymer and removed the coordinated water molecules to enhance the emission of Eu3+.
Determination mechanism
Upon the addition of OTC, O in carbonyl group of OTC showed strong bonding ability with Eu3+ and sensitized its luminescence by energy transfer, which caused the fluorescence enhancement of Eu3+. FTIR spectra were used to study the possible quenching mechanism of CD by OTC. In the FTIR spectra of CD@AMP/Eu-OTC (in Fig. 2), two strong peaks are observed centring at 1600 cm−1 and 3400 cm−1, and the peak of C=O at 1710 cm−1 dispears which may overlap with 1600 cm−1, indicating that the hydrogen bonds are formed between C=O groups of CD and OH groups of OTC. The formation of hydrogen bonds results in the shift of C=O adsorption band from high to low frequency [27]. The results show that hydrogen bond interaction exists between CD and OTC. The UV-vis absorption spectrum of OTC was compared with the excitation and emission spectrum of CD. The results in Fig. S5 reveal that the absorption spectrum of OTC exists a wide spectrum overlap with both excitation and emission spectrum of CD. Therefore, the fluorescence quenching of CD results from the inner filter effect (IFE) [28]. The lifetimes of CD in CD@AMP/Eu solution without and with OTC were measured to further study the quenching mechanism. The lifetimes of CD in CD@AMP/Eu and CD@AMP/Eu -OTC are 5.36 and 5.85 ns (Fig. S6), respectively. No obvious change in lifetime suggests that the fluorescence quenching of CD by OTC mainly results from IFE.
Performance of CD@AMP/Eu ratiometric probe
In order to gain a high determination sensitivity for OTC, some important experimental conditions including pH value, stability of the ratiometric probe and reaction time for OTC determination were optimized. At pH of 7–8 (Fig. S7), Eu3+ shows a strong emission peak, and CD displays moderate emission intensity, indicating that neutral conditions are more favorable for fluorescence determination of OTC. The stability of CD@AMP/Eu ratiometric probe was examined at room temperature over 50 days (Fig. S8). The result indicates that CD@AMP/Eu ratiometric probe in aqueous solution shows excellent optical stability and dispersion. The fluorescent response rate of CD@AMP/Eu toward OTC was determined at different time intervals. The fluorescence intensity ratio at 615 and 430 nm arrives at its maximum after 3 min (Fig. S9). The results indicate CD@AMP/Eu ratiometric probe is a fast response ratiometric probe toward OTC determination, and 3 min is chosen in subsequent experiments.
The sensitivity of CD@AMP/Eu ratiometric probe for OTC determination was evaluated. As indicated in Fig. 4a, the presence of OTC causes that the red fluorescence at 615 nm enhances obviously while the blue emission at 430 nm declines dramatically under the excitation of 310 nm. The intensity ratio of F615/F430 enhances linearly with the increase OTC concentration ranging from 0.2 to 60.0 μM (Fig. 4b). A good correlation coefficient square (R2 = 0.995) and a LOD of 25 nM (3σ) are obtained. The LOD value is much lower than most of the reported methods (Table S1). Although MIL-101, aptamer and RecJf mixed ratiometric probe [29] is sensitive for the OTC determination, the linearity range is narrower (0.02–20.0 μM) than that of CD@AMP/Eu ratiometric probe. Figure 4c shows that the fluorescence color of CD@AMP/Eu solution can be clearly observed under a UV lamp, which gradually changes from blue to red with OTC concentrations ranging from 0 to 80 μM. Accordingly, CD@AMP/Eu is a sensitive and reliable ratiometric probe for OTC determination.
