Abstract
A composite consisting of NiCo2S4 and reduced graphene oxide (rGO) was prepared via a hydrothermal process. Compared to individual NiCo2S4 nanomaterials or reduced graphene oxide, the composite exhibits enhanced oxidase-like activity. It is found that dopamine (DA) inhibits the ability of NiCo2S4-rGO to oxidize the substrate 3,3′,5′,5′-tetramethylbenzidine (TMB) to form blue colored ox-TMB. Based on these findings, a colorimetric method for determination of DA was worked out. The absorption, best measured at 652 nm, increases linearly in the 0.5–100 μM DA concentration range, and the limit of detection is 0.42 μM. This method was successfully applied to the detection of DA in spiked human serum samples.

A hierarchical NiCo2S4-rGO composite was prepared through two-step hydrothermal process. It exhibits enhanced oxidase-like activity which, however, is inhibited by dopamine (DA). Hence, less blue colored ox-TMB is formed by oxidation of 3,3′,5,5′-tetramethylbenzidine in the presence of dopamine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dopamine supports human sensing capability and physical activity, but abnormal level of dopamine often lead to agitation, addiction and diseases such as Parkinsonism, acquired immune deficiency syndrome (AIDS), senile dementia and schizophrenia [3,4,5]. A variety of physicochemical methods have been developed to detect dopamine, including field effect transistor (FET)-based biosensor [6], fluorometry [7,8,9,10], chromatography [11, 12], electroanalysis [13,14,15,16] and capillary electrophoresis [17]. These methods are of high precision and good reproducibility, but not easy to be observed by bare eyes. Colorimetry possesses several advantages, such as convenience, low cost and visual detection. However, natural enzymes can be easily influenced by environmental changes, which restricts their practical application. Much attention has been paid to the research of artificial enzyme with higher stability in the application in colorimetric detection. It was reported that bovine serum albumin (BSA) stabilized Au clusters have high intrinsic peroxidase-like activity, and a simple, high sensitive and selective method was developed for xanthine detection in harsh chemical environment [18]. A novel approach has been proposed to sensitively and selectively detect hydrogen peroxide based on a bimetallic (Co/2Fe) metal-organic framework with dual enzyme mimetic activity [19]. In addition, a colorimetric sensing of dopamine based on high oxidase-mimic activity of Fe/NC-800 hybrid was developed [20]. These works demonstrated that mimic enzymes have great potential in biomedical analysis and environmental monitoring.
The substrate recognition and catalytic function of enzymes are essentially determined by their supramolecular structure [21]. Particularly, it has been found that graphene is effective for anchoring active metal catalysts [22]. To mimic the special supramolecular structure, nano-sized hierarchical architecture of NiCo2S4 on reduced graphene oxide was synthesized through hydrothermal process. Several nanomaterials have been applied for colorimetric analysis with good response to dopamine detection [23,24,25,26]. We synthesized hierarchical NiCo2S4-rGO nanocomposites and evaluated the nanocomposite materials as an oxidase mimic, and the substrate TMB was transformed into oxidized TMB. The hierarchical NiCo2S4-rGO nanocomposites exhibit an enhanced oxidase-like activity compared to the individual components, demonstrating the synergistic catalytic effect between NiCo2S4 and reduced graphene oxide. The presence of dopamine causes a blue color fading of NiCo2S4-rGO-TMB system. Based on these results, a colorimetric method was developed to determine dopamine in human serum samples. This is the first report to use NiCo2S4-rGO nanocomposite as an oxidase mimic to detect dopamine.
Experiment and methods
Chemicals and reagents
Graphite, sulfuric acid (H2SO4), phosphorus pentoxide (P2O5), potassium persulfate (K2S2O8), potassium permanganate(KMnO4), Co(NO3)2·6H2O, Ni(NO3)2·6H2O, ethanol, Na2S·9H2O, hexamethylenetetramine, o-phenylenediamine (OPD), 3,3′,5,5′-tetramethylbenzidine (TMB), t-Butanol (TBA), p-benzoquinone (PBQ), superoxide dismutase (SOD), dopamine and dimethyl sulfoxide (DMSO) were purchased from Macklin Co Ltd. (Shanghai, China, http://www.macklin.cn/). Histidine, phenylalanine, L-cysteine,glutathione,ascorbic acid,bovine serum albumin,uric acid,glucose, glycine, tyrosine and lysine were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China, http://aladdin.company.lookchem.cn/). All chemical reagents used are analytical reagent grade without further treatment, and ultrapure water was used throughout the study.
