Abstract
A novel aluminum terephthalate/Fe2O3 nanocomposite was synthesized by the addition of Fe2O3 nanoparticles into a reaction solution containing aluminum terephthalate MOF. The synthesized nanocomposite was successfully used as a fiber coating material for solid-phase microextraction (SPME) of six organophosphorus compounds (OPPs) from river water, grape juice, and tea samples. The effect of different parameters on the efficiency of SPME including desorption temperature and time, extraction temperature and time, salt concentration, pH, and agitation were thoroughly studied. The OPPs were detected and determined using GC-MS. According to the findings, a wide linear range (0.15–800 μg kg−1), low limit of detection (0.04–10 μg kg−1), and high recoveries from spiked samples (87.5–112%) were achieved with low inter-day relative standard deviation (3.2–6.7%, n = 5). The MIL-53(Al)/Fe2O3 nanocomposite showed a high extraction ability towards OPPs, and hence, it can be considered a promising adsorbent for the extraction of various pesticides in complex matrices like tea and juice.
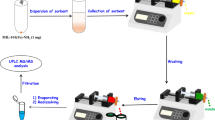
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides (OPPs) are characterized as low price and highly efficient pesticides that are enormously used in agriculture industry. However, OPPs are potentially toxic to human and may cause serious health problems such as neurodevelopmental disorders, cerebral palsy, and even death [1, 2]. In an attempt to regulate OPP application in agriculture industry, many countries have defined the maximum residue limits (MRLs) for OPPs in different types of fruits, tea, and water. For example, the EU has mandated a general MRL of 3.0 mg kg−1 for different kind of OPPs [1, 3]. The European committee has also set the MRL of 0.1 μg L−1 and 0.1 mg kg−1 for drinking water and tea, respectively [4]. Since the pesticide residue particularly OPPs appears to be very low in real samples such as tea, fruits, and vegetables, which are known as complicated matrices, it is very imperative to use a suitable sample preparation method before sample introduction to the analytical instrument [1, 5, 6].
So far, a wide variety of methods have been developed for the extraction of OPPs such as dispersive liquid–liquid microextraction (DLLME) [7], magnetic solid-phase extraction (MSPE) [4, 5], continuous sample drop flow microextraction [8], and solid-phase microextraction (SPME) [1, 9, 10]. Although liquid-phase microextraction methods proivde many advantages including lower consumption of organic solvent and higher EF and recovery compared to conventional microextraction methods [11, 12], they suffer from some drawbacks such as toxicity of organic solvents used in these methods and necessity of sample clean-up in samples with complex matrices. The main benefits associated with the MSPE method are as follows: very high ratio of surface area to extracting phase volume, better dispersion in the sample solution, and rapid magnetic separation of extracting phase. However, it has some disadvantages such as using organic solvents for the sake of analyte desorption. Besides, the synthesis of magnetic materials, especially the functional groups or special structures coated on them, is complex and difficult [13]. SPME is a simple and solvent-free extraction method which has gained a considerable amount of attention due to its special features such as providing both analyte extraction and sample introduction to analytical instrument using a single device as well as easier fiber coating [14, 15]. As a result, it is always among the best extraction methods for the analysis of samples with complex matrices such as food.
The extraction mechanism in SPME is based on the equilibrium partition of analyte between a coated fiber with an extracting phase and sample matrix [16]. Thus, the adsorbent is the most significant factor which plays a key role in the extraction process, and the efficiency, sensitivity, and selectivity of extraction is considerably linked to the adsorbent nature [10, 17]. Unfortunately, available commercial fibers fail to provide satisfactory results due to several limitations including low thermal stability, low solvent resistance, low adsorption capacity, poor durability, brittleness, and long extraction time which have significantly limited the practical application of SPME [18, 19]. So, it is very vital to improve the stability and sensitivity of fibers by developing a new coating material [16].
So far, different kinds of advanced material have been used as the SPME fibers such as aptamer-functionalized materials (AFMs), molecularly imprinted polymers (MIPs), carbon nanotubes(CNTs), metal–organic frameworks (MOFs), covalent organic frameworks (COFs), and ionic liquids (IL) [20] among which metal–organic frameworks (MOFs) appear to be more valuable due to their ability of improving adsorption capacity and equilibrium [16].
