Abstract
A fluorescence off-on sensing platform was developed based on thioglycolic acid-stabilized cadmium sulfide quantum dots (CdS QDs) as fluorescence probe for the sensitive and selective detection of 2,6-pyridinedicarboxylic acid (DPA) in spores. The fluorescence emission intensity of the quantum dots at 650 nm when excited at 460 nm was first quenched by mixing with europium ions (Eu3+) and then recovered after the addition of DPA. The interaction of DPA with Eu3+ relieved the quenching effect of Eu3+ toward CdS QDs. As the DPA concentration increases, the color of the probe changes from colorless to red. The method exhibits a wide linear range from 1 to 120 μM for DPA determination, with a detection limit of 0.2 μM. The CdS QDs based nanoprobe was successfully applied for sensitive determination of DPA released from bacteria spores. In this case, the detection limit is 3.5 × 104 CFU·mL−1.

An off-on fluorescence sensor for detecting anthrax markers -2,6-pyridinedicarboxylic acid though restoring the fluorescence of cadmium sulfide quantum dots quenching by europium ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthrax, the pathogen of which is anthrax bacillus, is a serious infectious diseases that threat to human health [1]. Once infected with anthrax bacillus, the spores of anthrax bacillus will enter the organism and multiply rapidly to form a capsule and release toxin [2]. More dangerously, people will die within 36 h if they inhaled more than 104 spores. So the prevention of anthrax have always been one of the focus of clinical medical research [3, 4].
Anthrax bacillus will be devitalized using disinfectant and some conventional sterilization methods. However, anthrax bacillus spores are highly resistant to high temperature, radiation, dryness, extreme pH, and other adverse conditions, so that they can survive for decades in the external environment [5]. The strong stress resistance mainly depends on the highly dehydrated and high density of 2,6-pyridinedicarboxylic acid (DPA) and its calcium salts (Ca-DPA) in the core area and cytoplasm of spores [6]. DPA, accounting for 5–15% of the dry mass of spores, is therefore an essential component in spores and is often chosen as a marker for spores germination analysis [7].
Effective detection of Bacillus anthracis spores is an important way for prevention and treatment of anthrax. Dormant spores may germinate when the surrounding micro-environment is favorable for their growth. They may eventually convert back into growing cells, with duration time of 20–30 min. The process of spores germination is accompanied by release of DPA, the concentration of which corresponded to the number of spores in real time. Therefore, sensors developed for effective detecting changes of DPA levels in spores should be fast in response and can be long-termly operated, which may provide an immediate response to DPA concentration fluctuations. In practice, however, monitoring DPA concentration fluctuations during spores germination is very challenging. In recent years, many researchers have made profound studies in this field. [8] Some methods, such as chromatography [9] and surface-enhanced Raman technology [10], have been developed to detect DPA. Although DPA concentration can be accurately detected by these methods, it was hard to achieve rapid on-site detection due to the cumbersome operation procedures of these traditional methods. Fluorescence detection has a great advantage compared with these methods due to its convenience and relative rapidity. [11, 12]
Quantum dots are widely used in the fields of photocatalysis, photoluminescence, and photoelectric conversion due to quantum restriction effect. For example, carbon quantum dots have been used to detect anthrax markers, although the detection range was not wide enough and still needs to be further improved, it has inspired good ideas [13, 14]. Compared with traditional organic luminous materials, quantum dots exhibit brilliant advantages including continuous excitation spectrum, symmetrical emission spectrum, high luminous efficiency, and very strong anti-light bleaching ability [15]. Moreover, as a result of large stoker displacement, the spectral overlap between fluorescence emission and excitation can be significantly avoided, and the detection sensitivity and stability of the fluorescence signal can be improved through quantum dots [13]. Due to the effect of quantum confinement, the size and chemical composition of quantum dots can be controlled through synthesis process, and nanoparticles with emission spectrum from visible light to near-infrared region can be obtained under a single excitation light source [16, 17]. Among them, water-dispersed CdS QDs with high quantum yield have shown great potential application value in many fields, such as biological imaging, water-phase ion detection, and especially bioluminescence labeling [18].
