Abstract
A novel polydopamine coated three-dimensional porous graphene aerogel sorbent carrying immobilized titanium(IV) ions (denoted as Ti4+@PDA@GA) was fabricated without using an organic solvent. The material is shown to be a viable carbon foam type of monolithic sorbent for selective lab-in-syringe enrichment of phosphoproteins and phosphopeptides. The phosphoproteins can be separated from a sample by aspiration and then bind to the sorbent. The analytes then can be dispensed within 5 min. The weight percent of titanium in the monolith typically is 14%, and the absorption capacities for the model proteins β-casein and κ-casein are 1300 and 1345 mg g−1, respectively. The absorption capacities for nonphosphoproteins are much smaller, typically 160 mg g−1 for β-lactoglobulin, 125 mg g−1 for bovine serum, and 4.8 mg g−1 for lysozyme. The results demonstrate that the selectivity for phosphoproteins was excellent on multiple biological samples including standard protein mixtures, spiked human blood serum, and drinking milk. The selective enrichment of phosphopeptides also makes the method a promising tool in phosphoproteomics.

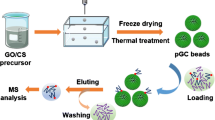
Schematic of a polydopamine coated three-dimensional porous graphene aerogel for immobilization of titanium(IV) ions. The material served as a monolithic sorbent for selective enrichment of phosphopeptides and phosphoproteins from biological samples. The enrichment process can be carried out conveniently using a lab-in-syringe way.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the most significant protein post-translational modifications (PTM), Protein phosphorylation plays an essential role in numerous biological events, including cell proliferation, protein-protein interaction, and signal propagation [1, 2]. Aberrant phosphorylation has often been implicated in the pathogenesis of human diseases, such as cancer, diabetes, neurodegenerative and cardiovascular disease [3]. Even though reversible phosphorylation is widespread in biological samples, phosphorylation is inherently a transient and dynamic process [4], so that phosphoproteins normally appear to be at relatively low stoichiometry and low abundances in contrast to nonphosphoproteins [5]. Therefore, the development of new methods for the phosphoproteins separation and enrichment has been intimately related to the comprehensive phosphoproteome analysis.
Currently, manifold approaches have been explored for purification and separation of endogenous phosphoproteins and phosphopeptides from complex biological samples [6]. Therein, immobilized metal ion affinity chromatography (IMAC) [7,8,9,10,11,12,13,14] based materials, which exploit the specific binding capacity of phosphate groups for metal ions, is one of the most prevailing methods for specific recognition and enrichment of phosphoproteins and phosphopeptides. For instance, He et al. rapid capture phosphopeptides from tryptic digests of rat brain lysate by carboxyl cotton chelator-titanium(IV) (CCC-Ti4+) fibers in a lab-in-syringe mode [7]. Zhang et al. initially appiled a novel titanium(IV)-immobilized hierarchically porous hybrid monoliths for specifical enrichment of phosphopeptides in complex samples [10]. Fei et al. highly and selectively enrich phosphorylated proteins by using Spore@Fe3+ microspheres, which successfully combined the organisms with IMAC material [11]. During the past few years, IMAC based magnetic nanoparticles have drawn much attention for specifically enrichment of phosphoproteins and phosphopeptides because of the outstanding magnetic responsivity, hypotoxicity and excellent biocompatibility, which avoid the co-sedimentation of impurities after high speed centrifugation. Ma et al. prepared Ti4+-modified polydopamine coated magnetic particles assisted with ethylene glycol for specific capture of phosphorylated proteins with a binding capicity of 1105.6 mg g−1 for κ-casein [8]. Xiong et al. used a Ti4+-immobilized multilayer polysaccharide coated magnetic nanoparticles for highly selective enrichment of phosphopeptides with a molar ratio (phosphopeptides/nonphosphopeptides) of 1: 2000 [12]. However, the synthesis of hybrid IMAC based magnetic particles is generally time-consuming and complex. And the uneven particle size and magnetism often lead to the possibility of incomplete separation of target molecules and tedious solid-phase extraction process. Therefore, it is very urgent and essential for the development of novel IMAC based materials in phosphorylation studies.
