Abstract
The authors describe the synthesis of a magnetic metal-organic framework (MOF) of type MIL-53(Fe) for coextraction of phenols and anilines from various environmental samples. A quick method for dispersive micro-solid phase extraction (D-μ-SPE) was developed for coextraction of the analytes 4-nitrophenol (4-NP), 4-chlorophenol (4-CP), 4-chloroaniline (4-CA), 1-amino-2-naphtol (1-A2N) and 2, 4-dichloroaniline (2, 4-DCA). The MOF was characterized by SEM, TEM, FT-IR, EDS, thermogravimetry, VSM and XRD. The method was optimized by response surface methodology combined with desirability function approach, specifically with respect to pH value of the sample, amount of sorbent, sorption time, salt concentration, sample volume, type and volume of the eluent, and elution time. Following elution with acetonitrile, the analytes were quantified by HPLC with photodiode array detection. Responses are linear in 0.1–2000 μg·L−1 concentration ranges. The limits of detection and relative standard deviations (for n = 5) are in the range of 0.03–0.2 μg·L−1 and 3.5–12.6%, respectively. Enrichment factors are 113, 61, 87, 144 and 114 for 4-NP, 4-CP, 4-CA, 1-A2N and 2,4-DCA, respectively. Recoveries from spiked samples ranged from 39.5 to 93.3%. The magnetic sorbent was successfully applied to the coextraction and determination of the analytes in river, rain and hookah water samples.

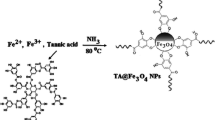
Schematic presentation for the synthesis of (a) Fe3O4 nanoparticles (NPs) and (b) Fe3O4@MIL-53(Fe). Fe3O4@MIL-53(Fe) was employed as a new nanosorbent in dispersive micro-solid phase extraction of phenols and anilines. The limits of detection are in the range of 0.03–0.2 μg·L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenols and anilines even at trace levels of concentration are suspected to be carcinogenic [1,2,3]. Thereby, it is extremely momentous to extend a sensitive, accurate and environmentally friendly determination method for measurement of these pollutants. Chloroanilines (CAs) and phenolic compounds are used in different industrial processes like pharmaceuticals, synthesis of chemical compounds like dyes or some pesticides, plastics and using them as reagents [1, 2, 4, 5]. Therefore, they can be discovered in waters, soils, and sediments [6,7,8]. Among these compounds, p-nitrophenol and 1-amino-2-naphthol can have irreparable harmful effects on the health of humans and animals. p-nitrophenol damages mitochondria and prevents energy metabolism in human and animals [9]. 1-amino-2-naphthol causes bladder tumors creation after bladder insemination, made by the reduction of Orange II [10]. Different analytical techniques such as high-performance liquid chromatography (HPLC) with photo diode array detection (PDA) [11], electrochemical detection [12], or mass spectrometry (MS) [13], gas chromatography (GC) with flame ionization detection (FID) [14], capillary electrophoresis (CE) with amperometric detection [3], have been applied to quantification of phenols, anilines and compounds derived from them.
Sample preparation is an essential step prior to the determination of these compounds in different samples [2, 15, 16]. D-μ-SPE is a well-established sample pretreatment method for both cleaning up samples and preconcentrating in a variety of matrices. This method boasts of high versatility due to the great diversity of solids accessible, covering a wide range of chemical functionalities [17, 18].
Micro or nano sorbents showing a dramatically larger surface area with shorter diffusion path in comparison with other sorbents [19]. Magnetic nanoparticles (MNPs) are member of this group of sorbents, they can disperse homogenously in the sample solution and separate by helping of an external magnet [3, 11, 20, 21]. MOFs, are a marvelous multifunctional inorganic-organic materials with various holes and functionalized 3D crystalline structures that formed by linkers and metal ions [22,23,24,25]. MOFs have interesting unique attributes like high surface area, high pore volume, adjustable porous sizes and chemical tenability. These attributes make MOFs applicable in many field such as materials with magnetic properties, separation, drug delivery and heterogeneous catalysis, gas storage and purification, luminescent properties, chemical sensing [24,25,26]. MIL-53(Fe), a Fe-based MOFs, has a three-dimensional porous structure with unlimited one-dimensional linkage of –Fe–O–O–Fe–O–Fe–, cross-linked by terephthalic acid (1, 4-BDC) as a linker [27]. According to the features given, magnetic MOFs are an ideal sorbent in D-μ-SPE method. We report the synthesis of a new high performance MOF [Fe3O4@MIL-53(Fe)] with entire characterization and confirmation of it. This MOF was used for coextraction of [4-nitrophenol (4-NP), 4-chlorophenol (4-CP), 4-chloroaniline (4-CA), 2, 4-dichloroaniline (2, 4-DCA) and 1-amino-2-naphtol (1-A2N)] based on D-μ-SPE method prior to their quantitation by HPLC. The synthesized magnetic MIL-53(Fe) has core-shell nanostructure with Fe3O4 core, which speeds up isolation of nanosorbent from the sample solution. The experimental results showed that magnetic MIL-53(Fe) have a high sorption capacity for intended analytes. This is because of the existence of some probably interactions like π-interaction, hydrophobic and hydrogen bonding between the intended analytes and the sorbent and cavities in MOF structure that can capture the intended analytes.
Experimental
Chemicals and reagents
HPLC grade acetonitrile and methanol were procured from Daejun (Seoul, South Korea, www.daejungchem.co.kr). 1, 4-BDC, FeCl3, (NH4)2Fe(SO4)2·6H2O, ammonium hydroxide (28% w.v−1), NaOH, HCl, NaCl, N, N-dimethyl formamide (DMF), 2-propanol, and acetone that all of these chemicals were of analytical grade were provided by Merck (Darmstadt, Germany, www.merck-chemicals.com). Ultrapure purity water obtained from a milli-Q system (Millipore, Bedford, MA, USA; www.emdmillipore.com) was used all over the experiments. 4-NP ((log P = 1.61, pKa = 7.07), 4-CP (log P = 2.27, pKa = 9.96), 4-CA (log P = 1.75, pKb = 3.49), 1-A2N (log P = 1.83, pKb = 4.16, pKa = 10.13) and 2, 4-DCA (log P = 2.35, pKb = 1.98) were purchased from Sigma-Aldrich (Milwaukee, WI, USA, www.sigmaaldrich.com).
Preparation of the samples and standards
Stock standard solutions of 4-NP, 4-CP, 4-CA, 1-A2N and 2, 4-DCA at a concentration of 1000 mg·L−1 were prepared in methanol. The working standard solutions were prepared daily dilution of the stock solution. Three different water samples (Hookah water, river water and rain water) were investigated for determination of intended analytes. Hookah water sample (five-time used) was obtained from a hookah lounge. River water sample was collected from Karaj River (Karaj, Iran). Rain water sample was collected during March 2018 (Tehran, Iran). 20 mL of each sample (spiked/non-spiked) was used without any dilution.
Instrumentation
The chromatographic analyses were conducted on a Shimadzu HPLC instrument model SCL-10AVP (Tokyo, Japan, www.shimadzu.com), consisting of a LC-10AVP pump, SPD-M10AVP photo diode array (PDA) detector, and a Rheodyne7725i (PerkinElmer, USA, www.perkinelmer.com). A 100 μL sample loop and 250 μL Hamilton HPLC syringe (Reno, NV, USA, www.hamiltoncomany.com) were used too. Data analysis was performed using LC-solution software. A Knauer HPLC column (Vertex Plus Column, Germany, www.knauer.net, 250 mm × 4.6 mm, i.d. 5 μm) consisting a C18 precolumn for protecting was employed for all separations, under an isocratic program using 50% acetonitrile and 50% water as mobile phase ingredients. Also, the flow