Traditional methods for quantum dots synthesis were almost carried out in organic phase. A lot of research has been devoted to develop new methods in order to increase their practical applications, mainly including the modification of ligands on the surface of quantum dots [19]. Commonly used ligands include sulfhydryl group stabilizers and chitosan. As a stabilizer, thioglycolic acid exhibits ability of modifying carboxylic groups on the surface of CdS quantum dots, increasing their water-solubility and providing active sites for reaction [20, 21]. It has been reported that the luminescence of quantum dots with a modified surface can be quenched by transition metal ions due to electron transfer and agglomeration, which were mostly irreversible [22, 23]. However, after quenching by lanthanide ions, it was found that the fluorescence could be restored, which was a reversible process [24].
Coincidentally, particularly strong coordination between lanthanide ions and DPA has been reported [25]. Especially, compared with Tb3+, Eu3+ has obvious advantages in eliminating second-order scattering interference and long-wavelength red light emission of visible spectrum. Coordination water will be replaced by the tridentate ligand of DPA in the solution to form Eu-DPA chelate, which is more stable [26].
This concept inspired us to design a novel fluorescent probe to detect DPA in spores based on CdS quantum dots stabilized by thioglycolic acid. Briefly, the surface of CdS quantum dots prepared in water system was modified with a large number of carboxylic groups. After europium ions were added into the system, fluorescence quenching of quantum dots was caused by electron transfer and agglomeration, which was a reversible process. DPA was then added to the system to compete with cadmium sulfide for europium ions, and the electron transfer process on the surface of the quantum dot was terminated, the quantum dot was also dispersed evenly again, leading to fluorescence recovery. Under optimized experimental conditions, the probe exhibits a stable response for DPA over a concentration ranging from 1 to 120 μM, with a detection limit of 0.2 μM. It is worth noting that this probe has good specificity for DPA, and the fluorescence of quantum dots was almost completely recovered with the increase of DPA concentration.
Experimental
Materials and reagents
Thioglycolic acid, sodium hydroxide, and sodium sulfide was bought from Macklin. Benzoic acid, terephthalic acid, dodecylamine, and isophthalic acid were purchased from Tokyo Chemical Industry Co., Ltd. (https://www.tcichemicals.com/). 2,6-Pyridinedicarboxylic acid, 2-dipicolinic acid, Cd(NO3)2·4H2O and Eu(NO3)2·6H2O were obtained from Sigma-Aldrich (https://www.sigmaaldrich.com/). Bacillus subtilis (reference number ATCC6633) were acquired from Shanghai Luwei Technology Co. Ltd. (Shanghai, China, http://www.luweibio.com/). All other reagents were of analytical grade and used without further purification. Ultra-pure water was used throughout all experiments.
Apparatus
Transmission electron microscope (TEM) characterization was performed on a Tecnai G2 F20 S-TWIN TMP microscopy. The fluorescence spectra and relative fluorescence intensity (FL) were recorded on a F-7000 spectrofluorometer (Hitachi, Japan) with a fixed excitation of 460 nm. Absorption spectra were recorded on a Shimadzu UV-2450 spectrophotometer. Fourier infrared spectra (FTIR) were scanned on a Bruker Vertex 70 FTIR spectrophotometer. X-ray diffraction (XRD) was recorded on an Empyrean X-ray diffractometer.
Preparation of TGA-CdS QDs
The thioglycolic acid capped CdS QDs (TGA-CdS QDs) were synthesized according to a previously reported method [27]. Briefly, 0.1190 g Cd(NO3)2·4H2O was dissolved in 50 mL ultra-pure water and 300 μL TGA was added under stirring. The solution was then heated to boil and refluxed under nitrogen protection for 30 min. After that, the pH was adjusted to alkalinity and 5 mL of Na2S·9H2O (0.1 M) solution was added. The solution continued to flow back in a nitrogen atmosphere for 200 min. The final QDs products were collected by precipitation with isopropyl alcohol three times and re-dispersed in equal volume of water.
Fluorescence detection for DPA concentration
To utilize the prepared probe for DPA concentration detection, 70 μL of DPA with different concentrations ranging from 0 to 200 μM were added to 200 μL of CdS solution mixed with 30 μL of 20 μM europium ion solution, and fluorescence spectra were recorded immediately with excitation wavelength of 460 nm.
Spores germination and fluorescence detection
To enable spores to germination, Bacillus subtilis spores suspension (7.1 × 109 CFU·mL−1) was diluted to solutions with different concentrations. 2 mM of dodecylamine was added to these diluted suspension after preheating at 90 °C for 30 min [28]. The spores were almost germinated completely after reacting at room temperature for 30 min. After that, 70 μL of germinated solution was added to 230 μL of CdS-Eu mixture. Fluorescence emission spectra ranging from 500 to 800 nm were recorded immediately when excited at 460 nm.
Results and discussion
Design of fluorescence nanoprobe for DPA detection
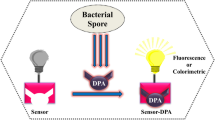
As Scheme 1 shows, soluble fluorescent probe for monitoring of DPA concentration in bacterial spores was designed. Fluorescent CdS QDs coated with thioglycolic acid were utilized as the probe due to their excellent optical properties as semiconductors, which enabling reliable detection results for biomarkers. Cationics can be used to induce fast quenching of fluorescent CdS QDs because they can combine with the carboxylic groups on the surface of quantum dots, resulting electron transfer and quantum dot aggregation. Different from irreversible quenching of quantum dot fluorescence by most other metal cations, the electron transfer of lanthanide ions occurred on the surface of quantum dot [29]. Once a new competing material was added, the quantum dot can be uniformly re-dispersed again, leading to recovery of the luminescence. So, europium ions were first added to the system to quench the fluorescence of CdS QDs. After the addition of DPA, DPA competed with CdS QDs for Eu3+ as the tridentate ligand of DPA has a stronger coordination effect on Eu3+ [30]. It enabled quick recovery of the fluorescence of quantum dots, even to the intensity before quenching, which provided a way for realizing high selective detection of DPA concentration in bacterial spores.
Preparation and characterization of fluorescent probe
CdS quantum dots stabilized by thioglycolic acid were synthesized by a classical hydrothermal method. The transmission electron microscope (TEM, Fig. 1a, c) images and the particle size distribution (Fig. S1) show morphology of the synthesized quantum dots, which are spherical and uniformly dispersed with an average particle size of about 2.7 nm. Further characterization of CdS was performed using powder X-ray diffraction (PXRD, Fig. 1d). It can be clearly seen that three strong diffraction peaks appeared at the positions of 2θ = 26°, 2θ = 44°, and 2θ = 51°, which correspond to three crystal planes of (111), (220), and (331) in the cubic structure of CdS, respectively [31]. These proved the synthesized CdS was a cubic zinc structure. To ascertain that the solvothermal synthesis technique indeed produced TGA-capped CdS QDs, the prepared CdS QDs was further characterized by Fourier-transform infrared spectroscopy (FTIR) (Fig. S2a). The FTIR broad absorption peaks at 3382 cm−1 in the spectrum of CdS QDs is the stretching vibration peak of O-H bond. The peaks at 2930 and 2853 cm−1 are assigned to the stretching and bending of CH2, and the two peaks at 1635 as well as 1386 cm−1 in the FTIR spectrum are attributed to the vibrations of C=O bond. Meanwhile, no characteristic peaks of the S-H bond are observed between 2600 and 2550 cm−1, indicating that the S-H bond is broken in thioglycolic acid, that is TGA is successfully modified on the surface of cadmium sulfide nanoparticles [32, 33]. Fluorescence spectrum and ultraviolet spectrum were utilized to record optical properties of CdS quantum dots (Fig. S2b). The excitation peak appeared around 460 nm, which is basically consistent with the absorption peak (Fig. S2c). Luminescence intensity at 650 nm is observed when excited at 460 nm. Moreover, luminescence intensity is contiguously monitored for 10 min and recorded for 5 weeks at intervals. As shown in Fig. S2d, although the fluorescence quenching of CdS is nearly 18% after 5 weeks, the fluorescence emission intensity remains basically unchanged within dozens of minutes, which satisfied the determination of DPA content within 30 min during spores germination.
Optimization of experimental parameters
To prove that the fluorescence quenching after adding europium ions was caused by aggregation, TEM images were also taken for CdS-Eu3+ complex (Fig. 1a, b). Under the same magnification, it is obvious that quantum dots changed from the original uniform dispersion to aggregation state. To further study the effect of concentration of europium ions on the fluorescence quenching of CdS, various concentrations of europium ions were mixed with quantum dot solution and the change of fluorescence intensity was immediately recorded. With the increase of Eu3+ concentration, fluorescence intensity of CdS decreased significantly, and remained stable until the concentration of Eu3+ reached 20 μM, which was therefore chosen as the optimal concentration of Eu3+ (Fig. 2a).
a Fluorescence intensity of CdS QDs with the increase of europium ions concentration. The inset is fluorescence spectra of CdS QDs mixed with different concentrations of Eu3+. b Fluorescence quenching efficiency of 20 μM Eu3+ for CdS QDs (red dots) and fluorescence recovery efficiency of 100 μM DPA for CdS-Eu3+ complex (blue dots) in different pH values. c Fluorescence recovery efficiency with time extension after adding 100 μM DPA. Error bars show standard deviations (n = 3)
Stern–Volmer equation was also used to analyze the quenching process [34].
Where I0 and I are the fluorescence intensity in the absence and presence of Eu3+. KSV is the quenching constant and [Q] is the concentration of the quencher. From the Stern-Volmer curve (Fig. S3), it can be seen that when the concentration of europium ion is lower than 10 μmol per liter, the linear relationship is good, while when the concentration of europium ion is higher, the curve begins to curve towards the X-axis and the correlation coefficient decreases. This means that the fluorescence quenching of europium ion on the quantum dots only occurs on the surface, so the fluorescence cannot be completely quenched. So the modified Stern-Volmer equation was used to analysis the quenching data [35].
Where f is the fraction of accessible fluorophore. The dependence of I0/I0-I on the reciprocal of Eu3+ concentration is linear (Fig. S4). The KSV was calculated to be 4.35 × 105 M−1. And the value 1/f is fixed on the ordinate so the f was calculated to be 91%. The incubation time of the CdS-Eu3+ complex with DPA and pH of the solution were also studied to further determine the optimal reaction conditions (Fig. 2b, c). For determination of 100 μM DPA, the results show that there is little difference in quenching efficiency between pH 5 and 7, but the maximum recovery efficiency is achieved between pH 6 and 7 after DPA was mixed with the probe. So water solution with pH of 7 was chosen for the following experiments. It is worth noting that the fluorescence of the quantum dots recovered immediately after the DPA was mixed with the probe and remained stable for a certain period of time, which provided the conditions for real-time determination for DPA.
Sensitivity of CdS QDs for DPA fluorescence determination
The response of the CdS-Eu3+ complex to different concentrations of DPA was then recorded to test the linear range of the assay to DPA. The higher the concentration of DPA, the stronger the fluorescence recovery at 650 nm, indicating a DPA concentration-dependent response of the fluorescent probe. The fluorescence intensity at 650 nm was linearly proportional to DPA concentration ranging from 1 to 120 μM (Fig. 3a, b), with a detection limit of 0.2 μM (3σ/S). The test results were verified by the classical colorimetric method, which were performed using ammonium iron(II) sulfate and ascorbic acid for detection of DPA (data was shown in Supporting Information, Fig. S5). Compared with other similar monitoring methods, our method has a wider linear range and a relatively lower detection limit (Table 1). In addition, with the change of DPA concentration, the color of the probe will change significantly under the ultraviolet lamp, which provides the conditions for rapid detection.
a Fluorescence intensity as a function of DPA concentration ranging from 0 to 120 μM. The inset is photo for probe-DPA taken under the ultraviolet lamp with increased concentration of DPA from left to right. b Optimized detection range of this assay on DPA (inset) the linear range of the probe to DPA. Fluorescence emission at 650 nm was acquired to draw the calibration plot. Each sample was measured three times (n = 3)
Specificity for DPA detection
Specificity is a vital index to evaluate the performance of a new method. To verify specificity of the probe for DPA detection, 1 mM of several common metal cations and aromatic compounds with similar structure to the DPA were incubated respectively with the probe under same conditions. Figure 4a shows responses of the probe to these materials can be nearly ignored relative to 100 μM DPA. Even if these compounds were mixed with DPA, fluorescence emission intensity is almost not affected. These results proved that the CdS-Eu3+ fluorescent probe in this work is specific for DPA detection, indicating the possibility of practical application.
Fluorescence detection of DPA in germinated spores
Considering the practical application of the probe, the release of DPA from germinated spores was studied. Bacillus subtilis spores were collected (7.1 × 109 CFU·mL−1) and diluted to different concentrations. DPA was released after germination induced by dodecylamine (DOD) [40]. And then spores were incubated with the probe and fluorescence emission spectra were recorded immediately. As shown in Fig. 4b, spores without DOD treatment do not leads to fluorescence enhancement, while fluorescence of CdS quantum dots recovered significantly when spores germinated after treating by DOD, and the degree of fluorescence recovery is significantly increased as the concentration of spores increased. However, at present, only ~ 3.5 × 104 spores could be detected at the minimum, and methods that can achieve a lower detection limit are still under development.
Conclusions
In conclusion, a sensitive off-on fluorescent probe based on CdS quantum dots was developed to detect DPA in bacterial spores. The switch of fluorescence emission of quantum dot was realized through the competition between DPA and carboxylic group on the surface of quantum dot for binding with Eu3+. The quenching and recovery efficiency are all very high. The fluorescence recovery intensity has a brilliant linear relationship with DPA concentration in a wide range of concentrations. What is more gratifying is that with the change of DPA concentration, the color of the probe will change significantly under the ultraviolet lamp, which provides the conditions for rapid prediction. The probe exhibits excellent specificity and potential value in practical application. However, the activity and stability of quantum dots in water phase still need to be enhanced, which is also a condition worth optimization.
References
Collier RJ, Young JA (2003) Anthrax toxin. Annu Rev Cell Dev Bi 19(1):45–70
Friebe S, Van der Goot FG, Bürgi J (2016) The ins and outs of anthrax toxin. Toxins 8(3):69
Liu S, Zhang Y, Moayeri M, Liu J, Crown D, Fattah RJ, Wein AN, Yu Z-X, Finkel T, Leppla SH (2013) Key tissue targets responsible for anthrax-toxin-induced lethality. Nature 501(7465):63–68
Zhu M, Li M, Li G, Zhou Z, Liu H, Lei H, Shen Y, Wan Y (2015) Nanobody-based electrochemical immunoassay for Bacillus thuringiensis Cry1Ab toxin by detecting the enzymatic formation of polyaniline. Microchim Acta 182(15–16):2451–2459
Yi X, Setlow P (2010) Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192(13):3424–3433
Wang S, Setlow P, Y-q L (2015) Slow leakage of Ca-dipicolinic acid from individual Bacillus spores during initiation of spore germination. J Bacteriol 197(6):1095–1103
Moir A (2006) How do spores germinate? J Appl Microbiol 101(3):526–530
Cao J, Nagl S, Kothe E, Köhler JM (2015) Oxygen sensor nanoparticles for monitoring bacterial growth and characterization of dose–response functions in microfluidic screenings. Microchim Acta 182(1–2):385–394
Chenau J, Fenaille F, Ezan E, Morel N, Lamourette P, Goossens PL, Becher F (2011) Sensitive detection of Bacillus anthracis spores by immunocapture and liquid chromatography–tandem mass spectrometry. Anal Chem 83(22):8675–8682
Cowcher DP, Xu Y, Goodacre R (2013) Portable, quantitative detection of Bacillus bacterial spores using surface-enhanced Raman scattering. Anal Chem 85(6):3297–3302
Rong M, Liang Y, Zhao D, Chen B, Pan C, Deng X, Chen Y, He J (2018) A ratiometric fluorescence visual test paper for an anthrax biomarker based on functionalized manganese-doped carbon dots. Sensor Actuat B Chem 265:498–505
Wang Q-X, Xue S-F, Chen Z-H, Ma S-H, Zhang S, Shi G, Zhang M (2017) Dual lanthanide-doped complexes: the development of a time-resolved ratiometric fluorescent probe for anthrax biomarker and a paper-based visual sensor. Biosens Bioelectron 94:388–393
Wegner KD, Hildebrandt N (2015) Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem Soc Rev 44(14):4792–4834
Rong M, Deng X, Chi S, Huang L, Zhou Y, Shen Y, Chen X (2018) Ratiometric fluorometric determination of the anthrax biomarker 2,6-dipicolinic acid by using europium (III) -doped carbon dots in a test stripe. Microchim Acta 185(3):201
Ju M-G, Dai J, Ma L, Zhou Y, Zeng XC (2018) Zero-dimensional organic–inorganic perovskite variant: transition between molecular and solid crystal. J Am Chem Soc 140(33):10456–10463
Kilina S, Velizhanin KA, Ivanov S, Prezhdo OV, Tretiak S (2012) Surface ligands increase photoexcitation relaxation rates in CdSe quantum dots. ACS Nano 6(7):6515–6524
Saa L, Díez-Buitrago B, Briz N, Pavlov V (2019) CdS quantum dots generated in-situ for fluorometric determination of thrombin activity. Microchim Acta 186(9):657
Zhai C, Zhu M, Pang F, Bin D, Lu C, Goh MC, Yang P, Du Y (2016) High efficiency photoelectrocatalytic methanol oxidation on CdS quantum dots sensitized Pt electrode. ACS Appl Mater Inter 8(9):5972–5980
Owen J, Brus L (2017) Chemical synthesis and luminescence applications of colloidal semiconductor quantum dots. J Am Chem Soc 139(32):10939–10943
Butwong N, Ngeontae W, Burakham R, Srijaranai S (2013) Detection of silver (I) ion based on mixed surfactant-adsorbed CdS quantum dots. Microchim Acta 180(11–12):1101–1107
Koneswaran M, Narayanaswamy R (2016) Retraction note to: CdS/ZnS core-shell quantum dots capped with mercaptoacetic acid as fluorescent probes for Hg (II) ions. Microchim Acta 183(4):1519–1519
Hosseini MS, Pirouz A (2014) Study of fluorescence quenching of mercaptosuccinic acid-capped CdS quantum dots in the presence of some heavy metal ions and its application to Hg (II) ion determination. Luminescence 29(7):798–804