Carbon materials, such as hybrid graphene composites [15], carbon nanotube [16] and ordered mesoporous carbon material [17, 18], have been investigated as novel sorbents for phosphoproteins/phosphopeptides enrichment with high sensitivity. Related research confirmed that the hydrophobic interactions and π-stacking interactions are the major driving forces for the affinity between carbon materials and peptides/proteins [19]. Graphene aerogel (GA), a representative type of three dimensional (3D) porous monolithic materials, has aroused great enthusiasm and persistent exploration of applications in both scientific and engineering fields due to its outstanding physical and chemical properties [20]. As a novel multifunctional material, the graphene aerogel not only exhibits intrinsically excellent properties of layered graphene sheets, such as high optical, electronic and catalytical properties, but also possesses outstanding characteristics of the 3D porous architecture including tunable pores, ultrahigh specific surface areas and great mechanical strength, actualizing the widespread applications in storage and conversion [21], separation [22], biological and chemical sensors [23]. Thus, 3D porous graphene aerogel can be an ideal candidate material for separation and life science own to these intriguing and excellent characteristics. The 3D porous graphene aerogel based sorbent manifests more advantages, such as the outstanding binding capability own to its ultrahigh specific surface area and the facile separating process without ultracentrifugation techniques, wherefore avoids tedious solid-phase extraction process and makes them more easily recyclable. However, the fact that it is hard to functionalize on the surface of GA greatly limits the application of the GA due to the lack of functional groups and its hydrophobic properties. Dopamine (DA) is well known as a neurotransmitter and hormone, which is also a small molecule mimicking the adhesion proteins secreted by mussels. Polydopamine (PDA), containing abundant amine and catechol hydroxyl groups, can coat on nearly any surface by the self-polymerization of dopamine in a weakly alkaline environment [8, 24]. Widespread applications of PDA in IMAC materials [25] and hybrid graphene materials [26, 27] have been reported last decade. It has been reported that there are wealth catechol functional groups existing in PDA, thereby making it possible to chelate with any transition metal ions to form a stabilized chelated structure [28, 29]. As a consequence, immobilization of transition metal ions on different materials functionalized by PDA layer can be likely to achieve.
In this work, we initially reported a green method in mild condition to fabricate three dimensional porous graphene aerogel coated by polydopamine for titanium(IV) immobilization without any organic solvent (Fig. 1). The 3D foam graphene aerogel was selected as a novel carbon substrate, which can be in-situ synthesized in the syringe equipment, and therefore avoided the tedious substrate synthesis processes of silica particles or polymer monolith. Here, the novel sorbent was subsequently applied for selective lab-in-syringe enrichment of phosphoproteins from standard protein mixture, drinking milk and human blood serum (Fig. 2). In this lab-in-syringe mode, phosphoproteins were rapidly extracted from sample homogenate through aspirating and dispensing for 20 times by functionalized graphene aerogel monolithic syringe. The result showed that the Ti4+@PDA@GA monolith containing a high amount of Ti4+ has high absorption capacities and remarkable selectivity for phosphoproteins because titanium ion has a high capacity and tendency to specifically coordinate with phosphate groups [30]. We have also demonstrated the excellent and specific selectivity of Ti4+@PDA@GA monolith for phosphopeptides from the tryptic digests of standard protein mixture. For the first time, we have shown the integrated Ti4+@PDA@GA monolithic syringe allows for the high selectivity enrichment of low-abundance phosphoproteins and phosphopeptides from complicated biological samples.
Experimental
Preparation of the Ti4+@PDA@GA monolithic syringe
Lab-in-syringe graphene aerogel (GA) was prepared on the basis of our group’s previous work with a slight modification [31] (Fig. 1). Briefly, 50 μL obtained homogeneous graphene oxide (GO) dispersion (5.0 mg mL−1) was added into the hub of a 2.5 ml syringe which has been placed the lower frit. After the syringes were freeze-dried for 24 h to form the 3D porous GO aerogel, the prepared monolith was then chemically reduced to graphene aerogel (GA) by hydrazine vapor at 95 °C for 24 h. Afterwards, the column bed was compressed to about 2 mm by the upper frit to avoid channeling phenomenon.
The polydopamine@graphene aerogel (PDA@GA) monolithic syringe was prepared as followed: 3.0 mg mL−1 dopamine (DA) solution (pH 8.5, 10 mM Tris-HCl buffer) was treated at 25 °C by ultrasonication for 5 min. 100 μL of the prepared solution was withdrawn through the syringe packed by GA and polymerized at room temperature for 20 h to form polydopamine (PDA) film. The functionalized PDA@GA monolithic syringes were washed with 500 μL deionized water for five times.
Here, Ti4+ was deposited by chelated with catechol hydroxyl groups to prepare the Ti4+@PDA@GA monolithic syringe. Briefly, 100 μL of Ti(SO4)2 solution (300 mM) was withdrew through the modified GA syringe at 35 °C for 2 h. The prepared monolithic syringes were washed with 400 μL deionized water for four times and vacuum-dried at 50 °C for 24 h.
Adsorption capacity experiments of Ti4+@PDA@GA
To optimize the condition to selectively enrich phosphoproteins, we investigated the effects of pH and initial concentration on adsorption capacity of Ti4+@PDA@GA monolithic sorbent. β-Cas was selected as a model phosphoprotein while BSA was selected as a model nonphosphoprotein. All the sample solutions were first diluted to certain concentrations by loading buffer (pH 6.0, 10 mM Tris-HCl buffer). The adsorption experiments were carried out by aspirating and dispensing a 500 μL solution for 20 times by the Ti4+@PDA@GA monolithic syringe. After the ultrafiltration process, the concentration of free β-Cas in the supernatant fraction was measured by UV-vis absorption spectroscopy at 276 nm. The amount of β-Cas adsorbed by the Ti4+@PDA@GA was calculated according to the following formula:
Where Q (mg g−1) is the amount of protein adsorbed by packed sorbent; C0 (mg mL−1) is the initial protein concentration; C (mg mL−1) is the protein concentration in supernatant; V (mL) is the volume of the initial solution; m (g) is the weight of the packed sorbent in syringes.