MOFs possess several distinctive features as follows: nanoscale porosity, tunable composition, various functional sites, great surface area and thermal stability, modification capability, and a great range of structures [16, 21]. In 2004, Loiseau et al. reported a porous aluminum terephthalate MOF called MIL-53(Al) synthesized by a hydrothermal process [22]. MIL-53(Al) is a three-dimensional aluminum terephthalate-based porous material with one-dimensional rhombic-shaped channels, pore diameters of 8.5 Å, and pore aperture windows of 2.6 × 13.6 Å2. Besides, it possesses accessible coordinative unsaturated sites, large breathing, high surface area (1140 m2g−1), and excellent chemical stability [22]. Several composites of MIL-53(Al) have been synthesized for different purposes as mentioned below. Xie and coworkers [23] demonstrated that amine-functionalized MIL-53(Al) could be used as an efficient fiber coating material for SPME of organochlorine pesticides and synthetic musks. Besides, Chen et al. [24] prepared three types of MIL-53(M) (M = Al, Fe, Cr) as a SPME fiber coating material and examined their efficiency in the microextraction of polycyclic aromatic hydrocarbons. The results showed that among the three MIL-53(M) coatings, MIL-53(Al) provided the highest extraction efficiency towards polycyclic aromatic hydrocarbons (PAHs) owing to its high thermal and chemical stability. Zhang et al. [25] solvothermally synthesized the Fe3O4/MIL-101 composite (in dimethylformamide solvent) and used it for MSPE of OPPs. They indicated that due to high porosity and the presence of a large amount of oxygen groups and π electrons in the Fe3O4/MIL-101 composite, their proposed method could provide good precision and satisfactory recoveries.
In this work, for the first time, MIL-53(Al)/Fe2O3 (25% wt) was employed as an SPME adsorbent for the extraction of six OPPs (phorate, diazinon, disulfoton, parathion, malathion, and chlorofenvinphos) from river water, fruit juice, and tea. Unlike Fe3O4/MIL-101 that was produced in DMF, which is a toxic solvent with a possibility of its encapsulataion inside MOFs and causing a secondary pollution [26], MIL-53(Al)/Fe2O3 was synthesized by a hydrothermal process which is more environmentaly freindly. Besides, MIL-53(Al) /Fe2O3 has a higher chemical and thermal stability than Fe3O4/MIL-101 making it a suitable adsorbent for coating SPME fibers.
Experimental
Solutions and chemicals
A full description of materials used in this study have been provided in supporting information.
Instrumentation
Please refer to supporting information to see the instrumentation details.
Synthesis of MIL-53(Al)
The aluminum terephthalate MOF (MIL-53(Al)) was prepared using hydrothermal process reported by Loiseau and coworkers (for more details, see ESM) [22].
Synthesis of α-Fe2O3
The iron oxide nanoparticles (α-Fe2O3) were synthesized using ultrasound-assisted protocol previously reported by Askarinejad and coworkers (for more details, see ESM) [27].
The synthesis of MIL-53(Al)/Fe2O3 (25% wt) nanocomposite
The same procedure described for synthesis of MIL-53(Al) was utilized to synthesize nanocomposite MIL-53(Al)/Fe2O3 (25% wt) with a minor change. A total of 360.0 mg of Fe2O3 nanoparticles were added to the reaction solution after addition of terephthalic acid, Al (NO3)3·9H2O, and water into the Teflon reactor. Once the reaction was over, the bright red-colored precipitate was separated from the solution by a centrifuge followed by rinsing with DI water. The collected sediment was then dried out under vacuum for 1 h. Finally, the dry sediment was calcined at 360 °C for 72 h in the air.
Fabrication of MIL-53(Al) and MIL-53(Al)/Fe2O3 fibers
In an effort to use the coated fibers for the extraction of the target compounds using SPME, a few pieces of stainless steel wires were cut into 2.5 cm pieces followed by successive sonication in water and acetone for 20 min. The wires were then dried out and dipped into an epoxy glue for a few seconds. The excess amount of glue on the wires was removed by a piece of filter paper, and only a thin film of glue was allowed to remain on the wire’s surface. Afterwards, the synthesized MIL-53(Al) and MIL-53(Al)/Fe2O3 nanocomposites were placed on the surface of modified wires by rotation of wires inside the mixture of nanocomposites. Finally, the SPME fibers were dried at 50 °C for 30 min. In order to prevent likely contamination of coated fibers and glue deterioration before extraction, they were placed in an in-house SPME device followed by inserting into the GC inlet and heating at 300 °C for 2 h under a stream of 99.999% helium gas.