Preparation of graphene oxide
To prepare graphene oxide, it is necessary to oxidize commercial graphite power. Specifically, graphite (3 g) and concentrated H2SO4 (12 mL) were heated to 80 °C, then P2O5 (2.5 g) and K2S2O8 (2.5 g) were added. The reaction liquid was cooled down naturally after stirring for 2 h, followed by dilution with 500 mL deionized water and kept overnight in ambient atmosphere. The final peroxidation product was obtained after centrifugation and natural drying. 15 g KMnO4 was gradually added into 120 mL H2SO4 and kept below 20 °C. Then the temperature of the suspension was set as 35 °C for another 2 h after stirring for 2 h. The mixture was transferred into ice bath under vigorous stirring, and 700 mL deionized water was added dropwise until no obvious smoke was generated. Subsequently 20 mL H2O2 was added, resulting in bright yellow color of the mixture. The final product was washed with deionized water and HCl (10%) for serval times.
Fabrication of NiCo2S4-rGO composites
Co(NO3)2·6H2O and Ni(NO3)2·6H2O were added in proportion to aqueous ethanol, then hexamethylenetetramine was added. The mixture was stirred for 30 min, and a transparent pink solution was obtained. The mixture was then transferred into an autoclave, kept at 80 °C for 10 h, cooled to room temperature and washed for several times with deionized water. The obtained precursor was naturally dried. Next, the Ni-Co precursor was added into ultrasound-treated GO suspension. Then 0.5 g of Na2S·9H2O was added under stirring. The dark brown solution was kept at 160 °C for 8 h, centrifuged, and washed for several times. The final product was vacuum-dried at 40 °C. Bare NiCo2S4 was prepared in similar way in the absence of GO.
Oxidase mimic activity and steady-state kinetic studies
The hierarchical NiCo2S4-rGO nanocomposites catalytically oxidize TMB and cause a color of blue, resulting in the characteristic absorption band at 652 nm. First of all, 60 μL TMB (30 mM in dimethyl sulfoxide, DMSO) was added into 895 μL acetate buffer (pH = 4.0). Subsequently, 45 μL hierarchical NiCo2S4-rGO suspension (1 mg·mL−1) was added into the analysis system. After the end of incubation for 35 min at 25 °C, the absorbance of the analysis system at 652 nm was measured with UV–Vis spectroscopy. To evaluate the kinetic of NiCo2S4-rGO composite, 60 μL TMB with various concentrations, 45 μL NiCo2S4-rGO (1 mg·mL−1) and 895 μL acetate buffer were added, and the absorbance of the mixed reaction solution at 652 nm within 25 min were recorded. The kinetic parameters were calculated by Michaelis-Menten equation:
in which [S] refers to the substrate concentration, Vmax and Km refers to the maximal reaction velocity and the Michael is constant, respectively.
Colorimetric detection of dopamine
To accurately investigate the capability of the NiCo2S4-rGO nanocomposite for determination of dopamine, 45 μL of catalyst suspension and equivalent amount of TMB were added into acetate buffer (pH = 4.0). Then, various concentrations of dopamine were introduced into the mixture to reach a total volume of 3 ml. The changes in the absorbance at 652 nm were recorded by UV–Vis spectrometry. The dopamine concentration was determined by a calibration plot of the absorbance (ΔA = A0-A) at 652 nm, where A refers to the absorbance of dopamine sample of different concentrations and A0 refers to the absorbance of the blank samples. For the real sample analysis, human serum samples from two donors were obtained with written informed consent (The project was approved by the hospital of Sichuan Agricultural University).
Characterization
The morphologies of NiCo2S4-rGO nanostructures were characterized by transmission electron microscope (TEM, JSM4800F, JEOL, Japan) and scanning electron microscope (SEM, JEOL2100F, JEOL, Japan). To analyze the crystalline structure, elemental composition and pore structure were analyzed by using X-ray diffractometer (XRD, DX-2700, Dan Dong, China). The chemical composition of the NiCo2S4-rGO composite was examined with X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi, Boyue, China). UV-Vis spectrophotometer (Beijing Purkinje General Instrument Co. Ltd., Beijing, China) was used to record the absorption spectra and evaluate the oxidase-like activity of the material.
Results and discussion
Choice of materials
Generally, in order to increase the sensitivity of metal sulfide nanoparticles, composites of these metal sulfide nanoparticles with other materials can be synthesized. