Abstract
Magnetic carbon nitride composites were synthesized via a solvothermal reaction and developed as an effective adsorbent for magnetic solid-phase extraction of trace hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) from urine samples prior to their determination by HPLC. The sorbent was characterized by Fourier transform infrared spectrometry, X-ray diffractometry, scanning electron microscopy, vibrating sample magnetometry and solvent stability experiments. The adsorption of hydroxy-PAHs is better by a factor or 20 to 49 compared to bare Fe3O4 and comparable that of a commercial C18 sorbent. The adsorbent amount, adsorption time and eluting solvent and volume were optimized. The complete extraction for the OH-PAHs at a level of 40 ng·mL−1 and by using 4 mg sorbent is completed within 3 min. With an enzymatic hydrolyzed urine sample loading volume of 2 mL, enhancement factors in the range of 9–10, and a limit of detection (at S/N = 3) of 0.08 ng·mL−1 were achieved. The method showed a linear response in the 0.25–250 ng·mL−1 hydroxy-PAH concentration range, and intra-day and inter-day precisions are 1.5–7.7% and 2.2–8.7%, respectively. The recovery from spiked urine samples ranged from 90.1% to 102%. The sorbent was stable over 10 successive cycles of extraction/desorption of urine sample without significant loss of extraction efficiency. The method was successfully applied for the determination of OH-PAHs in urine samples of smoking volunteers.

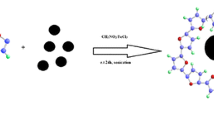
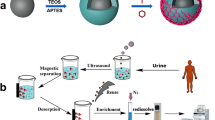
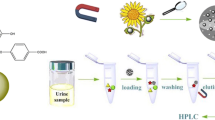
Schematic presentation of the preparation of graphitic carbon nitride (g-C3N4)/magnetite (Fe3O4) using a solvothermal reaction, and application for magnetic solid-phase extraction of three trace hydroxy polycyclic aromatic hydrocarbons (OH-PAHs) in urine samples of smoking volunteers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of ubiquitous environmental contaminants raising worldwide concerns. Human exposure to these contaminants may occur via dietary intake of smoked or grilled food, inhalation of polluted air and tobacco smoke, as well as dermal contact with soot, and polluted soils [1, 2]. Exposure to PAHs has been identified to associate with cardiovascular and pulmonary disease, immune impairment and adverse birth outcomes [3,4,5]. Additionally, some PAHs have been implicated with breast, bladder, and colon cancers in both humans and animal models [6,7,8]. In view of their increasing adverse health effects to humans, the accurate monitoring of these compounds from multiple entry routes is important not only for their studies on source profile and risk assessment, but also for the policy making and formulating regulations to reduce the exposure to these toxicants [9].
Hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) which are enzymatically converted from PAHs and excreted in the urine, are regarded as biomarkers for direct assessing total internal doses of PAHs exposure [10]. Particularly, the measurement of urinary metabolites represents a non-invasive and ready accessible approach. However, a preconcentration and enrichment step is usually required because of the complexity of raw urine and the low concentration of the analytes. Therefore, numerous sample pretreatment methods have been proposed to purify and capture OH-PAHs before instrumental analysis, including dispersive liquid-liquid microextraction (DLLME) [11], solid-phase microextraction (SPME) [12], solid-phase extraction (SPE) [13,14,15,16,17] and magnetic solid-phase extraction (MSPE) with different types of sorbents [18, 19].
MSPE technique has attracted great attention due to its speed, compatibility and selectivity in food, environmental, pharmaceutical, and biological analysis [20]. In MSPE, magnetized adsorbents are finely dispersed into crude sample solution for adsorption. Sufficient large contact area between the well-dispersed sorbents and the analytes facilitates the rapid equilibrium and enhances extraction efficiency. Additionally, a rapid and facile separation from the matrix can be achieved directly by a magnet without any additional centrifugation or filtration operations. For this powerful methodology, functionalized magnetic adsorption materials are of great interest to researchers because the adsorbent in the extraction process is crucial to capture and purify the analyte with high efficiency and good reproducibility [21]. To date, great varieties of functionalized adsorption materials such as magnetic carbon nanotube [22], graphene oxide [22], and metal organic frameworks [23] have been successfully developed.