For demonstration of the Ti4+@PDA@GA high specificity for phosphoproteins, α-casein (α-Cas), β-casein (β-Cas), κ-casein (κ-Cas) were selected as phosphoproteins and α-lactalbumin (α-Lac), β-lactoglobulin (β-Lg), bovine serum albumin (BSA) and lysozyme (Lyz) were selected as nonphosphoproteins with initial concentrations of 2.5 mg mL−1. The concentration of comparative proteins in the supernatant fraction was measured by a UV/Vis absorption spectroscopy at certain wavelength.
Selective enrichment of phosphoproteins from standard protein mixtures using Ti4+@PDA@GA monolithic syringe
The prepared Ti4+@PDA@GA lab-in-syringe was used for the phosphoproteins enrichment. For standard protein mixtures, a mixed protein solution (BSA and β-Cas or BSA, Lyz and β-Cas) was first made by the loading buffer (pH 6.0). The process was carried out by aspirating and dispensing a 1.0 mL mixed protein solution to fully ensure adsorption of proteins. After washing once by loading buffer, the captured proteins were eluted with 500 μL of elution buffer (pH 9.75, 10 mM Tris-HCl buffer). The loading solution, supernatant solution and eluent were all analyzed by HPLC and SDS-PAGE.
Selective capture of phosphopeptides from peptide mixtures using Ti4+@PDA@GA monolithic syringe
Selective enrichments of phosphorylated peptides from direct phosphorylated proteins and protein mixtures were investigated. A detailed procedure for the sample preparation of standard proteins is shown in the Electronic Supporting Material (ESM).
The phosphopeptide enrichment was carried out by aspirating and dispensing a 2.0 mL diluted solution of standard protein digests to ensure sufficient capture of target phosphopeptides. After washing once by loading buffer to deplete non-specific molecules, the captured phosphorylated peptides were eluted with 500 μL of elution buffer (5% ammonium hydroxide). Finally, the eluted solution was lyophilized to dryness and reconstituted in the 50 μL solution containing 50% acetonitrile and 0.1% trifluoroacetic acid (TFA). 1.0 μL of the final solution was mixed with 1.0 μL of saturated HCAA, and then 1.0 μL of mixture was pipetted onto the ground steel plate for MALDI-TOF-MS analysis.
Selective extraction of phosphoproteins using Ti4+@PDA@GA from real samples
We have also demonstrated the excellent selectivity of phosphoproteins on real biological samples, including drinking milk and spiked human blood serum. For the phosphoproteins enrichment from drinking milk, drinking milk was first diluted 20-fold by loading buffer before treatment. For the phosphoproteins enrichment from human serum, human serum was diluted 20-fold with loading buffer and the resultant solution was mixed with β-Cas to reach a protein concentration of 1.0 mg mL−1. The enrichment process was the same as the standard protein mixtures enrichment. Finally, the loading mixture, the supernatant and the elution were analyzed by SDS-PAGE and HPLC analyses.
Reusability of Ti4+@PDA@GA toward phosphoproteins
To estimate the reusability of Ti4+@PDA@GA, β-Cas solution (2.5 mg mL-1`) was first made. The absorption process was the same as before. After washing with 5% ammonium hydroxide and deionized water, the process of immobilization of titanium ion was repeated which was the same as mentioned before. The recovered Ti4+@PDA@GA was reused for protein adsorption.
Results and discussion
Preparation and characterization of Ti4+@PDA@GA
The synthesis strategy for the fabrication of Ti4+@PDA@GA is shown in Fig. 1. The syringe hub was chose as the model for the novel monolith, which facilitated the small injection volume and on-site sampling. The GA monolithic syringe through template-free “sol-cryo” method was rapidly in-situ made by freezing-dry process from the GO dispersion. As the GO solution was frozen, spontaneous phase segregation and separation facilitated the dispersed particles to the gaps between the ice crystals. After the ice crystals were removed through the sublimation, the GO aerogel constituting the skeleton of the 3D porous framework was made. The method for in-situ synthesis of GO comes with many advantages, such as uniform pore diameter, excellent mechanical properties, convenient operation and mild condition. Thus, it is an ideal method to prepare the sorbent in lab-in-syringe SPE thanks to all these characteristics. After the GO aerogel was chemically reduced to GA, GA was encapsulated by a thin hydrophilic polydopamine (PDA) layer through π-interaction on the surface of it through self-polymerization of dopamine in a slightly alkaline condition. The coating process can be conveniently operated and environmental-friendly because the self-polymerization of dopamine can be achieved under mild condition. In addition, the wealth double catechol groups on PDA made Ti4+ deposit on the surface of PDA@GA, which provide abundant functional sites for strongly bounding to phosphate groups of the phosphoproteins/phosphopeptides. These advantages made Ti4+@PDA@GA monolith favorably applied for isolation and enrichment of intact phosphorylated proteins and endogenous phosphopeptides from complex biological samples (Fig. 2).
