Abstract
We describe magnetite nanoparticles modified with polyfuran (PFu/Fe3O4) and their use as adsorbents for the solid-phase extraction of the polycyclic aromatic hydrocarbons (PAHs) naphthalene, fluorene and anthracene from water and urine samples. The PFu/Fe3O4 nanocomposite was characterized by Fourier transform infrared spectroscopy, scanning electron microscopy and vibrating sample magnetometry (VSM). The adsorbent was magnetically separated from the sample, extracted with dichloromethane, and the extracts were submitted to gas chromatographic analysis with flame ionization detection. The amount of adsorbent, adsorption and desorption time, salt addition and desorption solvent were optimized. The method displays detection limits (at an S/N ratio of 3) in the range from 5 to 20 pg mL−1, and the limits of quantification (at an S/N ratio of 10) are between 20 and 50 pg mL−1. Relative standard deviations (RSDs) for intra- and inter-day precision are 4.1–5.6, and 5.3–6.5 %, respectively. The feasibility of the method was demonstrated by extracting and determining PAHs in (spiked) real water and urine samples.

Nanoparticles consisting of a magnetite (Fe3O4) core and a polyfurane (PFu) coating were synthesized and used for the extraction of polycyclic aromatic hydrocarbons (PAHs) from environmental water samples and from urine of smokers and non-smokers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid phase extraction (SPE) is a sample preparation technique that is often combined with high performance liquid chromatography (HPLC) and gas chromatography (GC). It is widely used to extract organic pollutants from real samples [1, 2]. SPE excels by high recovery, low consumption of organic solvents and ease of operation. But this method still has drawbacks such as low efficiency of extraction of analytes with low amounts and time consuming of water samples in case of high volumes. To deal with these drawbacks, recently a new procedure for SPE, based on the use of magnetic or magnetically modified adsorbents called magnetic solid-phase extraction (MSPE) [3], has been developed. The separation process in MSPE can be performed directly in crude samples containing suspended solid material without the need of additional centrifugation or filtration, which makes separation easier and faster. In addition, owing to its nano-nature, magnetic nanoparticles possesses large specific surface area, and the equilibrium time between the sorbents and also the sample solutions are thus greatly shortened, and at the end resulting in relatively higher extraction capacity and detection sensitivity. Due to such advantages, MSPE has been widely used in sample preparation [4, 5]. Reviews on the analytical application of magnetic materials have been published [6, 7]. It is clear that the appropriate surface functionalization of magnetic nanoparticles is very important to get efficient extraction of target analytes.
Among different types of coating sorbents used for the extraction of organic analytes, conductive polymers due to their large surface area, the ability to establish π interactions, excellent chemical, mechanical, and thermal stability make them very attractive as SPE or SPME sorbents for extraction or isolation of trace amounts of compounds [8–10]. The ultrafine nanoparticles (such as Fe3O4, TiO2, SiO2, V2O5, Cu, Pd, Ag, and Pt) can be act as high surface area colloidal substrates for the precipitation of CPs. Therefore, these nanocomposites appear to exhibit considerable porosity and higher specific surface areas. This observation suggests that such nanocomposite particles might be interesting new stationary phase substrates for separation science [11]. Interest is focused on the materials that have combined magnetic nanoparticles and CPs. Magnetically responsive CP-based nanocomposites categorized as magnetopolymeric composites (MPCs), have been prepared by forming a layer of CP on the surface of inorganic magnetic nanoparticles. The potential applications of MPCs depend on their properties. The combined presence of CP and magnetic nanoparticles in MPCs imparts electrical and magnetic properties to the composites. Such MPCs find potential applications in electrochemical devices, electromagnetic interference, non-linear optics, microwave-absorbing materials, electromagnetic shielding, molecular electronics, catalysis, separation, etc. Fe3O4 nanoparticles have been coated with conductive polymers like polypyrrole [12], polythiophene [13] and polyaniline [14]. Among them, polyfuran (PFu) is known as a conjugated heterocyclic polymer which can be used as a humidity sensor, and acts as an optoelectronic device since its color changes from yellow-brown to black-brown upon doping [15]. Among the CPs, polyfuran (PFu) has been less investigated due difficulty of synthesis.