Liu ML, Chen BB, He JH, Li CM, Li YF, Huang CZ (2019) Anthrax biomarker: an ultrasensitive fluorescent ratiometry of dipicolinic acid by using terbium (III) -modified carbon dots. Talanta 191:443–448
Li W, Zhu J, Xie G, Ren Y, Zheng Y-Q (2018) Ratiometric system based on graphene quantum dots and Eu3+ for selective detection of tetracyclines. Anal Chim Acta 1022:131–137
Zhang Y, Li B, Ma H, Zhang L, Jiang H, Song H, Zhang L, Luo Y (2016) A nanoscaled lanthanide metal–organic framework as a colorimetric fluorescence sensor for dipicolinic acid based on modulating energy transfer. J Mater Chem C 4(30):7294–7301
Carr R, Evans NH, Parker D (2012) Lanthanide complexes as chiral probes exploiting circularly polarized luminescence. Chem Soc Rev 41(23):7673–7686
Chen J, Wu XJ, Yin L, Li B, Hong X, Fan Z, Chen B, Xue C, Zhang H (2015) One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew Chem Int Edit 54(4):1210–1214
Mi L, White AD, Shao Q, Setlow P, Li Y, Jiang S (2014) Chemical insights into dodecylamine spore lethal germination. Chem Sci 5(8):3320–3324
Zhang R, Chen W (2014) Nitrogen-doped carbon quantum dots: facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens Bioelectron 55:83–90
Yilmaz MD, Oktem HA (2018) Eriochrome black T-Eu3+ complex as a ratiometric colorimetric and fluorescent probe for the detection of dipicolinic acid, a biomarker of bacterial spores. Anal Chem 90(6):4221–4225
Malghe YS, Lavand AB (2016) Synthesis of C/ZnO/CdS nanocomposite with enhanced visible light photocatalytic activity. Adv Mater 7(3):239–245
Larkin P (2011) Infrared and Raman spectroscopy: principles and spectral interpretation. Stamford, CT, USA
Vikraman AE, Jose AR, Jacob M, Kumar KG (2015) Thioglycolic acid capped CdS quantum dots as a fluorescent probe for the nanomolar determination of dopamine. Anal Methods 7(16):6791–6798
Kuijpers KPL, Bottecchia C, Cambié D, Drummen K, König NJ, Noel T (2018) A fully automated continuous-flow platform for fluorescence quenching studies and Stern-Volmer analysis. Angew Chem Int Edit 130:11448–11452
Hu YJ, Liu Y, Pi ZB, Qu SS (2005) Interaction of cromolyn sodium with human serum albumin: a fluorescence quenching study. Bioorg Med Chem 13:6609–6614
Li Y, Li X, Wang D, Shen C, Yang M (2018) Hydroxyapatite nanoparticle based fluorometric turn-on determination of dipicolinic acid, a biomarker of bacterial spores. Microchim Acta 185(9):435
Tan H, Li Q, Ma C, Song Y, Xu F, Chen S, Wang L (2014) Lanthanide-functionalized silver nanoparticles for detection of an anthrax biomarker and test paper fabrication. J Nanpart Res 16(1):2151
Bhardwaj N, Bhardwaj S, Mehta J, Kim K-H, Deep A (2016) Highly sensitive detection of dipicolinic acid with a water-dispersible terbium-metal organic framework. Biosens Bioelectron 86:799–804
Baig MMF, Chen Y-C (2019) Gold nanocluster-based fluorescence sensing probes for detection of dipicolinic acid. Analyst 144(10):3289–3296. https://doi.org/10.1039/C9AN00240E40
Setlow P (2014) Germination of spores of Bacillus species: what we know and do not know. J Bacteriol 196(7):1297–1305
Acknowledgments
The authors thank the support of this work by the Hunan Provincial Science and Technology Plan Project, China (No. 2019TP1001) and Innovation-Driven Project of Central South University (2020CX002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1087 kb)
Rights and permissions
About this article
Cite this article
Li, X., Deng, L., Ma, F. et al. A luminous off-on probe for the determination of 2,6-pyridinedicarboxylic acid as an anthrax biomarker based on water-soluble cadmium sulfide quantum dots. Microchim Acta 187, 287 (2020). https://doi.org/10.1007/s00604-020-04272-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04272-0