rate was set at 1 mL·min−1 and the monitoring wavelength was 315 nm for 4-NP, 230 nm for 4-CP, 240 nm for 4-CA and 1-A2N and 245 nm for 2, 4-DCA. Under this condition, the retention time of 4-NP, 4-CP, 4-CA, 1-A2N and 2, 4-DCA was 5.1, 7.2, 8.4, 10.6 and 16.5 min, respectively. A digital pH meter (Metrohm, www.metrohm.com, model 827, with a glass calomel electrode) was utilized for measuring of pH values. Vortex mixing was accomplished with a MS3 digital vortex agitator (IKA Company, Staufen, Germany, www.ika.com). A 25 mL sample vial, a MR 3001 heating-magnetic stirrer (Heidolph Company, Kelheim, Germany, www.heidolph-instruments.com) were employed in extraction process. For characterization of magnetic MOF (MMOF), an EM 3200 KYKY scanning electron microscope (SEM, Zhongguancun, Beijing, China, www.kyky.com.cn) was used for morphological evaluation. Transmission electron microscopy (TEM) was performed on a Zeiss EM900 instrument at 150 kV (Carl Zeiss, Germany, www.zeiss.com). XRD patterns were recorded on a powder X-ray diffractometer (Philips-PW 12C, Amsterdam, the Netherlands; www.innovationservices.philips.com) armed with a Cu Kα radiation source. A VEGAII TESCAN instrument (www.tescan.com) was employed for accomplishing the energy dispersive X-ray spectroscopy (EDX). Fourier transform infrared (FT-IR) spectrometer (Bruker IFS-66 FT-IR, Bruker Optics, Karlsruhe, Germany, www.brukeroptics.com) was applied to examine the infrared spectra of MOF and MMOF using a pressed KBr tablet. Thermogravimetric analysis (TGA) was done on a Bahr STA-503 (Bahr-Thermoanalyse GmbH, Hüllhorst, Germany; www.tainstruments.com) instrument under air atmosphere. Magnetic measurements of the products were conducted with a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran; www.mdk-magnetics.com) at chamber temperature in a 1 Tesla magnetic field. For the purpose of sorbent collection and magnetic decantation an Nd-Fe-B strong magnet (15 cm × 12 cm × 5 cm, 1.4 T) was used.
Synthesis of magnetic MIL-53(Fe)
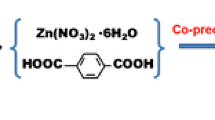
Fe3O4 NPs were synthesized by chemical co-precipitation method [17, 19, 28]. The Fe3O4@MIL-53(Fe) was synthesized via solvothermal method according to the literature with slight modifications [29]. The preparation steps of Fe3O4 NPs and Fe3O4@MIL-53(Fe) are described in detail in the Electronic Supplementary Material and Fig. 1S.
Synthesis of MIL-53(Fe)
The synthesis of MIL-53(Fe) is similar to the Fe3O4@MIL-53(Fe) preparation process, the only difference is the lack of Fe3O4 NPs in synthesis procedure.
Fe3O4@MIL-53(Fe) based D-μ-SPE procedure
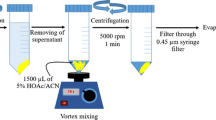
First, 20 mL solution of the intended analytes (1.0 mg·L−1) that containing 15% (w·v−1) NaCl was placed into the 25 mL sample vial and 20 mg Fe3O4@MIL-53(Fe) was appended to the vial. Then, sample solution pH was set to 5.0. The mixture was stirred for 8.0 min at 1250 rpm. Afterward, a strong external magnet was used for quickly collection of sorbent from the sample solution. When the suspension became lucid, the supernatant was decanted and the collected sorbent was eluted with 130 μL of acetonitrile by vortexing for 2.0 min with the purpose of desorbing the pre-concentrated intended analytes. Finally, for analyzing the intended analytes, the eluate was injected into the HPLC-PDA.
Zero charge (pHPZC) determination
In several beakers, a known amount (10 mg) of Fe3O4@MIL-53(Fe) was dispersed in 10 mL of the degassed 0.01 M NaNO3 aqueous solution at 25 °C. The sample solutions pH was set at 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0 using aqueous 0.1 mol L−1 HNO3 or 0.1 mol L−1 NaOH solutions as proper. This suspension was stirred for 24 h at 25 °C, after this time the pH of each beaker was measured. Finally, a plot of the primary solutions pH against ΔpH (differences between the primary and the final solutions pH) of the solutions gives the pHPZC value for the sample. The pHPZC related to the pH where ΔpH = 0 [5, 30].
Multivariate optimization
For optimizing the parameters that effect on D-μ-SPE, central composite design (CCD) was utilized by employing Design-Expert 7.0.0 (trial version) software.