Graphitic carbon nitride (g-C3N4), an emerging class of 2D graphene analogue composed of carbon and nitrogen, has aroused intense interest due to its exceptional electronic, mechanical and thermal properties [24]. The synthesis of g-C3N4 is facile and inexpensive due to the availability of various simple and green nitrogen-rich (N-rich) precursors [25, 26]. Particularly, the large specific surface area, satisfactory pH tolerance and the defected-rich and N-bridged molecular structure given by its tris-triazine connected double-sided polyaromatic scaffold make g-C3N4 qualified as an extraordinarily wonderful adsorbent to preconcentrate organic pollutants [27,28,29,30,31,32,33] and metals [34] in water, soil, cosmetics or food samples. Some efforts have been made to prepare magnetic g-C3N4 for MSPE. Chemical coprecipitation method has been commonly proposed using dispersed g-C3N4 as raw materials and ammonia as a precipitating agent [32,33,34,35,36,37]. However, such approaches involved in relatively tedious procedures and the prepared composites suffered solvent instability and thus resulted in oxidization of Fe3O4. A magnetization of g-C3N4 was also reported by simple physically blending of g-C3N4 onto Fe3O4 for the extraction of the organic pollutant from edible oil [38]. Nevertheless, such physical adsorption might not be stable enough for long-term application.
Herein, we report a one-step solvothermal strategy for the fabrication of magnetic g-C3N4 composites (g-C3N4/Fe3O4) and applied for fast and efficient extraction of 1-phenanthrol (1-OHPhe), 3-phenanthrol (3-OHPhe) and 1-pyrenol (1-OHPyr) in urine samples. Compared with the reported chemical co-precipitation or physical mixing approaches [32,33,34,35,36,37,38], the method allowed the solvothermal synthesis of g-C3N4/Fe3O4 with good stability and high reproducibility. The obtained composites were characterized by Fourier transform infrared spectrometry (FT-IR), X-ray powder diffractometry (XRD), vibrating sample magnetometer, and scanning electron microscopy (SEM). The impacts of some experimental factors on MSPE were discussed in detail. Coupling this MSPE technique with high performance liquid chromatography-fluorescence detector (HPLC-FLD), a sensitive method was established to determine OH-PAHs in urine samples.
Materials and methods
Chemicals and reagents
All reagents used were of analytical grade. Urea and ethylene glycol (EG) were purchased from Aladdin (Shanghai, China, www.aladdin-e.com). Iron (III) chloride hexahydrate (FeCl3·6H2O) and sodium acetate trihydrate (NaOAc·3H2O) were acquired from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China, www.guangfubiaowu.com). β-Glucuronidase from abalone (aqueous solution, ≥ 100,000 unites·mL−1) was provided by ANPEL Laboratory Technologies Inc. (Shanghai, China, www.anpel.com.cn). HPLC-grade methanol (MeOH), acetonitrile (ACN), acetone, and ethyl acetate were purchased from Fisher Scientific (Geel, Belgium, www.thermofisher.com). Ultrapure water was purchased from Hangzhou Wahaha Group Co., Ltd. (Hangzhou, China, www.wahaha.com.cn).
The standards of 1-OHPhe (98.0%), 3-OHPhe (98.0%) and 1-OHPyr (98.0%) were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany, www.analytical-standards.com). The stock solutions of the three analytes (500 μg·mL−1) were prepared with MeOH and stored at 4 °C in dark. Working solutions were prepared from the mixture of standard stock solutions by stepwise dilution with MeOH just before use.
Apparatus
Chromatographic analysis was performed on an Agilent 1200 HPLC (Santa Clara, USA, www.agilent.com), consisting a G1322A degasser, a G1311A pump system, a G1329A autosampler, a G1316A temperature control center and a G1321A FLD. SEM images of the prepared composites were acquired on a FEI JEM-2800F dual beam focused ion beam/field emission scanning electron microscope (Hillsboro, USA, www.fei.com). The FT-IR spectra were recorded on a Shimadzu FTIR-8400S Fourier transform infrared spectrometer (Kyoto, Japan, www.shimadzu.com). XRD patterns were characterized by a Brucker D8 VENTURE single crystal X-ray diffractometer (Karlsruhe, Germany, www.bruker.com). The magnetic property was investigated using an LDJ 9600–1 vibrating sample magnetometer (Troy, USA, www.digitalinstruments.net).