Sample preparation
Two grams of powdered dry tea sample supplied from the local market was sprayed with 150.0 μL of a 0.4 ppm OPP solution (in methanol). The sample was left in room condition for 12 h to ensure the penetration of OPPs into the sample [28, 29]. After 12 h, it was added into 40.0 mL of ultrapure boiling water and the solution was cooled down to the room temperature. Finally, 20.0 mL of this solution was transferred into a SPME extraction vial. The same procedure was conducted to prepare blank tea samples without an addition of OPP mixture. The grape juice samples were supplied from the local market, and water samples were collected from river Zakho. Then, 20.0 mL of each sample was spiked with OPPs to a final concentration of 10.0 μg L−1. Prior to analysis, the pH of real samples was cheked and adjusted between 4 and 7.5 using 0.5% NaOH and HCl solution. Before extraction, all the sample solutions were incorporated with 2.5% (w/v) NaCl solution.
SPME procedure
Twenty milliliters of working solution or real sample solution was transferred into a 20 mL headspace glass vial capped with septa. The solution was stirred at 700 r.p.m. for 35 min using a magnetic stirrer. The extraction temperature was adjusted at 40 °C using a thermostatic water bath. The needle of the SPME device was penetrated through the septum, and the coated fiber was pulled out to reach the sample solution. After 35 min, the fiber moved back into the needle, removed from the vial, and dried by nitrogen gas stream for 30 s. Then, it was inserted into the GC injector for thermal desorption of analytes at 280 °C for 2.0 min. Prior to each measurement, the fiber was conditioned at 280 °C for 6.0 min.
Results and discussion
Choice of materials
MIL-53(Al) is a carboxylate-based MOF with a high surface area, flexible framework, and notable thermal and chemical stability. The hydroxyl groups in the MIL-53(Al) structure can greatly increase the interaction between the sorbent and polar organic analytes. Besides, the benzene rings inside the pores can significantly increase the adsorption of the aromatic and heterocyclic analytes by MIL-53(Al). Hematite (α-Fe2O3) is the most stable iron oxide under ambient conditions that can easily be covered with H2O atoms and hydroxyl groups [30]. In this study, the MIL-53(Al)/Fe2O3 nanocomposite was fabricated via a hydrothermal method without using any hazardous organic solvents. Considering the special structure of MIL-53(Al), the MIL-53(Al)/Fe2O3 nanocomposite was chosen as the SPME coating material for the extraction of polar OPPs. This nanocomposite can be sensitive to OPPs with the π–π stacking, hydrogen bonding (HB), and host–guest interactions [31]. In addition, the presence of Fe2O3 in this composition seems to increase the surface area, possibility of the great HB between surface hydroxyls of Fe2O3 (as HB donor) and OPP molecules (as HB acceptor), pore volumes, and polarity making it very suitable for the adsorption of OPPs as polar compounds.
Characterization of MIL-53(Al) andMIL-53(Al)/Fe2O3 nanocomposite adsorbents
Evaluation and interpretation of TGA curves
The TGA curves of MIL-53(Al) and MIL-53(Al)/Fe2O3 nanocomposite particles are presented in Fig. S1 (see, ESM for more details).
Evaluation and interpretation of FT-IR spectrum
FT-IR spectra are known as useful tools to identify the presence of certain functional groups or chemical bonds in a compound of interest. Fig. S2 shows the FT-IR spectra of terephthalic acid, MIL-53(Al), and MIL-53(Al)/Fe2O3 (see, ESM for more details).
Evaluation and interpretation of SEM images and EDAX spectrum
Figure 1 a shows the SEM image of MIL-53(Al) at 30000x magnification. It shows that MIL-53(Al) has been created in the form of layered cubes with crystalline shape. The layers have a thickness of about 20–60 nm in some areas. The SEM image of the MIL-53(Al)/Fe2O3 nanocomposite is shown in Fig. 1b. The Fe2O3 nanoparticles can be seen in the shape of a coin at the surface surrounded by MIL-53(Al) cubes that are 50–70 nm in thickness and 100–600 in diameter.
The EDAX spectrum of the MIL-53(Al)/Fe2O3 nanocomposite is presented in Fig. 1c. It should be mentioned that the analysis was performed without metal coating because of the high thermal stability of the sample, and our findings proved the high purity of the product and presence of elements like C, O, Al, and Fe. the SEM image of the fiber made of the MIL-53(Al)/Fe2O3 nanocomposite is shown in Fig. 1d at 300x magnification.
