Abstract
The authors describe an efficient method for microextraction and preconcentration of trace quantities of cationic nitrogen compounds, specifically of anilines. It relies on a combination of electrochemically controlled solid-phase microextraction and on-line in-tube solid-phase microextraction (SPME) using polypyrrole-coated capillaries. Nanostructured polypyrrole was electrically deposited on the inner surface of a stainless steel tube and used as the extraction phase. It also acts as a polypyrrole electrode that was used as a cation exchanger, and a platinum electrode that was used as the anode. The solution to be extracted is passed over the inner surface of the polypyrrole electrode, upon which cations are extracted by applying a negative potential under flow conditions. This method represents an ideal technique for SPME of protonated anilines because it is fast, easily automated, solvent-free, and inexpensive. Under optimal conditions, the limits of detection are in the 0.10–0.30 μg L‾1 range. The method works in the 0.10 to 300 μg L‾1 concentration range. The inter- and intra-assay precisions (RSD%; for n = 3) range from 5.1 to 7.5 % and from 4.7 to 6.0 % at the concentration levels of 2, 10 and 20 μg L‾1, respectively. The EC-in-tube SPME method was successfully applied to the analysis of methyl-, 4-chloro-, 3-chloro and 3,4-dichloroanilines in (spiked) water samples.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic amines are widely used in industry for making dyes, cosmetics, pharmaceutical, as well as in the photography industry. They are also used as intermediates in many chemical syntheses such as antioxidants in the polymer industry [1–5]. Owing to their high solubility in water, amines can easily permeate through soil and contaminate groundwater [6–8]. Therefore, determination of aromatic amines in environmental water is very important because they are widely used in chemical industries and many of them are suspected carcinogens and are highly toxic to the aquatic life, most of them have been included in the list of priority pollutants by the US Environmental Protection Agency [9, 10]. High performance liquid chromatography (HPLC) is often used for determination of aromatic amines, and sample cleanup and preconcentration is usually required.
Several sample preparation techniques, mainly liquid–liquid extraction [11–14] and solid phase extraction, [15–17] have been applied for extraction of anilines from water and other matrices. This is while modern trends in analytical chemistry are moving toward simplified and miniaturized sample preparation methods providing sample clean-up and analyte preconcentration, simultaneously. Among these, SPME has become a popular sampling method for compounds due to its simplicity, solvent-free, reliable, and flexible properties. Since it was introduced by Pawliszyn and Arthur [18] in the beginning of 1990s, SPME has been widely accepted and applied as a sample preparation technique. Introduction of new polymeric fibers, development of new experimental configurations, and improvement of automatic devices will undoubtedly lead to the application of SPME to different fields of chemical analysis [19–22].
Organic conducting polymers, which are described as polymers with spatially extended conjugated-bonding systems obtained by electropolymerization or chemical oxidation, have been extensively studied for various technological applications over the past four decades [23]. Conducting polymers have attracted more and more attention due to their electrical properties, which are similar to metals, and some characteristics of organic polymers. Furthermore, they can be prepared in a way to be applied in different fields of requirements. Widely used conducting polymers are mainly of three types including polypyrrole, polythiophene, and polyaniline. In this respect, polypyrrole (PPY) is one of the most frequently investigated conducting polymers due to its facile synthesis, good environmental and thermal stability properties. Among various synthetic methods for conducting polymers, electrochemical polymerization plays an important role because the electrochemical approach has the advantage of one-step production of conducting polymer films onto inner surface of a metal electrode surface [24–26].
Combining solid phase microextraction with electrochemistry derives a sample preparation method, which is called electrochemically controlled solid phase microextraction (EC-SPME). The EC-SPME with modified solid phase electrodes has been employed for detecting the cations [27–29] and anions (chloride, nitrite, bromide, nitrate, sulfate, and phosphate). PPY with small counter-ions, e.g., Cl−, ClO4 −, and NO3 −, mainly exhibits an anion exchanger behavior due to the high mobility of these ions in the polymer matrix [30]. Cation exchanger behavior can be achieved by incorporating large polyanionic counter-ions, such as sodium dodecylbenzenesulfonate (SDBS), because of their immobility in the polymer matrix.