Results and discussion
Choice of material
Among diverse materials which can be used as D-μ-SPE sorbents, such as GO, Fe3O4@GO, RGO, CNTs, Fe3O4@CNTs, RCNTs, MOFs and Fe3O4@MOFs, Fe3O4@MOF was selected owing to its considerable properties. MIL-53(Fe) has a porous structure that can capture the analytes, resulted in the great increase of specific surface area and also due to the attendance of aromatic rings, hydroxyl groups and metal centers (Fe) in its structure, no modification is required. In spite of the fact that the other sorbents, should be modified to be able to interact with the analytes. Moreover, the duration of synthesis of MIL-53(Fe) is very short (2 h) compared to other sorbents. The attendance of Fe3O4 nanoparticles in MIL-53(Fe) structure, increases the stability of the MIL-53(Fe) and also facilitates the separation of this sorbent from the sample solution compared to non-magnetic sorbent such as GO, RGO, CNTs and RCNTs. Based on the diverse functional moieties, this sorbent has a potential applicability for the extraction of other analytes like pesticides, drugs, PAHs and any other pollutants in samples with complex matrix. The only limitation of this method is that the synthesized sorbent is non-selective. This problem can be resolved by optimization of the extraction conditions (such as pH of sample, salt concentration etc.) or by sorbent modification.
Characterization studies
The FT-IR, EDX, XRD, TGA and zeta-potential measurements information related to characterization of the sorbent are described in the Electronic Supplementary Materials (Fig. 2S–6S).
The SEM and TEM methods were utilized to explore the morphology of MIL-53(Fe) and magnetic MIL-53(Fe) (Fig. 1). As illustrated in Fig. 1a, the MIL-53(Fe) crystals have an average size of about 100 nm (n = 50 particles). Figure 1b shows the SEM image of the synthesized magnetic MIL-53(Fe). It is demonstrated that the sizes of magnetic MIL-53(Fe) crystals are obviously increased about 170 nm (n = 50 particles) than the MIL-53(Fe) crystals owing to the incorporation of Fe3O4 nanoparticles into the MIL-53(Fe) pores. Figure 1c displays the TEM image of MIL-53(Fe). TEM image shown in Fig. 1d reveals clearly that the Fe3O4@MIL-53(Fe) crystals are core-shell structures with Fe3O4 cores and MIL-53(Fe) shell with an average size of 40 nm (n = 10 particles) for Fe3O4 NPs.
The magnetic behavior of Fe3O4 NPs and Fe3O4@MIL-53(Fe) composite were studied and the VSM plots are illustrated in Fig. 1e. As illustrates in Fig. 1e, the saturation magnetization of Fe3O4 NPs and Fe3O4@MIL-53(Fe) was 64 and 38 emu·g−1 respectively, which depicts superparamagnetic properties of the sorbents and is enough for magnetic separation with a common magnetic field [11] (Fig. 1e).
Optimization of the extraction condition
The following parameters were optimized: (a) sorbent type; (b); eluent type and its volume; (c) effect of ionic strength; (d) pH effect; (e) sample volume; (f) amount of the sorbent; (g) sorption time. Respective data and Figures are given in the Electronic Supporting Material (Fig. 7S–8S). In short, the following experimental conditions were found to give best results: (a) optimal sorbent: Fe3O4@MIL-53(Fe); (b) optimal eluent and its volume: acetonitrile, 130 μL; (c) optimal ionic strength: 15% (w·v−1) NaCl; (d) best sample pH value: 5.0; (e) sample volume: 20 mL; (f) amount of the sorbent: 20 mg; (g) sorption time: 8 min.
Method analytical performance
Quantitative parameters of the D-μ-SPE-HPLC-PDA method, including the limits of detection (LODs), linear dynamic range (LDR), enrichment factors (EFs), and extraction recoveries (ER%) were determined, under the selected experimental conditions. The results are illustrated in Table 1. Excellent linearity with coefficients of determination higher than 0.999 were achieved for all the analytes. LODs and LOQs (limits of quantification) were calculated as 3 and 10 S/N (signal-to-noise), respectively. The EFs were computed as the ratio of the slopes of each analyte calibration plots before extraction and after extraction process. The repeatability and reproducibility of the method (as RSD) were obtained by performing five replicate experiments at four levels of analytes concentration (50, 100, 250 and 500 μg·L−1). The within day and between day RSDs of the mentioned method for determination of intended analytes are equal or less than 3.7% and 9.6%, respectively. The extraction recoveries percentage, which refers to a percentage of an efficient extraction of total analyte by the sorbent and eventually eluted with acetonitrile, are ranged from 39.5 to 93.3%. Combination of low detection limit with extensive linear range, providing a high potential for determination of the low concentration levels of intended analytes in water samples.
Real samples analysis
To evaluate the applicability of the Fe3O4@MIL-53(Fe) based D-μ-SPE-HPLC method on real samples, recovery studies were accomplished in water samples (rain water, river water and hookah wastewater) under the selected experimental conditions. The results of the determination and recovery studies with three replicate for the non-spiked and spiked samples are demonstrated in Table 2. As can be beheld, the relative recoveries are ranged from of 93.4 to 103.1% with RSDs below 8.1%. Therefore, the results confirm that the mentioned method is accurate and repeatable for enrichment and determination of the low concentration levels of phenols and anilines in diverse water samples. Fig. 2 displays typical HPLC chromatograms of non-spiked and spiked rain water, river water and hookah wastewater sample after extraction under the opted conditions.
Comparison of the D-μ-SPE-HPLC-PDA method with other reported approaches
Table 3 displays a comparison between the results achieved by this method and those achieved by some other formerly reported methods for coextraction and determination of 4-NP, 4-CP, 4-CA, 1-A2N and 2,4-DCA in various real samples. As can be beheld, the mentioned method indicated satisfactory wide linearity, acceptable RSDs and extraction recoveries compared with the reported methods. The achieved LODs of this study are lower than the other reported method. This method not only extracts phenols and anilines simultaneously at low concentration levels but also uses low amount of sorbent and little volume of organic solvents compared to the reported methods.
Conclusion
Fe3O4@MIL-53(Fe) was synthesized and utilized as an efficient nanoadsorbent for coextraction of phenols and anilines as pollutants at low concentration levels in diverse water samples. The synthesized Fe3O4@MIL-53(Fe) has core-shell structure with Fe3O4 cores and MIL-53(Fe) shell. The presence of Fe3O4 NPs in MIL-53(Fe) structure, makes the Fe3O4@MIL-53(Fe) structure more stable than MIL-53(Fe) without Fe3O4 NPs attendance and also helps to collect this sorbent from sample solution easily by a strong external magnet. This study introduces an efficient and environmentally friendly method which provides limits of detection in the low range and acceptable extraction recoveries and manifests the high potential of this method for applying in sample preparation step. Moreover, the duration of synthesis of MIL-53(Fe) is very short (2 h) compared to other sorbents. Based on the diverse functional moieties, this sorbent has a potential applicability for the extraction of other analytes like pesticides, drugs, PAHs and any other pollutants in samples with complex matrix. The only limitation of this method is that the synthesized sorbent is non-selective. This problem can be resolved by optimization of the extraction conditions (such as pH of sample, salt concentration etc.) or by sorbent modification.
References
Young A, Lai G, Hung B, Yuen A, He Y (2011) Determination of trace Chloroanilines in environmental water samples using hollow Fiber-based liquid phase microextraction. Chromatographia 74:83–88. https://doi.org/10.1007/s10337-011-2022-6
Yang Q, Chen X, Jiang X (2013) Liquid–liquid microextraction of Nitrophenols using supramolecular solvent and their determination by HPLC with UV detection. Chromatographia 76:1641–1647. https://doi.org/10.1007/s10337-013-2554-z
Pan YL, Chen F, Zhang MY, Wang TQ, Xu ZC, Zhang W, Chu QC, Ye JN (2013) Sensitive determination of chloroanilines in water samples by hollow fiber-based liquid-phase microextraction prior to capillary electrophoresis with amperometric detection. Electrophoresis 34:1241–1248. https://doi.org/10.1002/elps.201200320
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2017) Dispersive micro-solid phase extraction of aromatic amines based on an efficient sorbent made from poly(1,8-diaminonaphtalen) and magnetic multiwalled carbon nanotubes composite. J Chromatogr A 1499:38–47. https://doi.org/10.1016/j.chroma.2017.03.087
Babaee S, Daneshfar A (2016) Extraction of phenolic compounds from water samples by dispersive micro-solid-phase extraction. J Sep Sci 39:2508–2516. https://doi.org/10.1002/jssc.201500977
Sun XM, Sun Y, Wu LW, Jiang CZ, Yu X, Gao Y, Wang LY, Song DQ (2012) Development of a vortex-assisted ionic liquid microextraction method for the determination of aromatic amines in environmental water samples. Anal Methods 4:2074–2080. https://doi.org/10.1039/C2AY25056J
Baciocchi R, Attina M, Lombardi G, Boni MR (2001) Fast determination of phenols in contaminated soils. J Chromatogr A 911:135–141. https://doi.org/10.1016/S0021-9673(00)01249-8
Wennrich L, Popp P, Molder M (2000) Determination of chlorophenols in soils using accelerated solvent extraction combined with solid-phase microextraction. Anal Chem 72:546–551. https://doi.org/10.1021/ac990463r
Zhang WB, Xiao XM, An TC, Song ZG, Fu JM, Sheng GY, Cui MC (2003) Kinetics, degradation pathway and reaction mechanism of advanced oxidation of 4-nitrophenol in water by a UV/H2O2 process. J Chem Technol Biotechnol 78:788–794. https://doi.org/10.1002/jctb.864
Chung KT, Fulk GF, Egan M (1978) Reduction of azo dyes by intestinal anaerobes. Appl Environ Microbiol 35:558–562
Asgharinezhad AA, Ebrahimzadeh H (2016) A simple and fast method based on mixed hemimicelles coated magnetite nanoparticles for simultaneous extraction of acidic and basic pollutants. Anal Bioanal Chem 408:473–486. https://doi.org/10.1007/s00216-015-9114-3
Jauregui O, Galceran MT (1997) Determination of phenols in water by on-line solid-phase disk extraction and liquid chromatography with electrochemical detection. Anal Chim Acta 340:191–199. https://doi.org/10.1016/S0003-2670(96)00504-1
Cai MQ, Wei XQ, Du CH, Ma XM, Jin MC (2014) Novel amphiphilic polymeric ionic liquid-solid phase micro-extraction membrane for the preconcentration of aniline as degradation product of azo dye Orange G under sonication by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1349:24–29. https://doi.org/10.1016/j.chroma.2014.05.008
Sarafraz-Yazdi A, Sayyar Ardaki M, Amiri A (2013) Determination of monocyclic aromatic amines using headspace solid-phase microextraction based on sol gel technique prior to GC. J Sep Sci 36:1629–1635. https://doi.org/10.1002/jssc.201200940
Zhu L, Lee HK (2001) Liquid–liquid–liquid microextraction of nitrophenols with a hollow fiber membrane prior to capillary liquid chromatography. J Chromatogr A 924:407–414. https://doi.org/10.1016/S0021-9673(01)00906-2
Zhang D, Zhang L, Liu T (2018) A magnetic cellulose-based carbon fiber hybrid as a dispersive solid-phase extraction material for the simultaneous detection of six bisphenol analogs from environmental samples. Analyst 143:3100–3106. https://doi.org/10.1039/C8AN00544C
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2018) Determination of acidic, basic and amphoteric drugs in biological fluids and wastewater after their simultaneous dispersive micro-solid phase extraction using multiwalled carbon nanotubes/magnetite nanoparticles@poly(2-aminopyrimidine) composite. Microchem J 143:337–349. https://doi.org/10.1016/j.microc.2018.08.037