The prepared GA, PDA@GA and Ti4+@PDA@GA monolith were examined by different techniques. FT-IR was conducted to investigate the chemical structure of the prepared monoliths. The infrared spectra of GO, GA, PDA@GA and Ti4+@PDA@GA are shown in Fig. S1. From Fig. S1, it can be seen that the graphene oxide skeleton was embodied in 3368 cm−1 (O–H stretching vibrations of carboxyl groups) and 1738 and 1627 cm−1 (C=O stretching vibration), and the characteristic peak at 1049 cm−1 belonged to C-O vibration. After GA was chemically reduced, the absorption peak at 3368 cm−1 (O–H stretching vibrations of carboxyl groups) clearly disappeared and the color of aerogel was changed from light brown to black (Fig. S2). The typical peaks at 1502 cm−1 can be attributed to aromatic ring stretching vibration of PDA, which illustrated the PDA film was successfully modified on GA surface. After the Ti4+ was deposited on the PDA@GA surface, the absorption peak at 958 cm−1 can be attributed to Ti-O vibration, which proves the Ti ions were binding to the surface of PDA.
In addition, the morphological structures of GO, GA, PDA@GA and Ti4+@PDA@GA were determined by SEM (Fig. 3). The SEM images showed that the morphology of GO (Fig. 3a), GA (Fig. 3b), PDA@GA (Fig. 3c), and Ti4+@PDA@GA (Fig. 3d) did not change a lot, manifesting that the functionalization did not influence the morphology of graphene aerogel and that the mechanical strength of the aerogel was also kept well. The composition information of four resultant monoliths was also analyzed by EDS (Fig. S3), which illustrated that four resultant materials were successfully synthesized. As shown in Fig. S3d, Ti4+@PDA@GA monolith consisted of oxygen, carbon, nitrogen and titanium, illustrating successful modification of PDA and Ti4+ on PDA@GA monolith. The weight percentage of titanium on Ti4+@PDA@GA monolith were estimated to be 14.1%, indicating that the prepared Ti4+@PDA@GA monolith was composed of high titanium content and therefore would bring about high specificity for phosphorylated proteins and peptides. In our work, the initial concentration was 5.0 mg mL−1and the obtained Ti4+@PDA@GA was 1.29 mg, which was calculated through subtracting the initial weight of the syringe from the final weight of syringe.
Standard protein adsorption by Ti4+@PDA@GA monolith
For the optimization of the condition to selectively enrich phosphoproteins, we investigated the influence of pH and initial concentration on binding capacity of Ti4+@PDA@GA monolithic sorbent (Details are shown in the Electronic Supporting Material (ESM)).
The selectivity of Ti4+@PDA@GA monolith for different phosphoproteins was tested so as to illustrate the high specificity for phosphorylated proteins. κ-Cas, β-Cas and α-Cas were chosen as the phosphoproteins, while α-Lac, β-Lg, BSA and Lyz were chosen as the nonphosphorylated proteins. The binding capacities for these proteins were shown in Fig. 4a. The adsorption capacities of Ti4+@PDA@GA monolith for these proteins were measured (α-Cas: 829 mg g−1, β-Cas: 1290 mg g−1, κ-Cas: 1340 mg g−1, β-Lg: 159 mg g−1, α-Lac: 305 mg g−1, BSA: 124 mg g−1 and Lyz: 4.8 mg g−1). The adsorption capacities of Ti4+@PDA@GA towards phosphoproteins were much higher than those of nonphosphoproteins, indicating that Ti4+@PDA@GA had highly specific bio-recognition for phosphoproteins mainly thanks to abundant amount of Ti4+ in resultant monolithic sorbent and the strong affinity of transitional metal ion with phosphate groups. Among these three phosphoproteins, the result illustrated that Ti4+@PDA@GA exhibited relatively higher selectivity towards β-Cas and κ-Cas than α-Cas, mainly because the pI of α-Cas is a little lower than the pI of β-Cas and κ-Cas, respectively. When buffer of pH 2.5 was used (Fig. 4b), the adsorption capacity of Ti4+@PDA@GA for α-Cas is much higher than β-Cas and κ-Cas. This is because the pI of α-Cas (pI 4.0–4.1) is relatively lower than those of β-Cas (pI 4.5) and κ-Cas (pI 5.8–6.0). This phenomenon also demonstrated the adsorption capacity of Ti4+@PDA@GA is pH-dependent Table 1.
a Comparison of protein adsorption capacities of Ti4+@PDA@GA monolithic syringe for phosphoproteins/nonphosphoproteins (pH 6.0, n = 3). b The adsorption capacities of Ti4+@PDA@GA for different proteins at the low pH value (pH 2.5, n = 3). c Phosphoprotein and dephosphoprotein adsorption experiments (n = 3). d Protein adsorption capacities on GA, PDA@GA, Ti4+@PDA@GA and commercial P25 TiO2 (n = 3)
To further evaluate the specificity of Ti4+@PDA@GA for phosphoproteins, β-Cas, κ-Cas, dephosphorylated β-Cas (denoted as Dep-β-Cas) and dephosphorylated β-Cas (denoted as Dep-κ-Cas) were selected as model proteins. β-Cas and κ-Cas, as two types of well-known phosphoproteins, nearly have no surface-exposed phosphate groups after treated with alkaline phosphatase [32]. The maximum adsorption capacities of Ti4+@PDA@GA toward Dep-β/κ-Cas and β/κ-Cas were shown in Fig. 4c (Dep-β-Cas: 284 mg g−1; Dep-κ-Cas: 205 mg g−1; β-Cas: 1090 mg g−1; κ-Cas: 1080 mg g−1).It can be seen that the adsorption capacities of phosphoproteins were much lower than those of dephosphoproteins. It is proven that Ti4+@PDA@GA has outstandingly specific binding capacity with phosphorylated proteins.