SPME was combined with HPLC by Arthur and Pawliszyn in 1995 [31], and the original forms of SPME-HPLC and the automated form of in-tube SPME-HPLC, introduced in 1997 [32], providing an alternative choice for sample clean-up and enrichment for analysis of non-volatile analytes. After this report, many commercial (such as GC capillary column) and synthesized coated tubes were employed for in-tube SPME-HPLC [33–35]. In-tube SPME provides convenient automation of the extraction process, reduced analysis time, and improved precision and sensitivity in comparison with manual off-line techniques.
In the present study, a nanostructured polypyrrole coating was electrochemically deposited on the inner surface of a stainless steel tube. An EC-in-tube SPME method based on coupling of EC-SPME and in-tube SPME-HPLC was developed for extraction of anilines from aqueous solutions. The effect of different parameters on the extraction efficiency of the analytes were investigated and optimized.
Experimental
Chemicals and reagents
All chemicals were of analytical reagent grade. Standards of 2-methyl aniline (MA, pKb = 9.56, log Kow = 1.36), 4-chloro aniline (4-CA, pKb = 10.0, log Kow = 1.85), 3-chloro aniline (3-CA, pKb = 10.48, log Kow = 1.88), and 3,4- dichloro aniline (3,4-DCA, pKb = 11.1, log Kow = 2.8) were obtained from Sigma-Aldrich (St. Louis, MO, USA; www.sigmaaldrich.com). HPLC-grade methanol and acetonitrile were purchased from Caledon (Georgetown, ON, Canada; www.caledonlabs.com). The stock solutions of the anilines (1000 mg L−1) were prepared by dissolving 10 mg of the compounds in 10 mL methanol. A mixed standard solution of anilines was prepared by adding an appropriate amount of each stock standard solution to a 10 mL volumetric flask and diluting it to the mark by methanol. All standard solutions were stored at 4 °C and protected from light. Pyrrole of synthesis grade and sodium dodecyl benzene sulfonate and acetic acid were purchased from Sigma-Aldrich (Milwaukee, WI, USA; www.sigmaaldrich.com). The water consumed was purified on a Youngling ultrapure water purification system model Aqua Max TM-ultra (Seoul, South Korea; wk101406080.company.weiku.com). Other chemicals used were of reagent grade or of the highest purity available. Plastic and glassware used for the experiments were previously washed with acetone and rinsed carefully with ultra-pure water.
Apparatus
Particle size and morphology of the synthesized NPs were determined by a scanning electron microscope (SEM) model EM3200 from KYKY Zhongguancun (Beijing, China). Chromatographic analysis was performed with a HPLC instrument including a Varian 9012 HPLC pump (Walnut Creek, CA, USA), a six-port Cheminert HPLC valve from Valco (Houston, TX, USA) with a 20 μL sample loop and equipped with a Varian 9050 UV–vis detector. Chromatographic data were recorded and analyzed using Chromana software (version 3.6.4). The separations were run on an ODS-3 column (250 × 4.6 mm, with a 5 μm particle size) from Hector Company (Daejeon, Korea). The mobile phase consisted of 10 mM acetate buffer, pH 4.0, and acetonitrile (47:53). The flow rate of mobile phase was set at 1.0 mL min−1. Total analysis time was 15 min. The injection volume was 20 μL for all the samples and detection was performed at a wavelength of 240 nm. All the pH measurements were performed with a WTW Inolab pH meter (Weilheim, Germany). GPFA1-380 peristaltic pump from Ultra Voltammetry (Tehran, Iran) was used for passing the samples through the stainless steel capillary tube.
Preparation of polymer-coated capillary tubes
The PPY-DBS coating was prepared by electrochemical polymerization of pyrrole on the inner surface of the stainless steel tube (10 cm length and 0.75 mm diameter) using a three-electrode system by applying a potential of +0.80 V for 10 min in an aqueous solution containing 0.2 M pyrrole and 0.01 M sodium dodecyl benzene sulfonate as the supporting electrolyte and all potentials were adjusted related to an Ag/AgCl reference electrode [29]. Stainless steel tube, platinum electrode, and Ag/AgCl electrodes were used as the working, counter, and reference electrodes, respectively. Growth of the films was controlled based on the amount of charge consumed in the electropolymerization. A peristaltic pump was used to deliver the monomer solution from the inner surface of the stainless steel tube. Before electrochemical deposition, the stainless steel tubes were cleaned by acetone and HPLC grade water and finally air dried at the room temperature. After electrochemical deposition, the stainless tube coated with PPY-DBS film was washed with methanol, acetone, and water in sequence to remove excess pyrrole and electrolyte, and dried under nitrogen gas flow.