We describe the synthesis of PFu-coated Fe3O4 nanoparticles (PFu/Fe3O4) via in situ chemical polymerization. The PFu/Fe3O4 nanocomposite combines the properties PFu with the attractive magnetic property of Fe3O4 NPs that can be used in various fields of analytical chemistry, including electrochemistry and separation. Herein, we reported the preparation, characterization and application of PFu/Fe3O4 nanoparticles as MSPE adsorbents. To the best our knowledge, there is no publication on application of PFu/Fe3O4 as a sorbent for MSPE. The main aim of this study was to develop a SPE adsorbents preparation method and investigate the extraction performance of the synthesized adsorbents to target analytes from real water and urine samples. Since there are abundant furans on the PFu coatings, the PFu/Fe3O4 nanoparticles are expected to offer strong adsorption affinity to PAHs due to their π stacking interaction. Then, to demonstrate extraction performance of PFu/Fe3O4 nanoparticles, it was applied for the extraction of selected PAHs naphthalene, fluorene and anthracene as model compounds) from the real water and urine (smoker and non-smoker persons) samples using gas chromatography–flame ionization detector (GC–FID).
Experimental
Chemicals and materials
All reagents were of analytical grade and used without purification. Furan (Fluka) monomer was distilled under reduced pressure before use. Ferric chloride (FeCl3.6H2O), ferrous chloride (FeCl2.4H2O), anhydrous iron(III) chloride, perchloric acid (HClO4), nitromethane, and all solvents were all purchased from Merck (www.merck.de). Anthracene, fluorene and naphthalene were also purchased from Merck. A stock solution of PAHs (500 mg L−1) was prepared in methanol and kept in a refrigerator at 4 °C. Fresh working solutions were prepared daily by diluting of the stock solution in deionized water.
Instrumentation
GC analyses were performed using a gas chromatograph (Shimadzu-17A, Tokyo, Japan, www.shimadzu.com) with a split/splitless injection port and a flame ionization detection system (FID). Hydrogen gas (H2) was supplied for FID by using a Shimadzu OPGU-2200 s hydrogen generator (Tokyo, Japan). Nitrogen (99.999 %) was employed as a carrier gas, and its flow-rate was adjusted to 1 mL min−1. The gas chromatograph was equipped with a Shimadzu Hicap CBP-5-M25–025 capillary fused silica column (25 m, length; 0.25 mm I.D.; 0.22 m, film thickness; stationary phase, 5 % phenyl 95 % dimethyl polysiloxane). The column was held at 100 °C for 1 min and increased to 230 °C at a rate of 40 °C min−1 then holding for 1 min. The detector temperature was held at 270 °C and the injector temperature was set at 270 °C. A magnetic stirrer, model MR HCI-standard (Heidolph, Germany), (www.heidolph-instruments.com), with speed 0–1400 rpm, was employed for stirring samples during the extraction. For dispersion of nanoparticles in the sample solution, an ultrasonic bath (Parsonic 15S, Pars Nahand Engineering Co. Iran, www.pnec.co.ir), was used at a frequency of 28 kHz. An Nd-Fe-B magnet (2 × 2 × 1 cm, 1.4 T) was used for sorbent collection and magnetic decantation. The chemical structures of the nanocomposites were identified by a Fourier transform infrared spectroscopy (FT-IR, Burker Karlsrohe, Germany, www.bruker.com). The surface characteristics of the PFu/Fe3O4 nanocomposite were studied by scanning electron microscopy (SEM) (LEO, model 1450VP, http://www.leo-em.co.uk/).
Preparation of Fe3O4 magnetic nanoparticles
Fe3O4 nanoparticles were prepared by a chemical co-precipitation method. Briefly, a mixture of 1 g FeCl2.4H2O and 3.68 g of FeCl3.6H2O were dissolved in 50 mL distilled water under nitrogen gas with vigorous stirring for 30 min. Then, 10 mL of 25 % NH4OH was added to the solution dropwise using the dropping funnel during 30 min under nitrogen gas stream and vigorous stirring. The mixture was stirring at 80 °C for 1 h. During the whole process, the solution temperature was maintained at 80 °C and nitrogen gas was purged to prevent the intrusion of oxygen. After the reaction, the obtained Fe3O4 nanoparticles precipitate were separated from the reaction medium by the magnetic decantation, and then washed several times with degassed water and twice with ethanol. At the end Fe3O4 nanoparticles were dried at 80 °C under vacuum for 6 h.