The comparison of GA, PDA@GA, Ti4+@PDA@GA and commercial P25 TiO2 for proteins adsorption was investigated under the same condition (Fig. 4d). The absorption capacity of graphene aerogel for BSA did not vary too much before and after the modification of PDA and Ti4+. However, the Ti4+@PDA@GA exhibited excellent absorption towards β-Cas after the immobilization of Ti4+ in comparison of BSA. This was a result of electrostatic attraction between the positively charged Ti4+@PDA@GA with wealth titanium ions and the negatively charged phosphorylated protein molecules which contains abundant phosphate groups. For comparison, commercial P25 TiO2 particles were also used to absorb β-Cas and BSA. The absorption capacity of Ti4+@PDA@GA for phosphoprotein was 8 times more than that for nonphosphoprotein while the binding capacity of commercial P25 TiO2 for phosphoprotein was only 3 times more than that for nonphosphoprotein.
Specific enrichment of phosphorylated proteins from intact protein mixtures
The specific enrichment of phosphorylated proteins from intact protein mixtures was investigated by SDS-PAGE analysis. As shown in Fig. 5, β-Cas was selected as the phosphoprotein while BSA was selected as the nonphosphoprotein. The initial concentration of β-Cas was kept the same at 1.0 mg mL−1. A sequence of binary protein mixtures was pretreated in different mass ratios (nonphosphoprotein/phosphoprotein) of 1: 5, 1: 1 and 5: 1 to mimic complex samples. From Fig. 6, it can be observed that the quantity of eluted phosphoproteins rose as the quantity of nonphosphoproteins in the loading mixture was reduced. In this case, almost all phosphoprotein has been absorbed while little nonphosphoprotein had been absorbed, which demonstrated the great selective absorption capacity of Ti4+@PDA@GA for phosphoproteins. Moreover, there was still much elution of phosphoprotein even though there were abundant nonphosphoprotein intereference at the condition of mass ratios (nonphosphoprotein/phosphoprotein) of 5: 1. This indicated that Ti4+@PDA@GA has an outstanding capacity for the specific enrichment of phosphorylated protein from intact protein mixtures.
SDS-PAGE of BSA/β-Cas in different mass ratios treated with Ti4+@PDA@GA. Lane 1: maker; Lane 2: BSA & β-casein protein mixture (mass ratio = 1:5); Lane 3: the supernatant; Lane 4: the eluate; Lane 5: BSA & β-casein protein mixture (mass ratio = 1:1); Lane 6: the supernatant; Lane 7: the eluate; Lane 8: BSA & β-casein protein mixture (mass ratio = 5:1); Lane 9: the supernatant; Lane 10: the eluate
In order to assess the impact of the modification of the aerogel on the specific capture of phosphorylated proteins, the mixture of BSA/β-casein was treated with GA, PDA@GA, and Ti4+@PDA@GA, respectively (Fig. S5). The result clearly showed that the monolith can successfully separate the phosphoprotein from the mixture only after the immobilization of Ti4+.
To further test the selectivity of Ti4+@PDA@GA for the isolation of phosphorylated proteins, a mixture of Lyz, BSA and β-Cas (0.05 mg mL−1 Lyz, 0.35 mg mL−1 BSA and 1.00 mg mL−1 β-Cas) was adopted. The HPLC chromatogram of the untreated protein mixtures corresponding to Lyz, BSA and β-Cas, respectively, can be obtained (Fig. 6a). After enrichment with Ti4+@PDA@GA, 99% β-Cas was captured from the mixture with little loss of BSA and Lyz (10.4 and 15.1%, respectively) (Fig. 6b). The trapped β-Cas on Ti4+@PDA@GA can be eluted with 10% ammonium hydroxide, and 84.7% β-Cas was recovered in the eluent with minor non-specific absorption of Lyz and BSA (2.8 and 0.53%, respectively) (Fig. 6c).