On-line electrochemically controlled in-tube solid-phase microextraction-HPLC procedure
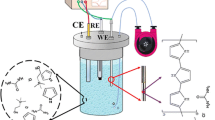
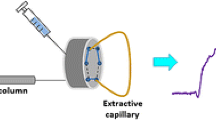
A schematic diagram of the complete assembly and operation mode of the extraction setup are shown in Fig. 1. The coated stainless steel tube was mounted on valve 1 (V1) in the position where the loop was originally positioned. Capillary connections were facilitated by 2.5 cm sleeve of 1/16-in polyether ether ketone tubing at each end of the capillary. Both V1 and valve 2 (V2) were initially set at the load position (Fig. 1a). Pump A is on to flow the sample solution through the tube at the flow rate of 10.0 mL min−1 and Pump B is off. The effluent of V1 was poured again into sample compartment after passing through the coated tube. On the other word, this procedure was carried out in a circulating path. Platinum electrode was placed into the sample solution and used as the anode electrode. By passing sample solution through the PPY-DBS coated tube, extraction of cations occurred by applying a negative potential under flow conditions. Also, the HPLC mobile phase was driven by pump C directly through the analytical column at the flow rate of 1.0 mL min−1 to obtain a stable baseline for chromatographic separation. After extraction for a given time interval, platinum electrode was moved into the desorption solvent and used as the cathode electrode (Fig. 1b). V1 was directed to the inject position, pump A was turned off while pump B was turned on to flow the desorption solvent (0.1 mol L−1 NaCl in methanol) through the tube at the flow rate of 6.0 mL min−1. By passing desorption solvent through the PPY-DBS coated tube, desorption of cations occurred by applying a positive potential. The effluent of desorption solvent was circulated as same as extraction procedure to reach the maximum desorption efficiency (blue arrows). Finally, after a given desorption time interval, pump B was turned off so that desorption solution was located into the HPLC loop, V1 was returned to the load position while V2 was directed to the inject position. Then, the extracted analytes collecting into the loop of V2 were eluted by the mobile phase into the HPLC column for analysis.
Sampling and sample preparation
-
a)
Water samples: different water samples, including tap water from our laboratory (Tehran, Iran), wastewater І from Industrial zone (Tehran, Iran), wastewater П from textile factory (Tabriz, Iran), and river water (Niasar, Iran) were collected and the EC-in-tube SPME method was applied to extract their anilines contents. For preconcentration, the pH of the samples was adjusted at 2.0; then each water sample was filtered to remove any suspended material and 30 mL of the prepared samples was used for analysis. Before the analysis, the water samples were stored in a dark place at 4 °C in an amber glass bottle that was previously rinsed with acetone and ultra-pure water and acetone.
-
b)
Acetate buffer (C = 0.01 mol L−1, pH = 4.0) was prepared by dissolving appropriate amounts of acetic acid and sodium acetate in water solution and the exact pH was adjusted by dropwise addition of 0.5 M nitric acid or 0.5 M sodium hydroxide solutions.
Results and discussion
In the present study, the applicability of EC-SPME combined with in-tube SPME-HPLC-UV was considered for quantitative analysis of anilines in water samples. There are several factors, which affect the extraction process. Optimization of EC-SPME conditions was carried out using one-variable-at-a-time procedure. The optimization was carried out using working solutions containing 100 μg L−1 of anilines. The chromatographic peak area, which is related to the number of moles of extracted analytes into the organic solvent, was used to evaluate the extraction efficiency under different experimental conditions. The injected volume of the extracted analytes into HPLC was kept constant at 20 μL throughout the experiments. Initial experimental conditions were as follow: extraction voltage, 1.0 V; desorption voltage, 1.0 V; extraction time, 20 min; desorption time, 5 min; extraction flow rate, 7 mL min−1; desorption flow rate, 3 mL min−1; and pH, 2.
The following parameters were optimized: (a) Sample pH value; (b) extraction voltage; (c) desorption voltage; (d) extraction time; (e) desorption time; (f) extraction flow rate and (g) desorption flow rate. Respective data and figure are given in the Electronic Supplementary Material. The following experimental conditions were found to give the best results: (a) a sample pH value of 2; (b) an extraction voltage of −0.8 V; (c) a desorption voltage of +0.8 V; (d) an extraction time of 13 min (e) a desorption time of 3 min (f) an extraction flow rate of 10 mL min−1 and (g) a desorption flow rate of 8 mL min−1.
Moreover, the effect of interfering inorganic cations on the extraction efficiency of the analytes was investigated by addition of different amount of NaCl into the sample solution. The results showed a negative effect on the extraction efficiency of aniline derivatives attributing to the competition among the target analytes and cationic interferences for electrochemically adsorption on the surface of PPY-DBS polymeric film.
Polymer film preparation and scanning electron microscope studies
Creation of a uniform and stable coating at the inner surface of a stainless steel tube is very important in in-tube SPME. Pyrrole and some of its derivatives can be polymerized easily with oxidation reactions by either an electrochemical or a chemical method. In electrochemical synthesis of PPY, the porous and uniform polymer film is directly electrodeposited on the inner surface of the stainless steel tube from an aqueous solution containing pyrrole and electrolyte using cyclic voltammetry or constant potential method and the morphology of the film can be controlled by the controlling electrochemical conditions. To take the SEM image from the inside of steel tube, a part of the tube was carefully cut with coping saw and the SEM image was taken. The image of the cut tube before and after polymer coating was shown in Fig. 2. The SEM micrographs of the synthesized PPY-DBS on the inner surface of the stainless steel tube using SDBS as the electrolyte was shown in Fig. 2. As can be seen in Fig. 2, the electrochemical coating possesses a porous structure and the fiber surface is very well distributed. A high surface area provides the fiber with large stationary phase loading and high extraction capacity. SEM was used to estimate the average thickness of the PPY-DBS coating and the size of the nano-structured particles which were found to be about 50 ± 2 μm and < 60 nm, respectively. Figure 2 shows the SEM micrographs of the PPY formed on the inner surface of the stainless steel tube indicating typical “cauliflower” morphology. The coating lifetime is important for the practical application. Since the PPY-DBS coating was used followed by a HPLC system, thermal stability of the polymer was not important in this study and just physical and chemical stability as well as extraction capability of the coating were investigated. The process of extraction in this study was carried out by using one fiber and the inter-day RSDs of the fiber for extraction of the anilines were lower than 7.5 %. So, the synthesized PPY-DBS coating exhibited very good chemical and physical characteristics. The tube-to-tube reproducibility was also evaluated by calculating the RSDs of the four repeated extractions of anilines spiked at the concentration of 20 μg L−1 in water. The results showed that RSDs% were between 6.5 and 7.8 %.
Method evaluation
The applicability of the EC-in-tube SPME method was examined for extraction of model anilines from water samples. To evaluate the performance of the EC-in-tube SPME technique, establishing dynamic range, extraction recovery (ER%), limits of detection (LODs), intra- and inter-assay precision (RSD%) and accuracy (Error%) were investigated utilizing standard solutions of anilines in ultrapure water. As provided in Table 1, calibration curves were plotted using ten spiking levels of the anilines at the concentrations ranging from 0.35 to 100 μg L−1 with three replicate measurements for each point. Calibration curves were found to be linear in the range of 0.50–100 μg L−1, 0.50–100 μg L−1, 0.35–100 μg L−1, and 0.30–50 μg L−1 for MA, 4-CA, 3-CA, and 3,4-DCA in water sample, respectively. The LODs, based on a signal-to-noise ratio (S/N) of 3, were 0.30, 0.30, 0.20, and 0.10 μg L−1 for MA, 4-CA, 3-CA and 3, 4-DCA, respectively. The total dynamic ranges (TDRs) in water sample were 0.30–300 μg L−1, 0.3–300 μg L−1, 0.2–300 μg L−1 and 0.10–300 μg L−1 for MA, 4-CA, 3-CA, and 3,4-DCA, respectively. Intra-day (n = 5) and inter-day standard deviations were calculated by extracting the analytes from water samples at the concentration levels of 2, 10, and 20 μg L−1 and RSDs% lower than 6.0 and 7.5 % were obtained, respectively. The ER% was calculated according to the following equation:
The n eluent and n 0 are the mole numbers of analyte in the eluent phase and the initial mole numbers of analyte in the sample solution, respectively. C eluent and C 0 are the concentration of analyte in the eluent phase and the initial concentration of analyte in the sample solution, respectively. PF is the preconcentration factor and V eluent and V aq are the volumes of the receiving and the source phases, respectively. The obtained ERs% were in the range of 34.8–49.1 % for the anilines.
Some representative analytical characteristics of the method are provided in Table S1 (Electronic Supplementary Material, ESM) and compared with the literature data obtained from other methods. As can be deducted, the method is quite comparable to the methods mentioned in Table S1. The EC-in-tube SPME method has some advantages over the other extraction methods (LPME, DLLME, etc.) including reduced extraction time, low detection limit, low cost of the extraction device, ability to extract from complex matrices, producing a clean extracting phase for subsequent analysis of the results, and ability to simultaneously use SPME and electrochemical methods for extraction of aninlines from complex water samples and rapid analysis with high performance liquid chromatography.
Analysis of real samples
In order to evaluate the applicability of the extraction method for analysis of anilines in real samples with complex matrices, different types of water samples including wastewaters, tap water, and river water were extracted and analyzed using the EC-in-tube SPME method under the optimal conditions. Sample preparation for the real samples was performed according to section Preparation of polymer-coated capillary tubes. The Error% and RSDs% for analysis of MA, 4-CA, 3-CA, and 3,4 -DCA in different water samples based on three replicate extractions and determinations are shown in Table 2.
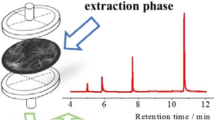
Neither dilution nor further treatment was applied to the samples before extraction. No target analytes were found in tap and river water samples, but analysis of wastewaters showed the presence of low concentration of anilines. The Error% values of the method were in the range of 3.0 to 10.0 % for water samples, indicating good performance of the presented method for determination of the anilines in complex matrices. RSDs% for determination of the anilines in the water samples examined located in the range of 5.0–8.6 %. The results demonstrated a good performance and accuracy of the presented method for the determination of anilines in complex water matrices. Figure 3 illustrates the HPLC-UV chromatograms of a waste І sample before (A) and after (B) spiking of anilines at the concentration level of 10 μg L−1, which shows the presence of 4-CA in the water sample.
Conclusions
A simple and effective approach based on EC-in-tube SPME methods were applied for the quantitative extraction of cationic analytes such as anilines from water samples. Moreover, in this study, an electroplating method was successfully used for the synthesis of a nanostructured PPY-DBS fiber as the extraction phase on the inner surface of a stainless steel tube for on-line preconcentration and determination of trace amount of anilines followed by HPLC-UV. The electroactivity of conducting polymers, especially PPY, have attracted great interest in development of electrochemically controlled extraction and desorption stages for charged species. The electrochemical synthesized fiber has remarkable advantages including ease of preparation, good mechanical strength, suitable chemical and physical characteristics, high extraction efficiency for extraction of ionic components, and low cost in comparison with the traditional fibers and detection limits as small as parts per trillion level were achieved for analytes under the optimized conditions. Based on the results obtained, it can be predicted that the EC-in-tube SPME technique based on PPY-DBS fiber may be used for enhanced extraction of cationic species in complex aqueous solution matrices without the need to modify fiber coating. The method was successfully applied for evaluation of aniline levels in some water samples. In the present work aniline derivatives were selected as the model analytes to investigate the applicability of the method for extraction of cationic compounds. On the other hand, poly pyrrole coating provides different intermolecular interactions such as acid–base, π–π, dipole-dipole, hydrophobic, and hydrogen bonding, as well as exchange among the polymer and the analytes. So, many cationic compounds including basic drugs such as thebaine, tramadol and nalmefene, pyridines or inorganic cationic metals such as Ca2+ (by cation-π interaction) that have the ability to interact with the polymer film may be extracted with this method.