XPS analysis
As observed in Fig. 2 and Fig. S3, the full-scan X-ray photoelectron spectroscopy (XPS) spectrum was used to exhaust studying of the surface elements and chemical states of the MIL-53(Al)/Fe2O3 nanocomposite. The survey XPS spectrum exhibits the presence of Al, C, O, and Fe elements in the MIL-53(Al)/Fe2O3, which is in accord with the EDAX result (Fig. 2) (see, ESM for more details).
Evaluation and interpretation of XRD pattern
XRD patterns of the metal–organic framework MIL-53(Al) and MIL-53(Al)/Fe2O3 nanocomposite in the range of 2θ = 5°-70° are shown in Fig. S4 (for more details, see the Electronic supplementary material).
The evaluation of adsorption–desorption of N2
Looking at the adsorption–desorption isotherm of nitrogen presented in Fig. S5, it is quite obvious that both MIL-53(Al) and MIL-53(Al)/Fe2O3 nanocomposites possess type I isotherms with a small hysteresis showing that synthesized samples have a microporous structure which completely comply with the Langmuir monolayer adsorption model (see ESM for more details).
Optimization of SPME parameters for extraction of pesticides
In an attempt to reach an optimized condition for extraction of OPPs by the fibers prepared for the DI-SPME, different parameters which may affect the extraction efficiency were assessed and optimized. So, extraction temperature and time, desorption temperature and time, stirring rate, pH, and salt effect were evaluated at the first step. During optimization process, the analyte concentrations were adjusted at 10.0 μg L−1 in aqueous solution and fruit juice and at 30.0 μg kg−1 in tea samples. The sample volume in all experiments was 20.0 mL.
The practical factors that can affect the efficiency of extraction including extraction temperature and time, desorption temperature and time, ionic strength, agitation, sample solution pH, and desorption temperature were preliminarily examined and optimized as follows: extraction temperature: 40 °C; extraction time: 35 min; desorption temperature: 280 °C; desorption time: 2 min; agitation: 700 rpm; sample solution pH: no adjustment; and sample ionic strength: 3% (w/v). Further information about the optimization procedure for each factor can be found in supplemetary information (Fig. S6–8).
Comparison of MIL-53(Al)/Fe2O3 with MIL-53(Al) and Fe2O3
The extraction efficiency of the proposed method was evaluated using three adsorbents described earlier including the MIL-53(Al)/Fe2O3 nanocomposite, MIL-53(Al), and Fe2O3 in order to identify the most suitable adsorbent. As indicated in Fig. 3, the highest extraction efficiency was achieved when MIL-53(Al)/Fe2O3 was used as the coating material for all studied OPPs under the same condition. As already discussed, the MIL-53(Al)/Fe2O3 nanocomposite possesses a three-dimensional nanoporous structure with a great specific surface area which may justify the high extraction efficiency achieved by this nanocomposite.
Method validation
The analytical performance of the presented the SPME-GC-MS method in analysis of concerned OPPs was evaluated through determination of figures of merit including limits of detection (LODs), limits of quantification (LOQs), linearity, coefficients of determination (r2), and relative standard deviations (RSDs). The concentration of the OPPs in real samples including tea, river water, and grape juice were adjusted at two different levels of 35.0 μg kg−1 and 35.0 μg L−1 by the addition of an appropriate amount of the OPPs into the sample. For tea, river water, and grape juice samples, the LODs were defined as the signal-to-noise ratio of 3 (S/N = 3) which was determined between 0.04 and 10.0 μg L−1, and the LOQs were calculated as signal-to-noise ratio of 10 (S/N = 10) varied from 0.15 to 35.0 μg L−1. The calibration curves stablished for each analyte were linear from their respective LOQs to 800.0 μg L−1 with r2 values between 0.990 and 0.999. For tea sample, the LODs were found to be between 0.1 and 10.0 μg kg−1 and the LOQs were estimated between 0.4 and 35.0 μg kg−1. The calibration curves obtained for each analyte were linear from their respective LOQs to 800.0 μg kg−1 with r2 values ranging from 0.990 to 0.995. The intra-day precision and inter-day precision were calculated at three different real samples. The intra- and inter-day precision of the proposed method was examined by running five successive measurements and five consecutive days without changing the fiber, respectively. The obtained RSDs were all below 7.9% indicating that this method has an acceptable precision in the analysis of OPPs. Also, the fiber-to-fiber reproducibility for three different fibers produced under the same conditions ranged from 4.8 to 9.7%. Table 1 summarizes the analytical figures discussed above. Selectivity study was also conducted according to the internationally accepted criteria of the Food & Drug Administration, 2018 (FDA) [32, 33]. As can be seen, no significant interfering peaks were observed at the same retention time of the OPPs in the chromatogram of the blank sample (OPP-free sample), indicating selectivity towards the MIL-53(Al)/Fe2O3 SPME and suitable chromatographic separation of OPPs (Fig. 4a). Finally, in order to check the applicability of the proposed method for the analysis of copmounds other than OPPs, some important pollutants were analyzed using this method and the results were included in supplementary information.