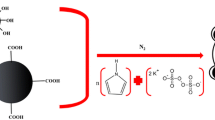
Synthesis of the polyfuran-magnetite (PFu/Fe3O4) nanocomposite
As shown in Fig. 1, the PFu/Fe3O4 nanocomposite synthesized via in situ chemical oxidation polymerization method. For PFu/Fe3O4 nanocomposite synthesis, first 0.8 g of Fe3O4 nanoparticles was added to 25 mL of dry, freshly distilled tetrahydrofuran (THF) and then disperse with an ultrasonic bath at room temperature for 1 h. After that, 1 mL of furan was added and stirred at room temperature for 30 min. In the next stage, 25 mL of THF containing 2.36 g anhydrous iron (III) chloride was added to the solution. The mixture was refluxed for 24 h and finally, a precipitate (PFu/Fe3O4) was obtained. The obtained dark powder dried at 50 °C for 24 h under vacuum. In order to doping nanocomposite 1.5 g of powder PFu/Fe3O4 nanocomposites was poured into 150 mL HClO4 (2 M) while the magnetically stirred for 3 h and then was isolated and washed with water. The doped PFu/Fe3O4 nanocomposite was dried in vacuum at 50 °C for 24 h.
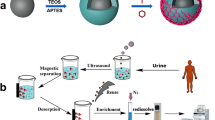
Extraction procedure
Firstly, 25 mg of PFu/Fe3O4 nanoparticles was added into 20 mL of sample solution containing 100 ng mL−1 of the analytes in a glass vial. After ultasonicated for 30 s, the mixture was stirred for 15 min. After the extraction, an external magnet collected the magnetic adsorbents and the supernatant was decanted. In order to desorption of target analytes from the surface of the sorbent, 1.5 mL of dichloromethane were added and stirred for 10 min. Finally, the magnet was used again to settle the nanoparticles, and the desorbed solution was evaporated under a gentle nitrogen flow. The residue was re-dissolved in 20 μL methanol, and 1 μL of this solution was injected into the GC system for analysis.
Results and discussion
Characterization of PFu/Fe3O4 nanocomposite
The morphology of the prepared PFu/Fe3O4 nanoparticles was investigated by SEM. As shown in Fig. 2, the prepared PFu/Fe3O4 nanoparticles are nearly spherical in shape and had a uniformly distributed particle size. Transmission electron micrograph (TEM) gave further insight into the structure of PFu-coated Fe3O4 nanoparticles. Typical TEM micrographs for PFu/Fe3O4 nanoparticles is shown in Fig. 3. The TEM image of PFu/Fe3O4 nanoparticles shows that particles possess spherical morphology having a crystallite size of 30 nm. The dark region is Fe3O4 nanoparticles and the light colored region is PFu in PFu/Fe3O4 nanocomposites. The TEM image shows that nanoparticles are monodisperse.
To ascertain the formation of polyfuran layer on the Fe3O4 nanoparticles, FT-IR spectra of the Fe3O4, PFu and the PFu/Fe3O4 were obtained and shown in Fig. 4. The FT-IR spectrum of Fe3O4 nanoparticles exhibits strong bands at 670 and 3369 cm−1 (stretching vibrations) which are due to Fe–O–Fe and O–H bonds of Fe3O4 [16]. The spectrum FT-IR of PFu shows a peak at 2893 cm−1 related to stretching of aliphatic C–H structures. The absorbance at 671 cm−1, 1012 cm−1 and 1600 cm−1 are correspond to the C–H out-of-plane vibration, C–O–C stretching vibration and C = C stretching vibration [17]. As shown in Fig. 4, the relatively strong band around 671 cm−1 resulted from Fe–O–Fe stretching modes in Fe3O4, confirms the presence of Fe3O4 nanoparticles in the PFu/Fe3O4 nanocomposite. The absorption bands around 3361, 1680, 1594, and 1010 cm−1 were assigned to the corresponding stretching vibrations of hydroxyl groups, carbonyl, C = C bond and C–O–C linkages, respectively. These absorption bands are introduced by the PFu, indicating successful functionalization of PFu on the surface of the Fe3O4 nanoparticles.