Selective enrichment of phosphorylated peptides from standard protein digests
Selective enrichment of phosphorylated peptides from direct phosphorylated proteins and protein mixtures were investigated. To evaluate the performance of Ti4+@PDA@GA monolith for phosphopeptide enrichment, 30 fmol μL−1 β-Cas digest was directly analyzed by MALDI-TOF MS (Fig. 7a). The result spectrum was dominated by intensive signals of nonphosphopeptides, and almost no signals of phosphopeptide were observed. After the treatment with Ti4+@PDA@GA monolithic syringe, three signal peaks of phosphopeptides (m/z 2061.7, 2556.4 and 3122.6) can be observed in the mass spectrum [7, 18]. The detailed sequences of amino acids of these phosphopeptides are shown in the Electronic Supporting Material (Table S1). The enrichment specificity of the Ti4+@PDA@GA monolith toward phosphopeptides from complex samples was also investigated, which was carried out using tryptic digests of 60 pmol μL−1 BSA and 300 fmol μL−1 β-Cas mixtures (the molar ratio of BSA to β-Cas is 200: 1). (Fig. 7b) Before enrichment with Ti4+@PDA@GA monolithic syringe, no phosphopeptides were detected due to the interference of highly abundant nonphosphopeptides. After the enrichment, three signal peaks of phosphopeptides (m/z 2061.7, 2555.9 and 3122.6) can be detected with a clean background in the mass spectrum. The detailed sequences of amino acids of these phosphopeptides are shown in the Electronic Supporting Material (Table S1). The result showed that the Ti4+@PDA@GA monolithic sorbent exhibited outstandingly high specificity for phosphopeptides even under the high interference of nonphosphopeptides.
MALDI-TOF MS analyses of peptides derived from (a) tryptic digest of β-Cas with a concentration of 60 fmol/μL: before and after enrichment by Ti4+@PDA@GA; (b) a peptide mixture of β-Cas and BSA at a molar ratio of 1: 200: before and after enrichment by Ti4+@PDA@GA. Phosphopeptides are labeled with their observed m/z
Regeneration of the Ti4+@PDA@GA
The regeneration of Ti4+@PDA@GA was also investigated (Fig. S6). There is a minor decrease in the binding capacity of Ti4+@PDA@GA towards β-Cas after five executive absorption-regeneration cycles. The EDS analysis of Ti4+@PDA@GA was also carried out (Fig. S7), indicating little leaching of Ti(IV) ions. The result shows that Ti4+@PDA@GA has an outstanding durability and recyclability.
Application in biological samples
Ti4+@PDA@GA monolith was further investigated to capture phosphoproteins from complex biological samples, including protein mixtures, drinking milk and spiked human serum (Fig. 8).
SDS-PAGE analyses of (a) BSA/β-Cas binary mixtures, (b) 10-fold diluted drinking milk and (c) spike-in 1 mg mL−1 β-Cas from 20-fold diluted human serum (Lane 1: PR1910 (11~180KD) maker, Lane 2: the untreated loading mixtures, Lane 3: the supernatant after treatment with Ti4+@PDA@GA, Lane 4: the eluate)
For protein mixtures (Fig. 8a), two major bands were clearly observed in the binary protein mixtures before treatment (Lane 2), which were ascribed to BSA (Mw 66 kDa) and β-Cas (Mw 24 kDa) proteins. After enrichment with Ti4+@PDA@GA, almost all β-Cas had been removed with minor absorption of BSA (Lane 3). After eluted by an alkaline solution, only one band corresponding to β-Cas appeared in the elution lane (Lane 4), illustrating the high specificity of Ti4+@PDA@GA toward phosphoproteins. Drinking milk containing abundant phosphoproteins was selected to further prove the specificity of Ti4+@PDA@GA in separation and enrichment of phosphoproteins from biological samples (Fig. 8b). As shown in Fig. 8b, four bands were revealed in lane 2, which attributed to α-Cas, β-Cas, β-Lg and α-Lac from up to bottom. After treatment with Ti4+@PDA@GA, there were much more β-Cas and α-Cas than nonphosphoproteins in the elute fraction (lane 4) compared with loading mixture (lane 2) and supernatant fraction (lane 3). Therefore, it proved that Ti4+@PDA@GA monolithic syringe has high adsorption affinity for phosphoproteins. Similar results were also revealed in the capture of β-Cas (Mw 24 kDa) from human serum with spiked-in β-Cas (Fig. 8c). Abundant nonphosphoproteins in loading mixture (lane 2) were effectively removed into supernatant fraction, as unfolded in the nearly same bands between loading mixture (lane 2) and supernatant fraction (lane 3). Compared to loading mixture and supernatant fraction, the high similarity of band patterns in the elution lanes demonstrates the predominance of enriched endogenous phosphoproteins apart from β-Cas. These results demonstrated that Ti4+@PDA@GA monolith syringe can effectively isolate and enrich phosphorylated proteins from biological samples with high specificity and sensitivity.
Conclusion
In summary, a novel three dimensional (3D) porous Ti4+@PDA@GA monolith was synthesized without any organic solvent and innovatively developed for the selective lab-in-syringe enrichment of phosphoproteins and phosphopeptides in multiple complex samples. The synthesis of Ti4+@PDA@GA monolithic sorbent was in mild condition, eco-friendly, and inexpensive. The titanium content of the novel material was high and the adsorption performance for phosphoproteins was impressively excellent. Ti4+@PDA@GA monolith exhibited good biological compatibility and excellent performance for specific and rapid enrichment of phosphoproteins and phosphopeptides from multiple samples. Taking advantages of the good specificity and biocompatibility of the Ti4+@PDA@GA monolithic material, Ti4+@PDA@GA manifested outstanding performance in phosphoproteins capture from spiked human serum, demonstrating its impressive application prospect in endogenous phosphoproteins in addition to spiked-in phosphorylated protein. Furthermore, the lab-in-syringe monolithic mode enhanced a great degree of simplification and rapidness for the whole enrichment process, which avoided the co-sedimentation of impurities and made it possible for bio-active compound analysis. We believe that such novel method will longer contribute to phosphorylated proteins and peptides enrichment in comprehensive phosphoproteomics studies and that three dimensional (3D) porous Ti4+@PDA@GA monolithic sorbent will be promising candidate for complicated biological analyses.