References
Yang J, Liu HC (2000) IR chemical sensor for detection of chlorinated anilines in aqueous solutions based on ATR waveguides coated with derivatized polystyrene. Analyst 125:1605–1610. doi:10.1039/B004315J
Voyksner RD, Straub R, Keever JT, Freeman HS, Hsu WW (1993) Determination of aromatic amines originating from azo dyes from chemical reduction combined with liquid chromatography/mass spectrometry. Environ Sci Technol 27:1665–1672. doi:10.1021/es00045a025
Dalene M, Skarping G (1985) Trace analysis of amines and isocyanates using glass capillary gas chromatography and selective detection IV. Determination of free aromatic amines using nitrogen-selective detection. J Chromatogr A 331:321–330. doi:10.1016/0021-9673(85)80038-8
Zhao L, Zhu L, Lee HK (2002) Analysis of aromatic amines in water samples by liquid–liquid–liquid microextraction with hollow fibers and high-performance liquid chromatography. J Chromatogr A 963:239–248. doi:10.1016/S0021-9673(02)00544-7
Chiang JS, Huang SD (2008) Simultaneous derivatization and extraction of anilines in waste water with dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometric detection. Talanta 75:70–75. doi:10.1016/j.talanta.2007.10.036
Lewin U, Efer J, Engewald W (1996) High-performance liquid chromatographic analysis with electrochemical detection for residues of explosives in water samples around a former ammunition plant. J Chromatogr A 730:161–167. doi:10.1016/0021-9673(95)01077-7
Adeyoju O, Iwuoha EI, Smyth MR, Leech A (1996) High-performance liquid chromatographic determination of phenols using a tyrosinase-based amperometric biosensor detection system. Analyst 121:1885–1889. doi:10.1039/AN9962101885
Liu XJ, Chen XW, Yang S, Wang XD (2007) Continuous-flow microextration coupled with HPLC for the determination of 4-chloroaniline in Chlamydomonas reinhardtii. Bull Environ Contam Toxicol 78:368–372. doi:10.1007/s00128-007-9200-0
EPA Method 1625, Fed. Reg. US Government Print Office, Washington, DC, 1994
EPA Method 8270B, Fed. Reg. US Government Print Office, Washington, DC, 1994
Sarfaraz-Yazdi A, Es’hagi Z (2006) Comparison of hollow fiber and single-drop liquid-phase microextraction techniques for HPLC determination of aniline derivatives in water. Chromatographia 63:563–569. doi:10.1365/s10337-006-0801-2
Wang X, Fu L, Wei G, Hu J, Zhao X, Liu X, Li Y (2008) Determination of four aromatic amines in water samples using dispersive liquid–liquid microextraction combined with HPLC. J Sep Sci 31:2932–2938. doi:10.1002/jssc.200800273
Börnick H, Grischek T, Worch E (2001) Determination of aromatic amines in surface waters and comparison of their behavior in HPLC and on sediment columns. J Anal Chem 371:607–613. doi:10.1007/s002160101011
Diao CP, Wei CH (2012) Rapid determination of anilines in water samples by dispersive liquid–liquid microextraction based on solidification of floating organic drop prior to gas chromatography–mass spectrometry. Anal Bioanal Chem 403:877–884. doi:10.1007/s00216-012-5907-9
Bhaskar M, Aruna P, Jeevan RJG, Radhakrishnan G (2004) β-Cyclodextrin-polyurethane polymer as solid phase extraction material for the analysis of carcinogenic aromatic amines. Anal Chim Acta 509:39–45. doi:10.1016/j.aca.2003.12.015
Patsias J, Papadopoulou-Mourkidou E (2000) Development of an automated on-line solid-phase extraction–high-performance liquid chromatographic method for the analysis of aniline, phenol, caffeine and various selected substituted aniline and phenol compounds in aqueous matrices. J Chromatogr A 904:171–188. doi:10.1016/S0021-9673(00)00927-4
Brede C, Skjevrak I, Herikstad H (2003) Determination of primary aromatic amines in water food simulant using solid-phase analytical derivatization followed by gas chromatography coupled with mass spectrometry. J Chromatogr A 983:35–42. doi:10.1016/S0021-9673(02)01652-7
Figueiredo EC, Sparrapan R, Sanvido GB, Santos MG, Arruda MAZ, Eberlin MN (2011) Quantitation of drugs via molecularly imprinted polymer solid phase extraction and electrospray ionization mass spectrometry: benzodiazepines in human plasma. Analyst 136:3753–3757. doi:10.1039/C1AN15198C