Fabrication of g-C3N4/Fe3O4
Graphitic carbon nitride (g-C3N4) was synthesized according to Dong et al. [39]. Typically, 10 g urea was put into a muffle furnace followed by heating at the ramp rates of 15 °C·min−1 to 550 °C and maintained at this temperature for 4 h to obtained bulk g-C3N4.
The g-C3N4/Fe3O4 composite was fabricated by the solvothermal reaction (Fig. 1a). g-C3N4 (270 mg) was dispersed in 40 mL EG under ultrasonication for 2 h. FeCl3·6H2O (270 mg) and NaOAc·3H2O (700 mg) were then added and stirred for 30 min to create a homogeneous dispersion. The mixture was transferred into a 100-mL Teflon-lined stainless-steel autoclave and maintained at 200 °C for 12 h. The collected magnetic nanoparticles were thoroughly washed with water and ethanol sequentially, and then dried in the vacuum under 60 °C for 30 min.
Sample collection and preparation
All individuals participating in this study were recruited on a voluntary basis. The urine samples were collected in the morning from 10 smoking volunteers in Tangshan, China.
To enzymatically hydrolyze of glucuronides and sulfates, 10 μL β-glucuronidase and 5 mL acetic acid-sodium acetate (pH 5.0, 0.5 mol·L−1) buffer solution were added in 5 mL urine. The sample was then shaken in a water bath at 37 °C overnight in dark. The resulting sample was centrifuged at 1500 rpm for 10 min. The supernatant was collected and stored at 4 °C.
Magnetic solid-phase extraction procedure
Four milligrams of g-C3N4/Fe3O4 composites were added to 2 mL hydrolyzed urine solution in a 5-mL glass vial. The mixture was shaken for 3 min to adsorb the analytes completely. Subsequently, an external magnet was placed to the outside bottom of the vial, and the g-C3N4/Fe3O4 composites were aggregated to the bottom of the vial. After the supernatant being discarded completely with a pipette, the isolated sorbent was vortexed in 0.5 mL acetone for 3 min for elution and the solvent was collected with the aid of a magnet. This elution procedure was repeated twice. Finally, the eluting solvent was combined and evaporated to dryness at 25 °C under a gentle stream of nitrogen gas. The residues were re-dissolved in 100 μL MeOH and filtered via a 0.45 μm membrane for HPLC-FLD determination.
Prior to the next use, g-C3N4/Fe3O4 composites were washed twice with 1 mL acetone and then with 1 mL water by vortex for 3 min. Figure 1b shows the flowchart of MSPE process using g-C3N4/Fe3O4 composites.
Chromatographic conditions
All HPLC experiments were performed on a PAH column (250 mm × 4.6 mm, 5 μm, Agilent, USA) at 25 °C. The mixture of water and ACN was used as mobile phase and the gradient elution was set as follows: 0–8 min 55% ACN, 8–10 min a linear increase to 65% ACN, 10–16 min 65% ACN. The flow rate was set at 1 mL min−1 and the injection volume was 20 μL. The Excitation (Ex)/Emission (Em) wavelengths for three OH-PAHs were, 284/383 nm (1-OHPhe), 250/360 nm (3-OHPhe) and 242/396 nm (1-OHPyr).