a Luminescence properties of CD@Eu/AMP ratiometric probe exposure to various OTC concentrations (0, 0.05, 0.1, 0.2, 0.3, 0.5, 0.8, 2, 5, 10, 15, 20, 25, 30, 40, 60, 100 μM, pH = 7); b Linear plot of F615/F430 versus OTC concentrations (0.2 to 60 μM, under 310 nm excitation, n = 3); c Fluorescence color photos of CD@AMP/Eu ratiometric probe over various OTC concentrations. (under 302 nm UV lamp)
The milk sample contains a variety of substances such as common metal ions, amino acids, glucose and vitamins which can influence the determination ability of CD@AMP/Eu ratiometric probe for OTC. Therefore, these coexistent substances were used to act as interferences to test the specificity of CD@AMP/Eu ratiometric probe for OTC. As displayed in Fig. 5, the fluorescence intensity ratio (F615/F430) presents a remarkable enhancement with only the addition of 60 μM OTC, while small or even negligible changes are observed in the presence of 60 μM amino acids (Lys, Phe, Tyr, Gly, Thr, Val, Pro, Glu, Ala, Cys and GSH), Aa, glucose, metal ions (Ca2+, Mg2+, Al3+, Fe3+, Zn2+, Cu2+ and K+) or Cl−, respectively. The recognition ability of CD@AMP/Eu ratiometric probe toward other antibiotics, such as TC, CTC, CAP, SMM, Trim and Cef were studied. Because of similar structure, the effects of TC and CTC on the fluorescence of CD@AMP/Eu are similar to that of OTC. Obviously, the fluorescence of CD@AMP/Eu shows little change in the presence of 60 μM CAP, SMM, Trim or Cef (Fig. S10). The competition experiments were also examined by addition of these coexisting substances. It can be observed that the fluorescence intensity ratios (F615/F430) of CD@AMP/Eu ratiometric probe exhibit no obvious influence upon addition of these coexisting substances (Fig. 5). Therefore, CD@AMP/Eu ratiometric probe displays an excellent selectivity toward OTC determination.
To reserve food security, the determination of OTC in milk is particularly important. Therefore, the applicability of CD@AMP/Eu ratiometric probe for OTC determination was investigated. Standard solutions with five various OTC concentrations were added to liquid milk samples (0.3, 0.5, 10, 20 and 50 μM final concentration). The results are shown in Table 1, the recoveries of the added standard OTC in milk ranged from 93.1 to 97.7% with relative standard derivation (RSD) values no more than 3.57% (n = 3). Compared with CD@AMP/Eu ratiometric probe, AMP/Eu as reference material shows weak responses and lower sensitivity in the presence of various OTC concentrations (Fig. S11). Therefore, AMP/Eu is not suitable to be used for monitoring OTC concentration in real sample. The results suggest that CD@AMP/Eu ratiometric probe possesses more advantages and can provide accurate and credible measurement for OTC concentration in milk.
Visual determination of OTC
Currently, Many analytical methods for OTC are seriously restricted because of their tedious operation or costly instrument. Thus, the development of a fast, convenient, sensitive method for OTC determination is really necessary. In view of this, a simple, portable paper-based visual ratiometric probe was developed by using CD@AMP/Eu immobilized on test paper for OTC determination. Under 302 nm UV irradiation, the paper-based ratiometric probe loaded with various amouts of OTC are observed significant fluorescence color changes from blue to red (the inset of Fig. 6). To make the fluorescence color switch easy for on-site determination, a smartphone was used to capture the fluorescence color image. A smartphone color detector APP was employed to calculate the color intensities of the image. The images of the paper-based ratiometric probe mainly show red (R) and blue (B) colors. The intensity ratio of R/B shows good linear relationship with the OTC concentration (1–100 μM) and LOG for OTC is calculated approximately to be 0.5 μM. The correlation coefficient square (R2) is 0.980 (Fig. 6). Compared with the assay of CD@AMP/Eu ratiometric probe in solution, a lower R2 is attributed to that the sensitivity of color detector APP is lower than that of fluorescence spectrometer. To improve the accuracy, a more advanced color detector APP with high sensitivity should be designed. Although the R2 is a little low, it is still high enough to accurately measure the content of OTC. Further study has been carried out about the stability of the paper-based ratiometric probe. Sealed in the dark at room temperature for 2 months, the paper-based ratiometric probe was used to detect OTC concentrations in spiked milk samples (Table 2). The fluorescence color changes of the test papers with different OTC concentrations were found from deep blue to light blue or light red under UV lamp. The recoveries were calculated in the range of 92.0 to 108.0%. The satisfactory results demonstrate that ratiometric fluorescent (F615/F430) paper-based assay can eliminate the interference of spontaneous fluorescence of the biomatter and the influence of weaken signal caused by UV absorbers in treated milk. Therefore, the paper-based ratiometric probe is suitable for visual monitoring of OTC concent in practical samples.