The magnetic property of pure Fe3O4 nanoparticles and PFu/Fe3O4 nanocomposites was measured by vibrating-sample magnetometer (VSM). The superparamagnetic nature of as-prepared bare Fe3O4 nanoparticles is evident from the inset of Fig. 5. In the case of PFu/Fe3O4 nanocomposites also, this superparamagnetic nature remains unchanged (evident from Fig. 5), although, with a decrease in saturation magnetization, which could be due to the presence of polyfuran coating around the Fe3O4 nanoparticles after functionalization.
Choice of materials
The sorbent type is indeed an effective parameter on the extraction efficiency. In this study, the ability of Fe3O4 nanoparticles coated with polyfuran was tested for the extraction of PAHs in water and urine samples. Recently, conductive polymers (CPs) have attracted a great deal of attention due to their multifunctional properties including hydrophobicity, acid base character, π–π interaction, polar functional groups, ion exchange property, hydrogen bonding and electro-activity. CPs with different chemical structures such as polyene-type, aromatic, heteroaromatic and mixed aromatic (or heteroaromatic) systems, polyaniline (PANI), polypyrrole (PPy), polythiophene (PTh), poly(p-phenylene), poly(phenylene vinylene) have been studied extensively. PFu has been less extensively studied among the common conductive polymers because the PFu suffers from a higher potential of oxidation than other conducting polymers. Nevertheless, the resource of furan is much greater, and thus, furan is much less expensive than pyrrole, aniline and thiophene, because the furan comes from an agricultural and sideline product that is a reproducible resource. These characteristics will attract the attention of more scientists in chemistry and materials science [18].
Optimization of method
The following parameters were optimized: (a) the amount of sorbent; (b) desorption conditions; (c) extraction time and (d) salting effect. Respective data and Figures are given in the Electronic Supporting Material (ESM). The following experimental conditions were found to give best results: (a) 25 mg of the PFu/Fe3O4 sorbent (Fig. S1, ESM); (b) desorption solvent, dichloromethane (Fig. S2, ESM); volume of the desorption solvent, 1.5 mL; desorption time, 10 min (c) extraction time, 15 min (Fig. S3, ESM); (d) the concentration of NaCl, 25 % w/v (Fig. S4, ESM).
Reusability of adsorbent
Reusability is another important factor for evaluating the performance of the magnetic nano-adsorbents. The adsorbent was regenerated by washing with 2 mL of methanol and then with 5 mL water before the next MSPE application. In such way, no carry-over of the analytes on the adsorbent was detected. The results showed that no obvious changes were observed in the extraction efficiency after fifteen successive extraction process. The good reusability indicates that this magnetic sorbent is stable and durable during the extraction procedure.
Analytical performance
Analytical characteristics of the developed method were evaluated under the selected optimum extraction conditions (25 mg of the sorbent, 1.5 mL of dichloromethane as the elution solvent, 15 min extraction time, and the concentration of salt 25 % w/v). The results of the analysis are listed in Table 1. As shown in Table 1, all analytes exhibited a linear range from 0.02 to 200 ng mL−1 with the determination coefficients (r2) varying between 0.9984 and 0.9996. The limits of detection (LODs), based on signal-to-noise ratio (S/N) of 3, were 5–20 pg mL−1, and the limits of quantification (LOQs), based on signal-to-noise ratio of 10, were 20–50 pg mL−1. The intra-day repeatability was evaluated by analyzing five spiked water samples at three concentration levels (0.05, 5 and 100 ng mL−1). The relative standard deviations (RSDs) were in the range of 4.1–5.6 % for all analytes. The inter-day precision was determined by performing three consecutive extractions each day over a period of three working days. The water samples were spiked at 0.05 ng mL−1. The inter-day RSDs varied from 5.3 to 6.5 %.