Results and discussion
Characterization of g-C3N4/Fe3O4
The g-C3N4, Fe3O4 and g-C3N4/Fe3O4 composites were characterized by FT-IR and XRD. As shown in Fig. 2a, the observed representative bands at 808 cm−1 and 1200–1650 cm−1 of g-C3N4/Fe3O4 composites are attributed to the typical stretching of the triazine and skeletal vibrations of the s-triazine or tri-s-triazine of g-C3N4 [24]. And the peak at 570 cm−1 corresponds to the typical adsorption of Fe-O [35]. Figure 2b indicates the XRD patterns of the g-C3N4, Fe3O4 and g-C3N4/Fe3O4 composites. The pattern of g-C3N4/Fe3O4 shows all typical diffraction peaks at 13.0° (100) and 27.4° (002) of g-C3N4 and 30.2° (220), 35.6° (311), 43.3° (400), 53.7° (422), 57.3° (511), 62.8° (440) and 74.9° (533) of Fe3O4. Both FT-IR and XRD results confirm the successful combination of g-C3N4 and Fe3O4. Particularly, there are no changes in XRD patterns of g-C3N4/Fe3O4 composites, and the composites soaked in ultrapure water and hexane for 30 days respectively (Fig. S1). The high solvent stability in both polar and nonpolar solvents of the prepared g-C3N4/Fe3O4 is available for loading and eluting steps in MSPE and favorable for method reproducibility. The saturation magnetization values for Fe3O4 and g-C3N4/Fe3O4 are 77.8 and 36.4 emu·g−1, respectively (Fig. 2c). Such adequate magnetic property of g-C3N4/Fe3O4 makes it susceptible to magnetic field and easy to isolate the target analytes from matrices solution with the aid of an external magnet. The SEM images of g-C3N4, Fe3O4 and g-C3N4/Fe3O4 composites are shown in Fig. 2d–f, respectively. It can be clearly observed that Fe3O4 nanoparticles are well distributed on the layered surface of g-C3N4. Thus, g-C3N4/Fe3O4 composites have the magnetic properties originating from Fe3O4 nanoparticles, enabling the easy and robust removal of the material after dispersion, g-C3N4 in the composites still has its own properties, providing potential adsorption ability for target analytes.
Optimization of MSPE procedure
In order to confirm the adsorptive performance of the prepared g-C3N4/Fe3O4 composite, the extractions of three OH-PAHs on bare Fe3O4 and commercial C18 were compared. 10 mg of sorbents were equilibrated with 2 mL aqueous solution containing 100 ng·mL−1 of each analyte for 15 min to ensure a complete adsorption. As obviously shown in Fig. 3, the adsorption efficiency of g-C3N4/Fe3O4 for all analytes gets 98.0%–100%, 20 to 49-fold that of bare Fe3O4, while reaches a comparable efficiency with that of commercial C18 (99.2%–100%).
Considering the magnetic property, good adsorption performance and solvent stability, g-C3N4/Fe3O4 composites were used as an adsorbent to extract trace level of three OH-PAHs from the complicated urine sample. Parameters affecting the extraction efficiency were investigated in detail, including the sorbent amount, adsorption time, eluting solvent and its volume. 2 mL aqueous solution spiked with 40 ng·mL−1 of each analyte was used, and all the experiments were carried out in triplicate.
g-C3N4/Fe3O4 amount and adsorption time
The appropriate adsorbent amount and adsorption time allow adequate mass transfer between the sorbents and the target analytes from the solution, giving a high accuracy of the MSPE method. The amount of g-C3N4/Fe3O4 in the range of 0.5–5 mg was investigated to extract three OH-PAHs. As shown in Fig. 4a, the adsorption efficiency of 1-OHPyr increases rapidly as the amount of adsorbent increased, and achieves maximum adsorption in only 1 mg of the sorbent. After that, excess sorbent does not increase the adsorption efficiency. For 1-OHPhe and 3-OHPhe, as the adsorbent amount increases, their adsorption efficiencies slightly enhance from 0.5 to 4 mg and the maximum plateau is reached when the amount of g-C3N4/Fe3O4 increases up to 4 mg. According to the result, only 4 mg of sorbent is adequate to extract all OH-PAHs from the aqueous solution. Thus, 4 mg of g-C3N4/Fe3O4 is employed for the following experiment.
The effect of the adsorption time on the extraction efficiency of the OH-PAHs was investigated from 0.5 min to 4 min with 4 mg of adsorbent. Figure 4b illustrates that the extraction efficiency reaches a maximum for 1-OHPyr at 1 min, and for 1-OHPhe and 3-OHPhe at 3 min. Further prolonged adsorption time leads to no significant change in the extraction efficiency, suggesting a desorption time of 3 min is enough to elute all OH-PAHs from g-C3N4/Fe3O4. The extraction equilibrium between the aqueous phases and the sorbents when using 4 mg g-C3N4/Fe3O4 is accomplished in short time. This reduces the cost of the MSPE.