Chisvert A, Cardenas S, Lucena R (2019) Dispersive micro-solid phase extraction. TrAC Trends Anal Chem 112:226–233. https://doi.org/10.1016/j.trac.2018.12.005
Asgharinezhad AA, Jalilian N, Ebrahimzadeh H, Panjali Z (2015) A simple and fast method based on new magnetic ion imprinted polymer nanoparticles for the selective extraction of Ni(II) ions in different food samples. RSC Adv 5:45510–45519. https://doi.org/10.1039/C5RA05639J
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA, Molaei K (2017) Extraction and determination of trace amounts of gold(III), palladium(II), platinum(II) and silver(I) with the aid of a magnetic nanosorbent made from Fe3O4-decorated and silica-coated graphene oxide modified with a polypyrrole-polythiophene copolymer. Microchim Acta 184:2191–2200. https://doi.org/10.1007/s00604-017-2170-y
Zhang H, Yuan Y, Sun Y, Niu C, Qiao F, Yan H (2018) An ionic liquid-magnetic graphene composite for magnet dispersive solid-phase extraction of triazine herbicides in surface water followed by high performance liquid chromatography. Analyst 143:175–181. https://doi.org/10.1039/C7AN01290J
Yang Z, Xu X, Liang X, Lei C, Wei Y, He P, Lv B, Ma H, Lei Z (2016) MIL-53(Fe)-graphene nanocomposites: efficient visible-light photocatalysts for the selective oxidation of alcohols. Appl Catal B 198:112–123. https://doi.org/10.1016/j.apcatb.2016.05.041
Hashemi B, Zohrabi P, Raza N, Kim KH (2017) Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. TrAC Trends Anal Chem 97:65–82. https://doi.org/10.1016/j.trac.2017.08.015
Rocio-Bautista P, Gonzalez-Hernandez P, Pino V, Pasan J, Afonso AM (2017) Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. TrAC Trends Anal Chem 90:114–134. https://doi.org/10.1016/j.trac.2017.03.002
Ghorbani-Kalhor E (2016) A metal-organic framework nanocomposite made from functionalized magnetite nanoparticles and HKUST-1 (MOF-199) for preconcentration of Cd(II), Pb(II), and Ni(II). Microchim Acta 183:2639–2647. https://doi.org/10.1007/s00604-016-1896-2
Azizi Vahed T, Naimi-Jamal MR, Panahi L (2018) (Fe)MIL−100-met@alginate: a hybrid polymer–MOF for enhancement of metformin's bioavailability and pH-controlled release. New J Chem 42:11137–11146. https://doi.org/10.1039/C8NJ01946K
Araya T, Jia M, Yang J, Zhao P, Cai K, Ma W, Huang Y (2017) Resin modified MIL-53(Fe) MOF for improvement of photocatalytic performance. Appl Catal B 203:768–777. https://doi.org/10.1016/j.apcatb.2016.10.072
Rezvani M, Asgharinezhad AA, Ebrahimzadeh H, Shekari N (2014) A polyaniline-magnetite nanocomposite as an anion exchange sorbent for solid-phase extraction of chromium(VI) ions. Microchim Acta 181:1887–1895. https://doi.org/10.1007/s00604-014-1262-1
Banerjee A, Gokhale R, Bhatnagar S, Jog J, Bhardwaj M, Lefez B, Hannoyerc B, Ogale S (2012) MOF derived porous carbon–Fe3O4 nanocomposite as a high performance, recyclable environmental superadsorbent. J Mater Chem 22:19694–19699. https://doi.org/10.1039/C2JM33798C
Madrakian T, Afkhami A, Zadpour B, Ahmadi M (2015) New synthetic mercaptoethylamino homopolymer-modified maghemite nanoparticles for effective removal of some heavy metal ions from aqueous solution. J Ind Eng Chem 21:1160–1166. https://doi.org/10.1016/j.jiec.2014.05.029
Asgharinezhad AA, Ebrahimzadeh H (2015) Coextraction of acidic, basic and amphiprotic pollutants using multiwalled carbon nanotubes/magnetite nanoparticles@polypyrrole composite. J Chromatogr A 1412:1–11. https://doi.org/10.1016/j.chroma.2015.07.087
Asiabi H, Yamini Y, Rezaei F, Seidi S (2016) Nanostructured polypyrrole for automated and electrochemically controlled in-tube solid-phase microextraction of cationic nitrogen compounds. Microchim Acta 183:1449–1458. https://doi.org/10.1007/s00604-015-1534-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 997 kb)
Rights and permissions
About this article
Cite this article
Jalilian, N., Ebrahimzadeh, H. & Asgharinezhad, A.A. A nanosized magnetic metal-organic framework of type MIL-53(Fe) as an efficient sorbent for coextraction of phenols and anilines prior to their quantitation by HPLC. Microchim Acta 186, 597 (2019). https://doi.org/10.1007/s00604-019-3698-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3698-9