Conclusions
A highly selective and sensitive ratiometric fluorescent probe CD@AMP/Eu has been developed for OTC determination. The presence of OTC resulted in distinct quenching of CD fluorescence because of the inner filter effect, while the fluorescence of Eu3+ increased gradually due to antenna effect of OTC. The CD@AMP/Eu ratiometric probe showed a wide response range (0.2–60.0 μM) for OTC determination and a low LOD of 25 nM. More significantly, a visual paper-based ratiometric probe based on CD@AMP/Eu nanocomposites has been fabricated for monitoring OTC under a portable UV lamp with the assistant of a smartphone. Fluorescence color of the paper-based probe can be observed changing from blue to red with naked eyes. Although the sensitivity of the paper-based ratiometric probe is not high enough (LOD = 0.5 μM for OTC), it presents a wide linear range (1–100 μM). The visual paper-based ratiometric probe is convenient, rapid and inexpensive and meets the requirement of accurate monitoring of OTC concentration in real sample.
References
Dai JD, Wei X, Cao ZJ, Zhou ZP, Yu P, Pan JM, Zou TB, Li CX, Yan Y (2014) Highly-controllable imprinted polymer nanoshell at the surface of magnetic halloysite nanotubes for selective recognition and rapid adsorption of tetracycline. RSC Adv 4(16):7967–7978
Chen GY, Liu GY, Qin F (2011) Use of a portable time-resolved fluorometer to determine oxytetracycline residue in four fruit crops. Food Chem 127(1):264–269
Kusano T, Kanda M, Kamata K, Miyazaki T (2004) Microbiological method for the determination of antibiotic residues in meat using mixed-mode, reverse-phase and cation-exchange cartridge. J Food Hyg Soc Jpn 45(4):191–196
Aga DS, Goldfish R, Kulshrestha P (2003) Application of ELISA in determining the fate of tetracyclines in land-applied livestock wastes. Analyst 128(6):658–662
Li YT, Qu LL, Li DW, Song QX, Fathi F, Long YT (2013) Rapid and sensitive in-situ determination of polar antibiotics in water using a disposable Ag-graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens Bioelectron 43:94–100
Zhu J, Snow DD, Cassada DA, Monson SJ, Spalding RF (2001) Analysis of oxytetracycline, tetracycline, and chlortetracycline in water using solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A 928(2):177–186
Tan B, Zhao HM, Du L, Gan XR, Quan X (2016) A versatile fluorescent biosensor probe based on target-responsive graphene oxide hydrogel for antibiotic determination. Biosens Bioelectron 83:267–273
Ma XH, Du CC, Zhang JL, Shang MX, Song WB (2019) A system composed of vanadium(IV) disulfide quantum dots and molybdenum(IV) disulfide nanosheets for use in an aptamer-based fluorometric tetracycline assay. Microchim Acta 186:837
Tan HL, Ma CJ, Song YH, Xu FG, Chen SH, Wang L (2013) Determination of tetracycline in milk by using nucleotide/lanthanide coordination polymer-based ternary complex. Biosens Bioelectron 50:447–452
Esmaelpourfarkhani M, Abnous K, Taghdisi SM, Chamsaz M (2019) A fluorometric assay for oxytetracycline based on the use of its europium(III) complex and aptamer-modified silver nanoparticles. Microchim Acta 186(5):290
Qu F, Sun Z, Liu DY, Zhao XE, You JM (2016) Direct and indirect fluorescent determination of tetracyclines using dually emitting carbon dots. Microchim Acta 183(9):2547–2553
Yang XM, Luo YW, Zhu SS, Feng YJ, Zhuo Y, Dou Y (2014) One pot synthesis of high fluorescent carbon nanoparticles and their applications as probes for determination of tetracyclines. Biosens Bioelectron 56:6–11
Zhu A, Qu Q, Shao X, Kong B, Tian Y (2012) Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent probe for in vivo imaging of cellular copper ions. Chem Int Ed Engl 51(29):7185–7189