Particularly, the extraction equilibrium of 1-OHPyr on g-C3N4/Fe3O4 is achieved in 1 min or even using 1 mg sorbent, which is much lower than those of 1-OHPhe and 3-OHPhe. The adsorption ability of g-C3N4/Fe3O4 for the selected compounds follows an increasing order of their oil-water partition coefficient (logP) values (logP1-OHPyr 4.29 ˃ logP1-OHPhe 3.94 ˃ logP3-OHPhe 3.70). The results indicate that the hydrophobicity of the analytes plays a significant role in MSPE. Besides, the large p-electron system of g-C3N4 also enhances a strong affinity for aromatic rings structures, typical of these selected analytes, OH-PAHs [26].
Elution conditions
Proper elution condition is a crucial step for MSPE to release the analytes sufficiently from the magnetic sorbent. The organic solvents including MeOH, ACN, acetone and ethyl acetate were optimized. Figure 4c shows that under the same extraction condition, acetone provides the best results. Thus, acetone is chosen as the eluting solvent. The effect of eluting volume (0.5 mL × 1, 0.5 mL × 2, 0.5 mL × 3 and 0.5 mL × 4) on desorption efficiency of the target analytes was also investigated by rinsing the adsorbent with each 0.5 mL of acetone. The eluting volume increased from 0.5 mL × 1 to 0.5 mL × 2 provides a positive effect on the recovery of all the analytes. Further increase of the eluting volume leads to a slight change of the recovery of 1-OHPyr (98.7%–92.6%), but significant decreases of the recovery of 1-OHPhe and 3-OHPhe. The excess use of solvents results in the prolonged evaporation time, which prone to make the loss of the volatile analytes including 1-OHPhe and 3-OHPhe. To provide high and stable recovery, 0.5 mL × 2 is chosen for further experiments (Fig. 4d).
On the basis of the results discussed above, the optimized conditions for the MSPE of three OH-PAHs are performed as follows: sorbent amount, 4 mg; adsorption time, 3 min; elution solvent, acetone; elution volume, each 0.5 mL, two times.
Analytical performance of the MSPE coupled with HPLC-FLD using g-C3N4/Fe3O4 as sorbent
The analytical figures of merit of the g-C3N4/Fe3O4 composites for the MSPE of three OH-PAHs under the optimized are listed in Table 1. The method exhibits a good linear range of 0.25–250 ng·mL−1 for each analyte with correlation coefficients (r) higher than 0.9985. The limits of detection (LOD) (S/N = 3) and limits of quantification (LOQ) (S/N = 10) for three OH-PAHs in spiked urine samples are found to be 0.08 ng·mL−1 and 0.25 ng·mL−1, respectively. Method accuracy was evaluated with recovery and determined using independently spiked urine samples at three different levels of 0.5, 1.25 and 2.5 ng·mL−1. The recoveries of three OH-PAHs are in the range of 90.1%–102%. The intra-day and the inter-day precisions (relative standard deviation, RSD) for three replicate extractions of OH-PAHs are in the range of 1.5–7.7%. and 2.2–8.7%, respectively. In addition, the extraction efficiency of the g-C3N4/Fe3O4 composites MSPE for three OH-PAHs in urine changes less than 9.8% for 10 successive cycles of extraction/desorption, confirming a good stability of the g-C3N4/Fe3O4 composites (Fig. S2).
Figure 5a–d presents the typical chromatograms of the urine sample and the corresponding urine sample spiked with 2.5 ng·mL−1 of each analyte by direct analysis and MSPE pretreatment. Compared with the chromatograms of the urine samples obtained by direct injection, the results of pretreated urine samples demonstrate that the target compounds are obviously purified and effectively enriched via MSPE process. With an enzymatic hydrolyzed urine sample loading volume of 2 mL, the enhancement factors are in the range of 9–10, which were defined as the ratio of the concentration of the analyte in the pretreated sample to that in the original sample. Such results clearly confirmed that the strategy is powerful for efficient purification and concentration of OH-PAHs in urine samples.