References
Hunter T (2000) Signaling—2000 and beyond. Cell 100(1):113–127
Humphrey SJ, Azimifar SB, Mann M (2015) High-throughput phosphoproteomics reveals in vivo insulin signaling dynamics. Nat Biotechnol 33(9):990–995. https://doi.org/10.1038/nbt.3327
Tan CSH, Bodenmiller B, Pasculescu A, Jovanovic M, Hengartner MO, Jørgensen C, Bader GD, Aebersold R, Pawson T, Linding R (2009) Comparative analysis reveals conserved protein phosphorylation networks implicated in multiple diseases. Sci Signal 2 (81):ra39-ra39
Thingholm TE, Jensen ON, Larsen MR (2009) Analytical strategies for phosphoproteomics. Proteomics 9(6):1451–1468
Witze ES, Old WM, Resing KA, Ahn NG (2007) Mapping protein post-translational modifications with mass spectrometry. Nat Methods 4(10):798–806
Li X-S, Yuan B-F, Feng Y-Q (2016) Recent advances in phosphopeptide enrichment: strategies and techniques. Trends Anal Chem 78:70–83. https://doi.org/10.1016/j.trac.2015.11.001
He XM, Chen X, Zhu GT, Wang Q, Yuan BF, Feng YQ (2015) Hydrophilic carboxyl cotton Chelator for titanium(IV) immobilization and its application as novel fibrous sorbent for rapid enrichment of Phosphopeptides. ACS Appl Mater Interfaces 7(31):17356–17362. https://doi.org/10.1021/acsami.5b04572
Ma X, Ding C, Yao X, Jia L (2016) Ethylene glycol assisted preparation of Ti4+-modified polydopamine coated magnetic particles with rough surface for capture of phosphorylated proteins. Anal Chim Acta 929:23–30. https://doi.org/10.1016/j.aca.2016.04.058
Yang X, Xia Y (2016) Urea-modified metal-organic framework of type MIL-101(Cr) for the preconcentration of phosphorylated peptides. Microchim Acta 183(7):2235–2240. https://doi.org/10.1007/s00604-016-1860-1
Zhang H, Ou J, Yao Y, Wang H, Liu Z, Wei Y, Ye M (2017) Facile preparation of titanium(IV)-immobilized hierarchically porous hybrid monoliths. Anal Chem 89(8):4655–4662. https://doi.org/10.1021/acs.analchem.7b00242
Fei R, Zhang T, Huang Y, Hu Y (2017) Highly selective enrichment of phosphorylated proteins by using spore@ Fe3+ microspheres. Anal Chim Acta 986:161–170. https://doi.org/10.1016/j.aca.2017.07.030
Xiong Z, Zhang L, Fang C, Zhang Q, Ji Y, Zhang Z, Zhang W, Zou H (2014) Ti4+-immobilized multilayer polysaccharide coated magnetic nanoparticles for highly selective enrichment of phosphopeptides. J Mater Chem B 2(28):4473–4480. https://doi.org/10.1039/c4tb00479e
Capriotti AL, Cavaliere C, Ferraris F, Gianotti V, Laus M, Piovesana S, Sparnacci K, Zenezini Chiozzi R, Lagana (2018) A new Ti-IMAC magnetic polymeric nanoparticles for phosphopeptide enrichment from complex real samples. Talanta 178:274–281
Kailasa SK, Wu HF (2014) Recent developments in nanoparticle-based MALDI mass spectrometric analysis of phosphoproteomes. Microchim Acta 181(9–10):853–864. https://doi.org/10.1007/s00604-014-1191-z
Ma W, Zhang F, Li L, Chen S, Qi L, Liu H, Bai Y (2016) Facile synthesis of Mesocrystalline SnO2 Nanorods on reduced graphene oxide sheets: an appealing multifunctional affinity probe for sequential enrichment of endogenous peptides and Phosphopeptides. ACS Appl Mater Interfaces 8(51):35099–35105. https://doi.org/10.1021/acsami.6b14597
S-f R, Y-l G (2006) Carbon nanotubes (2,5-dihydroxybenzoyl hydrazine) derivative as pH adjustable enriching reagent and matrix for MALDI analysis of trace peptides. J Am Soc Mass Spectrom 17(7):1023–1027
Qin H, Gao P, Wang F, Zhao L, Zhu J, Wang A, Zhang T, Wu Ra, Zou H (2011) Highly efficient extraction of serum peptides by ordered mesoporous carbon. Angew Chem Int Ed 50 (51):12218–12221
Zhang L, Gan Y, Sun H, Yu B, Jin X, Zhang R, Zhang W, Zhang L (2017) Magnetic mesoporous carbon composites incorporating hydrophilic metallic nanoparticles for enrichment of phosphopeptides prior to their determination by MALDI-TOF mass spectrometry. Microchim Acta 184(2):547–555. https://doi.org/10.1007/s00604-016-2046-6
Matsuura K, Saito T, Okazaki T, Ohshima S, Yumura M, Iijima S (2006) Selectivity of water-soluble proteins in single-walled carbon nanotube dispersions. Chem Phys Lett 429(4):497–502