Analysis of real samples
The performance the proposed method was tested for the analysis of six real environmental water (tap, lake, well, river and wastewater) and urine samples. These water samples were collected from Sabzevar, Razavi Khorasan Province, Iran. Water samples were stored in amber-glass bottles without headspace and maintained in the dark at 4 °C until their analysis. The quantitative results of this water samples are listed in Table 2. As a result, among these water samples, no residues of the PAHs were detected in four water samples (i.e., tap, lake, well, and river water). A low concentration of PAHs was found in two wastewater samples. The recoveries of PAHs were studied by spiking the target analytes into water samples at 0.05 ng mL−1 and the results are given in Table 2, which indicated that the recoveries for the analytes were in the range from 93.2 to 99.2 % with RSDs between 5.4 and 7.3 %.
We recruited a smoker and a nonsmoker for the determination of the PAHs in their urine in this study. Due to the use of urine samples immediately after collection, urine samples of both persons were stored in polyethylene bottles in the dark at 4 °C. Before the extraction, urine samples were adjusted to pH 7 with HCl and NaOH solution. Standard addition experiments were carried out to simulate any matrix effects in the analysis of PAHs in urine samples. The results of these urine samples are listed in Table 2. As you can see, all the investigated compounds were found in the urine of smoking people with excretion values greater than those observed for no-smoking person. The presence of PAHs in greater amount in the smoker’s urine sample is due to the presence of toxic substances in cigarette smoke. For these urine samples, the relative recoveries ranged from 87.3 to 97.8 % and the RSDs were between 6.6 and 8.3 %. Figure 6 shows the typical chromatograms obtained from smoker urine samples before and after being spiked at 0.05 ng mL−1 each of the target analytes.
The results show that PAHs can be determined in different matrices with good accuracy and precision.
Comparison of MSPE with other reported methods
A comparison between the figures of merit of the proposed method and some of the reported methods for the extraction and determination of PAHs are summarized in Table 3. Considering the results, the MSPE method proved to be a sensitive, efficient, reliable, and easy to use technique with good precision and linear range in extraction and preconcentration of the PAHs from aqueous samples. MSPE had comparable to or even lower LODs than other reported methods. However, sensitivity of the current method can be improved using sensitive detection method such as mass spectrometry. All the results reveal that the developed method is a good sample preconcentration technique that can be used for ultra-trace analysis of the target analytes in real samples.
Conclusion
In this work, PFu/Fe3O4 nanoparticles successfully synthesized and used for the separation PAHs in the environment water samples and urine of smoking and no-smoking people. The coating of nanoparticles with PFu not only can increase the adsorption ability of the target analytes, due to the existence of π–π interactions between sorbent and target analytes, but also improves stability of the nanoparticles and their dispersibility in aqueous media. The magnetic separation greatly improved the phase separations while avoided the time-consuming column passing or filtration operations encountered in SPE. Since nanoparticle sorbents possess high adsorption capacity and rapid adsorption rates, low amount of sorbents can be used and short equilibrium time is required to extract the analytes from samples. The results showed the repeatability, low detection limit, good relative extraction recovery and wide linear dynamic range of the method are acceptable.