In order to further validate the feasibility of this method, a comparison with the reported methods was performed, including DLLME [11], SPE [13,14,15], and MSPE approach using different sorbent [18]. It can be seen from Table 2 that the MSPE method based on the g-C3N4/Fe3O4 gives lower LODs and better recovery for OH-PAHs than other SPE and MSPE methods with similar FLD detector in our work and DLLME method with mass spectrometry (MS) detector. In addition, with the consumption of 2 mL sample solution, this approach needs much shorter adsorption time (3 min) and less sorbent (4 mg) than other SPE methods. This procedure enables easy operation by an external magnetic field without additional centrifugation or filtrations. In conclusion, these results show that the present method is sensitive, accurate and reliable to measure trace levels of OH-PAHs in urine samples.
Analysis of OH-PAHs in urine samples of smoking volunteers
Smoking is a major source of exposure to PAHs, thus the feasibility of the method was further illustrated to determine the urine samples of smoking volunteers (n = 10). As listed in Table 3, 1-OHPyr is found to be 4.24 ± 0.02 ng·mL−1 and 4.26 ± 0.06 ng·mL−1 in two cases in group 1 (smoked <10 cigarettes per day), while 3-OHPhe and 1-OHPyr are detected in all five samples of group 2 (smoked ≥10 cigarettes per day) with the concentrations of 1.81 ± 0.10 ng·mL−1 to 2.26 ± 0.10 ng·mL−1, and 4.27 ± 0.02 ng·mL−1 to 4.92 ± 0.05 ng·mL−1, respectively. 1-OHPhe is detected in two urine samples with a concentration of 1.65 ± 0.04 ng·mL−1 and 1.75 ± 0.02 ng·mL−1. The recoveries obtained by spiking 2.5 ng·mL−1 of each OH-PAH in urine samples are in the ranged of 90.1%–110%. These satisfactory results demonstrate that this method is feasible for the monitoring of OH-PAHs in the urine samples.
Conclusion
In conclusion, g-C3N4/Fe3O4 composites were first fabricated by a one-step solvothermal strategy and successfully applied as an effective adsorbent for MSPE of trace OH-PAHs in urine samples prior to HPLC-FLD. The method not only allowed the solvothermal synthesis of g-C3N4/Fe3O4 with good stability, but also provided satisfactory recovery and low detection limit in a rapid and simple way. Since such adsorbent offers good feasibility, it has great potential in dealing with other organic pollutants or pesticides from complicated samples.
References
WHO, IARC (2010) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum 92:1–853
Kim KH, Jahan SA, Kabir E, Brown RJC (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Yang B, Deng Q, Zhang W, Feng Y, Dai X, Feng W, He X, Huang S, Zhang X, Li X, Lin D, He M, Guo H, Sun H, Yuan J, Lu J, Hu FB, Zhang X, Wu T (2016) Exposure to polycyclic aromatic hydrocarbons, plasma cytokines, and heart rate variability. Sci Rep 6:19272
Yang P, Wang YX, Sun L, Chen YJ, Liu C, Huang LL, Lu WQ, Zeng Q (2017) Urinary metabolites of polycyclic aromatic hydrocarbons, sperm DNA damage and spermatozoa apoptosis. J Hazard Mater 329:241–248
Shen M, Xing J, Ji Q, Li Z, Wang Y, Zhao H, Wang Q, Wang T, Yu L, Zhang X, Sun Y, Zhang Z, Niu Y, Wang H, Chen W, Dai Y, Su W, Duan H (2018) Declining pulmonary function in populations with long-term exposure to polycyclic aromatic hydrocarbons-enriched PM2.5. Environ Sci Technol 52:6610–6616
Bandowe BAM, Meusel H, Huang RJ, Ho K, Cao J, Hoffmann T, Wilcke W (2014) PM2.5-bound oxygenated PAHs, nitro-PAHs and parent-PAHs from the atmosphere of a Chinese megacity: seasonal variation, sources and cancer risk assessment. Sci Total Environ 473-474:77–87
Liu B, Xue Z, Zhu X, Jia C (2017) Long-term trends (1990-2014), health risks, and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the U.S. Environ Pollut 220:1171–1179
Rodgers KM, Udesky JO, Rudel RA, Brody JG (2018) Environmental chemicals and breast cancer: an updated review of epidemiological literature informed by biological mechanisms. Environ Res 160:152–182