Matrix effect
The matrix effect (ME%) was evaluated by analyzing water, juice, and tea samples under the optimized conditions by blew by the equation [34]:
As, An, and Aw are the peak areas of the analyte in the spiked and nonspiked samples and spiked ultrapure water, respectively. The results are summarized in Table 1. As can be seen, the determination of analytes in water and grape juice were not affected by the matrix significantly, while in more complex samples such as tea drink, it can be impacted very slightly.
Determination of OPPs in real samples
In order to examine the versatility of the proposed method in real sample analysis, six OPPs were analyzed in water, grape juice, and tea samples using this method. Table 2 shows the analytical figures associated with the analysis of these OPPs in mentioned samples. As it can be seen, only river water (4.0 μg L−1) and tea samples (15.0 μg kg−1) contained measurable amount of chlorofenvinphos both of which lower than the MRLs set by EU. The samples were then spiked with 4.0 and 40.0 μg kg−1 of the OPPs to evaluate the accuracy and recovery of the method. The respective analytical data are summarized in Table 2. The GC-MS chromatograms related to the analysis of each OPPs in the blank and spiked real samples (grape juice and tea) are shown in Fig. 4. The chromatogram in Fig. 4a, A is related to blank grape juice which was free of OPPs and the chromatogram in Fig. 4b, B belongs to blank tea which contained 14.5 μg kg−1 chlorofenvinphos.
Comparison study
The analytical figures of the proposed method were compared to those of previously reported methods and the same were listed in Table 3. As it can be seen, the linear range of the proposed method is greater than SBSE-GC-FPD ([35]), SPE-HPLC-UV ([5]), MSPE-GC-NPD ([25]), and Md-SPE-GC-MS ([37]), but narrower than SPME-GC-MS ([1]), HS-SPME-GC-MS ([10]), and MSPE-GC/NPD ([37]). Although the LOD appears to be lower than SPME-GC-MS ([1]), MSPE-GC-NPD ([25]), and MSPE-GC/MS ([38]), it is higher than HS-SPME-GC-MS ([5]) and MSPE-GC/NPD ([25]) and comparable with SBSE-GC-FPD ([35]). The extraction time in the current method seems to be longer than some of the listed methods. However, it is still comparable to SPME-GC-MS ([1]), HS-SPME-GC-MS ([10]), and MSPE-GC-NPD ([25]).
In summary, it seems that the synthesized MIL-53(Al)/Fe2O3 nanocomposite is a suitable adsorbent for the extraction of studied OPPs using SPME, and the established DI-SPME-GC-MS method can be considered a proper alternate for pesticide quantification in juice, water, and tea samples.
Conclusions
The MIL-53(Al)/Fe2O3 nanocomposite was used as a coating material for trace analysis of OPPs using DI-SPME-GC-MS in water, grape juice, and tea samples. It was demonstrated that coating the SPME fiber with MIL-53(Al)/Fe2O3 nanocomposite enhances its porosity and provides extractions with high efficiency and good precision for OPPs. Using the proposed method, six OPPs were successfully extracted and determined in three different real samples with good recoveries and acceptable RSDs. Despite all the mentioned benefits, the thermal stability of the introduced nanocompoite is limited due to the presence of iron oxide inside its structure. So, the proposed method may not be applicable for the compounds with desorption temperatures higher than 450 °C. As a conclusion, we believe that the MIL-53(Al)/ Fe2O3 nanocomposite is a promising adsorbent for SPME, and it can be used for extraction of many pesticides and organic pollutions in food and environmental samples. On the other hand, little is known about the extraction mechanism using MIL-53(Al)/Fe2O3 nanocomposite-coated fibers which means that a lot more studies can be conducted in this area.