Vonderheide AP, Montes-Bayon M, Caruso JA (2002) Solid-phase microextraction as a sample preparation strategy for the analysis of seleno amino acids by gas chromatography-inductively coupled plasma mass spectrometry. Analyst 127:49–53. doi:10.1039/B107781C
Yuan B, Li F, Xu D, Fu ML (2013) Comparison of two methods for the determination of geosmin and 2-methylisoborneol in algae samples by stable isotope dilution assay through purge-and-trap or headspace solid-phase microextraction combined with GC/MS. Anal Methods 5:1739–1746. doi:10.1039/C3AY26626E
Mitani K, Kataoka H (2006) Determination of fluoroquinolones in environmental waters by in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. Anal Chim Acta 562:16–22. doi:10.1016/j.aca.2006.01.053
Silva BJG, Lanças FM, Queiroz MC (2008) In-tube solid-phase microextraction coupled to liquid chromatography (in-tube SPME/LC) analysis of nontricyclic antidepressants in human plasma. J Chromatogr B 862:181–188. doi:10.1016/j.jchromb.2007.12.006
Shirakawa H, Louis E, MacDiarmid A, Chiang C, Heeger A (1977) Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J Chem Soc Chem Commun 16:578–580. doi:10.1039/C39770000578
Gou Y, Eisert R, Pawliszyn J (2000) Automated in-tube solid-phase microextraction–high-performance liquid chromatography for carbamate pesticide analysis. J Chromatogr A 873:137–147. doi:10.1016/S0021-9673(99)01125-5
Mohammadi A, Yamini Y, Alizadeh N (2005) Dodecylsulfate-doped polypyrrole film prepared by electrochemical fiber coating technique for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. J Chromatogr A 1063:1–8. doi:10.1016/j.chroma.2004.11.087
Wang YH, Li YQ, Feng JF, Sun C (2008) Polyaniline-based fiber for headspace solid-phase microextraction of substituted benzenes determination in aqueous. Anal Chim Acta 619:202–208. doi:10.1016/j.aca.2008.05.003
Liljegren G, Nyholm L (2003) Electrochemically controlled solid-phase microextraction and preconcentration using polypyrrole coated microarray electrodes in a flow system. Analyst 128:232–236. doi:10.1039/B211398H
Tamer U, Yates B, Galal A, Gbatu T, LaRue R, Schmiesing C, Temsamani K, Ceylan O, Mark HB Jr (2003) Electrochemically aided control of solid phase micro-extraction (EASPME) using conducting polymer-coated solid substrates applicable to neutral analytes. Microchim Acta 143:205–215. doi:10.1007/s00604-003-0056-7
Kalhor H, Alizadeh N (2013) Enhancing sensitivity of ion mobility spectrometry determination of aldehydes by in situ gas phase derivatization with dibutylamine. Int J Ion Mobil Spectrom 16:199–205. doi:10.1007/s12127-013-0119-3
Wu J, Pawliszyn J (2004) Solid-phase microextraction based on polypyrrole films with different counter ions. Anal Chim Acta 520:257–264. doi:10.1016/j.aca.2004.05.019
Wu JC, Pawliszyn J (2001) Preparation and applications of polypyrrole films in solid-phase microextraction. J Chromatogr A 909:37–52. doi:10.1016/S0021-9673(00)01025-6
Eisert R, Pawliszyn J (1997) Automated in-tube solid-phase microextraction coupled to high-performance liquid chromatography. Anal Chem 69:3140–3147. doi:10.1021/ac970319a
Ovais Aziz-Zanjani M, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta 181:1169–1190. doi:10.1007/s00604-014-1265-y
Kataoka H (2002) Automated sample preparation using in-tube solid-phase microextraction and its application – a review. Anal Bioanal Chem 373:31–45. doi:10.1007/s00216-002-1269-z
Alhooshani K, Kim TY, Kabir A, Malik A (2005) Sol–gel approach to in situ creation of high pH-resistant surface-bonded organic–inorganic hybrid zirconia coating for capillary microextraction (in-tube SPME). J Chromatogr A 1062:1–14. doi:10.1016/j.chroma.2004.10.103
Acknowledgments
The authors gratefully acknowledge financial support from Tarbiat Modares University
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 429 kb)
Rights and permissions
About this article
Cite this article
Asiabi, H., Yamini, Y., Rezaei, F. et al. Nanostructured polypyrrole for automated and electrochemically controlled in-tube solid-phase microextraction of cationic nitrogen compounds. Microchim Acta 182, 1941–1948 (2015). https://doi.org/10.1007/s00604-015-1534-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1534-4