Srogi K (2007) Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett 5:169–195
Woudneha MB, Benskina JP, Grace R, Hamilton MC, Magee BH, Hoeger GC, Forsberg ND, Cosgrove JR (2016) Quantitative determination of hydroxy polycyclic aromatic hydrocarbons as a biomarker of exposure to carcinogenic polycyclic aromatic hydrocarbons. J Chromatogr A 1454:93–100
Gupta MK, Jain R, Singh P, Ch R, Mudiam MKR (2015) Determination of urinary PAH metabolites using DLLME hyphenated to injector port silylation and GC-MS-MS. J Anal Toxicol 39:365–373
Yang BC, Fang SF, Wan XJ, Luo Y, Zhou JY, Li Y, Li YJ, Wang F, Huang OP (2017) Quantification of monohydroxylated polycyclic aromatic hydrocarbons in human urine samples using solid-phase microextraction coupled with glass-capillary nanoelectrospray ionization mass spectrometry. Anal Chim Acta 973:68–74
Li Y, Li X, Zhou Z (2014) A novel facile method using polyetheretherketone as a solid phase extraction material for fast quantification of urinary monohydroxylated metabolites of polycyclic aromatic hydrocarbons. RSC Adv 4:39192–39196
Chauhan A, Bhatia T, Singh A, Saxena PN, Kesavchandran C, Mudiama MKR (2015) Application of nano-sized multi-template imprinted polymer for simultaneous extraction of polycyclic aromatic hydrocarbon metabolites in urine samples followed by ultra-high performance liquid chromatographic analysis. J Chromatogr B 985:110–118
Yin W, Hou J, Xu T, Cheng J, Li P, Wang L, Zhang Y, Wang X, Hu C, Huang C, Yu Z, Yuan J (2018) Obesity mediated the association of exposure to polycyclic aromatic hydrocarbon with risk of cardiovascular events. Sci Total Environ 616-617:841–854
Yang DH, Shin MJ, Kim M, Kim YD, Kim H, Shin JS (2016) Molecularly imprinted titania microbeads for extraction of the metabolite 1-hydroxypyrene from urine prior to its determination by HPLC. Microchim Acta 183:1601–1609
Shin JS, Lankova D, Urbancova K, Sram RJ, Hajslova J, Pulkrabova J (2016) A novel strategy for the determination of polycyclic aromatic hydrocarbon monohydroxylated metabolites in urine using ultra-high-performance liquid chromatography with tandem mass spectrometry. Anal Bioanal Chem 408:2512–2525
Zhou L, Hu Y, Li G (2016) Conjugated microporous polymers with built-in magnetic nanoparticles for excellent enrichment of trace hydroxylated polycyclic aromatic hydrocarbons in human urine. Anal Chem 88:6930–6938
Zhu L, Xu H (2014) Magnetic graphene oxide as adsorbent for the determination of polycyclic aromatic hydrocarbon metabolites in human urine. J Sep Sci 37:2591–2598
Wierucka M, Biziuk M (2014) Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC Trends Anal Chem 59:50–58
Kudr J, Haddad Y, Richtera L, Heger Z, Cernak M, Adam V, Zitka O (2017) Magnetic nanoparticles: from design and synthesis to real world applications. Nanomaterials 7:243
Li N, Jiang HL, Wang X, Wang X, Xu G, Zhang B, Wang L, Zhao RS, Lin JM (2018) Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC Trends Anal Chem 102:60–74
Liu C, Yu LQ, Zhao YT, Lv YK (2018) Recent advances in metal-organic frameworks for adsorption of common aromatic pollutants. Microchim Acta 185:342
Miller TS, Jorge AB, Suter TM, Sella A, Corà F, McMillan PF (2017) Carbon nitrides: synthesis and characterization of a new class of functional materials. Phys Chem Chem Phys 19:15613–15638
Inagaki M, Tsumura T, Kinumoto T, Toyoda M (2019) Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 141:580–607
Sun YP, Ha W, Chen J, Qi HY, Shi YP (2016) Advances and applications of graphitic carbon nitride as sorbent in analytical chemistry for sample pretreatment: a review. TrAC Trends Anal Chem 84:12–21
Khosrowshahi EM, Razmi H (2018) Application of sunflower stalk-carbon nitride nanosheets as a green sorbent in the solid-phase extraction of polycyclic aromatic hydrocarbons followed by high-performance liquid chromatography. J Sep Sci 41:2020–2028