References
Li N, Lee HK (2001) Solid-phase extraction of polycyclic aromatic hydrocarbons in surface water. Negative effect of humic acid. J Chromatogr A 921:255–263
Limam I, Driss MR (2013) Off-line solid-phase extraction procedure for the determination of polycyclic aromatic hydrocarbons from aqueous matrices. Int J Environ Sci Technol 10:973–982
Safarikova M, Safarik I (1999) Magnetic solid-phase extraction. J Magn Magn Mater 194:108–112
Bunkoed O, Proespichaya P (2015) Extraction of polycyclic aromatic hydrocarbons with a magnetic sorbent composed of alginate, magnetite nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182:1519–1526
Sarafraz-Yazdi A, Rokhian T, Amiri A, Ghaemi F (2015) Carbon nanofibers decorated with magnetic nanoparticles as a new sorbent for the magnetic solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. New J Chem 39:5621–5627
Giakisikli G, Anthemidis AN (2013) Magnetic material as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal Chim Acta 789:1–16
Wierucka M, Biziuk M (2014) Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. Trends Anal Chem 59:50–58
Wu J, Mullett WM, Pawliszyn J (2002) Electrochemically controlled solid-phase microextraction based on conductive polypyrrole films. Anal Chem 74:4855–4859
Bagheri H, Ayazi Z, Naderi M (2013) Conductive polymer-based microextraction methods: a review. Anal Chim Acta 767:1–13
Abolghasemi MM, Parastari S, Yousefi V (2015) Microextraction of phenolic compounds using a fiber coated with a polyaniline-montmorillonite nanocomposite. Microchim Acta 182:273–280
Maeda S, Armes SP (1995) Surface area measurements on conducting polymer-inorganic oxide nanocomposites. Synth Met 73:151–155
Tahmasebi E, Yamini Y, Seidi S, Rezazadeh M (2013) Extraction of three nitrophenols using polypyrrole-coated magnetic nanoparticles based on anion exchange process. J Chromatogr A 1314:15–23
Tahmasebi E, Yamini Y, Moradi M, Esrafili A (2013) Polythiophene-coated Fe3O4 superparamagnetic nanocomposite: synthesis and application as a new sorbent for solid-phase extraction. Anal Chim Acta 770:68–74
Asgharinezhad AA, Ebrahimzadeh H, Mirbabaei F, Mollazadeh N, Shekari N (2014) Dispersive micro-solid-phase extraction of benzodiazepines from biological fluids based on polyaniline/magnetic nanoparticles composite. Anal Chim Acta 844:80–89
Shilabin AG, Entezami AA (2000) Electrochemical behaviour of conducting polyfuran derivatives containing pyrrole, thiophene and ethylenic spacers. Eur Polym J 36:2005–2020
Aydın M, Durmus Z, Kavas H, Esat B, Sözeri H, Baykal A, Yılmaz F, Toprak MS (2011) Synthesis and characterization of poly(3-thiophene acetic acid)/Fe3O4 nanocomposite. Polyhedron 30:1120–1126
Şen S, Bardakçı B, Yavuz AG, Gök AU (2008) Polyfuran/zeolite LTA composites and dsorption properties. Eur Polym J 44:2708–2717
González-Tejera MJ, de la Blanca ES, Carrillo I (2008) Polyfuran conducting polymers: synthesis, properties, and applications. Synth Met 158:165–189
Mehdinia A, Khojasteh E, Barardaran Kayyal T, Jabbari A (2014) Magnetic solid phase extraction using gold immobilized magnetic mesoporous silica nanoparticles coupled with dispersive liquid–liquid microextraction for determination of polycyclic aromatic hydrocarbons. J Chromatogr A 1364:20–27
Saleh A, Yamini Y, Faraji M, Rezaee M, Ghambarian M (2009) Ultrasound-assisted emulsification microextraction method based on applying low density organic solvents followed by gas chromatography analysis for the determination of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A 1216:6673–6679
Mohammadi A, Yamini Y, Alizadeh N (2005) Dodecylsulfate-doped polypyrrole film prepared by electrochemical fiber coating technique for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. J Chromatogr A 1063:1–8
Kefi BB, Atracheh LL, Kochkar H, Ghorbel AB (2011) TiO2 nanotubes as solid-phase extraction adsorbent for the determination of polycyclic aromatic hydrocarbons in environmental water samples. Magnetic solid phase extraction using gold immobilized magnetic mesoporous silica nanoparticles coupled with dispersive liquid–liquid microextraction for determination of polycyclic aromatic hydrocarbons. J Environ Sci 23:860–867
Wei MC, Jen JF (2007) Determination of polycyclic aromatic hydrocarbons in aqueous samples by microwave assisted headspace solid-phase microextraction and chromatography/flame ionization detection. Talanta 72:1269–1274
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 5510 kb)
Rights and permissions
About this article
Cite this article
Amiri, A., Baghayeri, M. & Kashmari, M. Magnetic nanoparticles modified with polyfuran for the extraction of polycyclic aromatic hydrocarbons prior to their determination by gas chromatography. Microchim Acta 183, 149–156 (2016). https://doi.org/10.1007/s00604-015-1622-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1622-5