References
Pang Y, Zang X, Li H, Liu J, Chang Q, Zhang S, Wang C, Wang Z (2020) Solid-phase microextraction of organophosphorous pesticides from food samples with a nitrogen-doped porous carbon derived from g-C3N4 templated MOF as the fiber coating. J Hazard Mater 384:121430. https://doi.org/10.1016/j.jhazmat.2019.121430
Li ZD, Liu Z, Xu Y, Wang D (2018) Synthesis, characterization of a ternary Cu(II) Schiff base complex with degradation activity of organophosphorus pesticides. Inorg Chim Acta 471:280–289. https://doi.org/10.1016/j.ica.2017.11.024
Mol HGJ, Van Dam RCJ, Steijger OM (2003) Determination of polar organophosphorus pesticides in vegetables and fruits using liquid chromatography with tandem mass spectrometry: selection of extraction solvent. J Chromatogr A 1015(1–2):119–127. https://doi.org/10.1016/s0021-9673(03)01209-3
Amiri A, Tayebee R, Abdar A, Narenji Sani F (2019) Synthesis of a zinc-based metal-organic framework with histamineas an organic linker for the dispersive solid-phase extraction of organophosphorus pesticides in water and fruit juice samples. J Chromatogr A 1597:39–45. https://doi.org/10.1016/j.chroma.2019.03.039
Gao L, Liu L, Sun Y, Zhao W, He L (2020) Fabrication of a novel azamacrocycle-based adsorbent for solid-phase extraction of organophosphorus pesticides in tea drinks. Microchem J 153:104364. https://doi.org/10.1016/j.microc.2019.104364
Shizuka S-S, Tomoko H, Satoru N, Hiroshi A (2018) Multiresidue determination of pesticides in tea by liquid chromatography-high resolution mass spectrometry: comparison between Orbitrap and time-of-flight mass analyzers. Food Chem 256:140–148. https://doi.org/10.1016/j.foodchem.2018.02.123
Moinfar S, Hosseini MRM (2009) Development of dispersive liquid-liquid microextraction method for the analysis of organophosphorus pesticides in tea. J Hazard Mater 169(1–3):907–911. https://doi.org/10.1016/j.jhazmat.2009.04.030
Moinfar S, Jamil LA, Sami HZ (2020) Determination of organophosphorus pesticides in juice and water by modified continuous sample drop flow microextraction combined with gas chromatography–mass spectrometry. Food Anal Methods 13:1050–1059. https://doi.org/10.1007/s12161-020-01723-5
Pellicer-Castell E, Belenguer-Sapiña C, Amorós P, El Haskouri J, Herrero-Martínez JM, Mauri-Aucejo A (2018) Study of silica-structured materials as sorbents for organophosphorus pesticides determination in environmental water samples. Talanta 189:560–567. https://doi.org/10.1016/j.talanta.2018.07.044
Bagheri H, Amanzadeh H, Yamini Y, Masoomi MY, Morsali A, Salar-Amoli J, Hassan J (2018) A nanocomposite prepared from a zinc-based metal-organic framework and polyethersulfone as a novel coating for the headspace solid-phase microextraction of organophosphorous pesticides. Microchim Acta 185:62. https://doi.org/10.1007/s00604-017-2607-3
Timofeeva I, Shishov A, Kanashina D, Dzema D, Bulatov A (2017) On-line in-syringe sugaring-out liquid-liquid extraction coupled with HPLC-MS/MS for the determination of pesticides in fruit and berry juices. Talanta 167:761–767. https://doi.org/10.1016/j.talanta.2017.01.008
Alexovič M, Horstkotte B, Solich P, Sabo J (2016) Automation of static and dynamic non-dispersive liquid phase microextraction. Part 1: approaches based on extractant drop-, plug-, film- and microflow-formation. Anal Chim Acta 906:22–40. https://doi.org/10.1016/j.aca.2015.11.038
Xu B, Wang Y, Jin R, Li X, Song D, Zhang H, Sun Y (2015) Magnetic solid-phase extraction based on Fe3O4@polyaniline particles followed by ultrafast liquid chromatography for determination of Sudan dyes in environmental water samples. Anal Methods 7:1606–1614. https://doi.org/10.1039/C4AY02645D
Arthur CL, Pawliszyn (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62(19):2145–2148. https://doi.org/10.1021/ac00218a019