Singh N, Kaur N, Mulrooney RC, Callan JF (2008) A ratiometric fluorescent probe for magnesium employing excited state intramolecular proton transfer. Tetrahedron Lett 49(47):6690–6692
Freeman R, Willner I (2012) Optical molecular sensing with semiconductor quantum dots (QDs). Chem Soc Rev 41:4067–4085
Ma XF, Sun R, Cheng JH, Liu JY, Gou F, Xiang HF, Zhou XG (2016) Fluorescence aggregation-caused quenching versus aggregation induced emission: a visual teaching technology for undergraduate chemistry students. J Chem Educ 93:345–350
Li Q, Sun K, Chang K, Yu J, Chiu DT, Wu C, Qin WP (2013) Ratiometric luminescent determination of bacterial spores with terbium chelateds emiconducting polymer dots. Anal Chem 85(19):9087–9090
Lu LL, Feng CC, Xu J, Wang FY, Yu HJ, Xu ZA, Zhang W (2017) Hydrophobic-carbon-dot-based dual-emission micelle for ratiometric fluorescence biosensing and imaging of Cu2+ in liver cells. Biosens Bioelectron 92:101–108
Tan HL, Ma CJ, Chen LL, Xu FG, Chen SH, Wang L (2014) Nanoscaled lanthanide/nucleotide coordination polymer for detection of an anthrax biomarker. Sensors Actuators B Chem 190:621–626
Tan HL, Liu BX, Chen Y (2012) Luminescence nucleotide/Eu3+ coordination polymer based on the inclusion of tetracycline. J Phys Chem C 116:2292–2296
Guo XJ, Xu LP, Zhang LZ, Wang HY, Wang XM, Liu XH, Yao J, Hao AJ (2018) One-pot solid phase pyrolysis synthesis of highly fluorescent nitrogen-doped carbon dots and the interaction with human serum albumin. J Lumin 196:100–110
Iqbal A, Iqbal K, Xu L, Li B, Gong D, Liu X, Guo Y, Liu W, Qin W, Guo H (2018) Heterogeneous synthesis of nitrogen-doped carbon dots prepared via anhydrous citric acid and melamine for selective and sensitive turn on-off-on determination of Hg(II), glutathione and its cellular imaging. Sensors Actuators B: Chem 255:1130–1138
Hou J, Wang L, Zhang P, Xu Y, Ding L (2015) Facile synthesis of carbon dots in an immiscible system with excitation-independent emission and thermally activated delayed fluorescence. Chem Commun 51(100):17768–17771
Shen L, Chen M, Hu L, Chen X, Wang J (2013) Growth and stabilization of silver nanoparticles on carbon dots and sensing application. Langmuir 29(52):16135–16140
Nishiyabu R, Hashimoto N, Cho T, Watanabe K, Yasunaga T, Endo A, Kaneko K, Niidome T, Murata M, Adachi C (2009) Nanoparticles of adaptive supramolecular networks self-assembled from nucleotides and lanthanide ions. J Am Chem Soc 131(6):2151–2158
Qin ZX, Wang WH, Zhan XZ, Du XJ, Zhang QM, Zhang R, Li K, Li JH, Xu WP (2019) One-pot synthesis of dual carbon dots using only an N and S co-existed dopant for fluorescence determination of Ag+. Spectrochim Acta A Mol Biomol Spectrosc 208:162–171
Wang P, Li Y, Zhang C, Feng FQ, Zhang H (2020) Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem 308:125599
Miao H, Wang YY, Yang XM (2018) Carbon dots derived from tobacco for visually distinguishing and detecting three kinds of tetracyclines. Nanoscale 10(17):8139–8145
He JC, Li GK, Hu YL (2017) Aptamer-involved fluorescence amplification strategy facilitated by directional enzymatic hydrolysis for bioassays based on a metal-organic framework platform: highly selective and sensitive determination of thrombin and oxytetracycline. Microchim Acta 184:2365–2373
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21465015), the program of Scientific Research Fund of Jiang Xi Provincial Education Department (grant no. 150366).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 794 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Xu, H., Wang, L. et al. Portable ratiometric probe based on the use of europium(III) coordination polymers doped with carbon dots for visual fluorometric determination of oxytetracycline. Microchim Acta 187, 125 (2020). https://doi.org/10.1007/s00604-019-4104-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4104-3