Han Q, Yang L, Liang Q, Ding M (2017) Three-dimensional hierarchical porous graphene aerogel for efficient adsorption and preconcentration of chemical warfare agents. Carbon 122:556–563
Han S, Wu D, Li S, Zhang F, Feng X (2014) Porous graphene materials for advanced electrochemical energy storage and conversion devices. Adv Mater 26(6):849–864
Uzzaman A, Shang Z, Qiao Z, Cao CX, Xiao H (2018) Graphene and graphene oxide as a solid matrix for extraction of membrane and membrane-associated proteins. Microchim Acta 185(2):123. https://doi.org/10.1007/s00604-017-2658-5
Sun W, Cao L, Deng Y, Gong S, Shi F, Li G, Sun Z (2013) Direct electrochemistry with enhanced electrocatalytic activity of hemoglobin in hybrid modified electrodes composed of graphene and multi-walled carbon nanotubes. Anal Chim Acta 781:41–47
Wan W, Han Q, Zhang X, Xie Y, Sun J, Ding M (2015) Selective enrichment of proteins for MALDI-TOF MS analysis based on molecular imprinting. Chem Commun 51(17):3541–3544. https://doi.org/10.1039/c4cc10205c
Yan Y, Zheng Z, Deng C, Li Y, Zhang X, Yang P (2013) Hydrophilic polydopamine-coated graphene for metal ion immobilization as a novel immobilized metal ion affinity chromatography platform for phosphoproteome analysis. Anal Chem 85(18):8483–8487. https://doi.org/10.1021/ac401668e
Gao H, Sun Y, Zhou J, Xu R, Duan H (2013) Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl Mater Interfaces 5(2):425–432
Wang X, Deng C (2015) Preparation of magnetic graphene@polydopamine@Zr-MOF material for the extraction and analysis of bisphenols in water samples. Talanta 144:1329–1335
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318(5849):426–430
Moser J, Punchihewa S, Infelta PP, Graetzel M (1991) Surface complexation of colloidal semiconductors strongly enhances interfacial electron-transfer rates. Langmuir 7(12):3012–3018
Zhou H, Ye M, Dong J, Corradini E, Cristobal A, Heck AJ, Zou H, Mohammed S (2013) Robust phosphoproteome enrichment using monodisperse microsphere-based immobilized titanium(IV) ion affinity chromatography. Nat Protoc 8(3):461–480
Han Q, Liang Q, Zhang X, Yang L, Ding M (2016) Graphene aerogel based monolith for effective solid-phase extraction of trace environmental pollutants from water samples. J Chromatogr A 1447:39–46. https://doi.org/10.1016/j.chroma.2016.04.032
Liu W, Zheng J, Li S, Wang R, Lin Z, Yang H (2015) Aluminium glycinate functionalized silica nanoparticles for highly specific separation of phosphoproteins. J Mater Chem B 3(31):6528–6535. https://doi.org/10.1039/c5tb01055a
Deng Q, Wu J, Chen Y, Zhang Z, Wang Y, Fang G, Wang S, Zhang Y (2014) Guanidinium functionalized superparamagnetic silica spheres for selective enrichment of phosphopeptides and intact phosphoproteins from complex mixtures. J Mater Chem B 2(8):1048–1058. https://doi.org/10.1039/c3tb21540g
Ma X, Jia L (2016) Polydopamine assisted preparation of Ti4+-decorated magnetic particles for selective and rapid adsorption of phosphorylated proteins. J Chem Technol Biot 91(4):892–900. https://doi.org/10.1002/jctb.4654
Cheng G, Wang ZG, Liu YL, Zhang JL, Sun DH, Ni JZ (2013) Magnetic affinity microspheres with meso−/macroporous shells for selective enrichment and fast separation of phosphorylated biomolecules. ACS Appl Mater Interfaces 5(8):3182–3190
Acknowledgements
This work was supported by the Natural Science Foundation of China [grant numbers 21575076, 21621003]; the National Key Research and Development Program of China [grant numbers 2016YFA0203101,2017YFC0906902]; and the Beijing Municipality Science and Technology Program [grant numbers D161100002116001].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1.39 mb)
Rights and permissions
About this article
Cite this article
Tan, S., Wang, J., Han, Q. et al. A porous graphene sorbent coated with titanium(IV)-functionalized polydopamine for selective lab-in-syringe extraction of phosphoproteins and phosphopeptides. Microchim Acta 185, 316 (2018). https://doi.org/10.1007/s00604-018-2846-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2846-y