Ghaemmaghami M, Yamini Y, Zavar Mousavi K (2020) Accordion-like Ti3C2Tx MXene nanosheets as a high-performance solid phase microextraction adsorbent for determination of polycyclic aromatic hydrocarbons using GC-MS. Microchim Acta 187(2):151. https://doi.org/10.1007/s00604-020-4123-0
Guo Y, He X, Huang C, Chen H, Lu Q, Zhang L (2020) Metal–organic framework-derived nitrogen-doped carbon nanotube cages as efficient adsorbents for solid-phase microextraction of polychlorinated biphenyls. Anal Chim Acta 1095:99–108. https://doi.org/10.1016/j.aca.2019.10.023
Zheng J, Huang J, Yang Q, Ni C, Xie X, Shi Y, Sun J, Zhu F, Ouyang G (2018) Fabrications of novel solid phase microextraction fiber coatings based on new materials for high enrichment capability. TrAC Trends Anal Chem 108:135–153. https://doi.org/10.1016/j.trac.2018.08.021
Ma T-T, Shen X-F, Yang C, Qian H-L, Pang Y-H, Yan X-P (2019) Covalent immobilization of covalent organic framework on stainless steel wire for solid-phase microextraction GC-MS/MS determination of sixteen polycyclic aromatic hydrocarbons in grilled meat samples. Talanta 201:413–418. https://doi.org/10.1016/j.talanta.2019.04.031
Spietelun A, Pilarczyk M, Kloskowski A, Namieśnik J (2010) Current trends in solid-phase microextraction (SPME) fibre coatings. Chem Soc Rev 39(11):4524–4537. https://doi.org/10.1039/C003335A
Chen Y, Xia L, Liang R, Lu Z, Li L, Huo B, Li G, Hu Y (2019) Advanced materials for sample preparation in recent decade. Trends Anal Chem 120:115662. https://doi.org/10.1016/j.trac.2019.115652
Qin Z, Jiang Y, Piao H, Li J, Tao S, Ma P, Wang X, Song D, Sun Y (2020) MIL-101(Cr)/MWCNTs-functionalized melamine sponges for solid-phase extraction of triazines from corn samples, and their subsequent determination by HPLC-MS/MS. Talanta 211:120676. https://doi.org/10.1016/j.talanta.2019.120676
Loiseau T, Taulelle F, Serre C, Henry M, Huguenard C, Bataille T, Fink G, Ferey G (2004) A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem Eur J 10(6):1373–1382. https://doi.org/10.1002/chem.200305413
Xie L, Liu S, Han Z, Jiang R, Zhu F, Xu W, Su C, Ouyang G (2017) Amine-functionalized MIL-53(Al)-coated stainless steel fiber for efficient solid-phase microextraction of synthetic musks and organochlorine pesticides in water samples. Anal Bioanal Chem 409:5239–5247. https://doi.org/10.1007/s00216-017-0472-x
Chen X-F, Zang H, Wang X, Cheng JG, Zhao RS, Cheng CG, Lu XQ (2012) Metal–organic framework MIL-53(Al) as a solid-phase microextraction adsorbent for the determination of 16 polycyclic aromatic hydrocarbons in water samples by gas chromatography–tandem mass spectrometry. Analyst 137:5411–5419. https://doi.org/10.1039/C2AN35806A
Zhang S, Jiao Z, Yao W (2014) A simple solvothermal process for fabrication of a metal-organic framework with an iron oxide enclosure for the determination of organophosphorus pesticides in biological samples. J Chromatogr A 1371:74–81. https://doi.org/10.1016/j.chroma.2014.10.088
Lin K-YA, Chang HA, Hsu CJ (2015) Iron-based metal organic framework, MIL-88A, as a heterogeneous persulfate catalyst for decolorization of Rhodamine B in water. RSC Adv 5:32520–32530. https://doi.org/10.1039/C5RA01447F
Askarinejad A, Bagherzadeh M, Morsali A (2011) Sonochemical fabrication and catalytic properties of α-Fe2O3 nanoparticles. J Exp Nanosci 6(3):217–225. https://doi.org/10.1080/17458080.2010.489583
Wang F, Li L, Feng H, Yang Y, Xiao B, Chen D (2019) An enhanced sensitivity and cleanup strategy for the nontargeted screening and targeted determination of pesticides in tea using modified dispersive solid-phase extraction and cold-induced acetonitrile aqueous two-phase systems coupled with liquid chromatography-high resolution mass spectrometry. Food Chem 275:530–538. https://doi.org/10.1016/j.foodchem.2018.09.142