Speltinia A, Maraschi F, Govoni R, Milanese C, Profumo A, Malavasi L, Sturini M (2017) Facile and fast preparation of low-cost silica-supported graphitic carbon nitride for solid-phase extraction of fluoroquinolone drugs from environmental waters. J Chromatogr A 1489:9–17
Wu T, Wang JT, Liang WQ, Zang XH, Wang C, Wu QH, Wang Z (2017) Single layer graphitic carbon nitride-modified graphene composite as a fiber coating for solid-phase microextraction of polycyclic aromatic hydrocarbons. Microchim Acta 184:2171–2180
Yang YX, Qin PG, Zhang J, Li WQ, Zhu JH, Lu MH, Cai ZW (2018) Fabrication of nanoscale graphitic carbon nitride/copper oxide hybrid composites coated solid-phase microextraction fibers coupled with gas chromatography for determination of polycyclic aromatic hydrocarbons. J Chromatogr A 1570:47–55
Zhang N, Huang CH, Tong P, Feng ZM, Wu XP, Zhang L (2018) Moisture stable Ni-Zn MOF/g-C3N4 nanoflowers: a highly efficient adsorbent for solid-phase microextraction of PAHs. J Chromatogr A 1556:37–46
Zhang MS, Huang GB, Huang JR, Chen WL (2018) Three-dimensional multi-walled carbon nanotubes@g-C3N4@Fe3O4nanocomposites-based magnetic solid phase extraction for the determination of polycyclic aromatic hydrocarbons in water samples. Microchem J 142:385–393
Li D, Zhu J, Wang M, Bi W, Huang X, Chen DDY (2017) Extraction of trace polychlorinated biphenyls in environmental waters by well-dispersed velvet-like magnetic carbon nitride nanocomposites. J Chromatogr A 1491:27–35
Fahimirad B, Asghari A, Rajabi M (2017) Magnetic graphitic carbon nitride nanoparticles covalently modified with an ethylenediamine for dispersive solid-phase extraction of lead (II) and cadmium (II) prior to their quantitation by FAAS. Microchim Acta 184:3027–3035
Wang M, Yuan H, Deng W, Bi W, Yang X (2015) A Taiji-principle-designed magnetic porous C-doped graphitic carbon nitride for environment-friendly solid phase extraction of pollutants from water samples. J Chromatogr A 1412:12–21
Rajabi M, Moghadam AG, Barfi B, Asghari A (2016) Air-assisted dispersive micro-solid phase extraction of polycyclic aromatic hydrocarbons using a magnetic graphitic carbon nitride nanocomposite. Microchim Acta 183:1449–1458
Fan S, Zhu J, Ren L, Wang M, Bi W, Li H, Huang X, Chen DDY (2017) Co-solvent enhanced adsorption with magnetic velvet-like carbon nitride for high efficiency solid phase extraction. Anal Chim Acta 960:63–71
Zheng HB, Ding J, Zheng SJ, Zhu GT, Yuan BF, Feng YQ (2016) Facile synthesis of magnetic carbon nitride nanosheets and its application in magnetic solid phase extraction for polycyclic aromatic hydrocarbons in edible oil samples. Talanta 148:46–53
Dong F, Wang Z, Sun Y, Ho WK, Zhang H (2013) Engineering the nanoarchitecture and texture of polymeric carbon nitride semiconductor for enhanced visible light photocatalytic activity. J Colloid Interface Sci 401:70–79
Acknowledgements
This work was supported by the Natural Science Foundation of Hebei Province, China [grant numbers H2017209232]; the Research Foundation of Education Bureau of Hebei Province, China [grant number ZD2018014]; the Training Foundation of North China University of Science and Technology [grant number JQ201717].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests. The study has been approved by the Research Council and Ethics Committee of North China University of Science and Technology, China and has been performed in accordance with the ethical standards. All participating volunteers were informed that the samples provided will be used for scientific research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 209 kb)
Rights and permissions
About this article
Cite this article
Nian, Q., Wang, X., Wang, M. et al. A hybrid material composed of graphitic carbon nitride and magnetite (Fe3O4) for magnetic solid-phase extraction of trace levels of hydroxylated polycyclic aromatic hydrocarbons. Microchim Acta 186, 497 (2019). https://doi.org/10.1007/s00604-019-3607-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3607-2