Zhu B, Xu X, Luo J, Jin S, Chen W, Liu Z, Tian C (2019) Simultaneous determination of 131 pesticides in tea by on-line GPC-GC–MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem 276:202–208. https://doi.org/10.1016/j.foodchem.2018.09.152
Pabisiak T, Kiejna A (2019) Incipient adsorption of water and hydroxyl on hematite (0001) surface. J Phys Commun 3:035023. https://doi.org/10.1088/2399-6528/ab0fa7
Deng J, Zhang P, Jin T, Zhou H, Cheng J (2017) Graphene oxide/b-cyclodextrin composite as fiber coating for high efficiency headspace solid phase microextraction of organophosphate ester flame retardants in environmental water. RSC Ad 7:54475–54484. https://doi.org/10.1039/C7RA07903F
FDA (2018) Bioanalytical method validation guidance, FDA. 44. http://www.ich.org/fileadmin/Public WebSite/ICH_Products/Guidelines/Quality/Q2R1/Step4/Q2R1__Guideline.pdf
Shokrollahi M, Seidi S, Fotouhi L (2020) In situ electrosynthesis of a copper-based metal–organic framework as nanosorbent for headspace solid-phase microextraction of methamphetamine in urine with GC-FID analysis. Microchim Acta 187:548. https://doi.org/10.1007/s00604-020-04535-w
Amini S, Ebrahimzdeh H, Seidi S, Jalilian N (2020) Preparation of electrospun polyacrylonitrile/Ni-MOF-74 nanofibers for extraction of atenolol and captopril prior to HPLC-DAD. 187:508. https://doi.org/10.1007/s00604-020-04483-5
Xiao Z, He M, Chen B, Hu B (2016) Polydimethylsiloxane/metal-organic frameworks coated stir bar sorptive extraction coupled to gas chromatography-flame photometric detection for the determination of organophosphorus pesticides in environmental water samples. Talanta 156-157:126–133. https://doi.org/10.1016/j.talanta.2016.05.001
Cruz Fernandes V, Freitas M, Grosso Pacheco J, Maria Oliveira J, Fernandes Domingues V, Delerue-Matos C (2018) Magnetic dispersive micro solid-phase extraction and gas chromatography determination of organophosphorus pesticides in strawberries. J Chromatogr A 1566:1–12. https://doi.org/10.1016/j.chroma.2018.06.045
Mahpishanian S, Sereshti H (2016) Three-dimensional graphene aerogel-supported iron oxide nanoparticles as an efficient adsorbent for magnetic solid phase extraction of organophosphorus pesticide residues in fruit juices followed by gas chromatographic determination. J Chromatogr A 1443:43–53. https://doi.org/10.1016/j.chroma.2016.03.046
Deng X, Guo Q, Chen X, Xue T, Wang H, Yao P (2014) Rapid and effective sample clean-up based on magnetic multiwalled carbon nanotubes for the determination of pesticide residues in tea by gas chromatography-mass spectrometry. Food Chem 145(15):853–858. https://doi.org/10.1016/j.foodchem.2013.08.137
Wang P, Luo M, Liu D, Zhan J, Liu X, Wang F, Zhou Z, Wang P (2018) Application of a magnetic graphene nanocomposite for organophosphorus pesticide extraction in environmental water samples. J Chromatogr A 1535:9–16. https://doi.org/10.1016/j.chroma.2018.01.003
Niu M, Li Z, He W, Zhou W, Lu R, Li J, Gao H, Zhang S, Pan C (2020) Attapulgite modified magnetic metal-organic frameworks for magnetic solid phase extraction and determinations of benzoylurea insecticides in tea infusions. Food Chem 317:126425. https://doi.org/10.1016/j.foodchem.2020.126425
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
Solutions and chemicals, instrumatations, some experimental results are provided. (DOCX 2015 kb)
Rights and permissions
About this article
Cite this article
Moinfar, S., Khodayari, A., Sohrabnezhad, S. et al. MIL-53(Al)/Fe2O3 nanocomposite for solid-phase microextraction of organophosphorus pesticides followed by GC-MS analysis. Microchim Acta 187, 647 (2020). https://doi.org/10.1007/s00604-020-04621-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04621-z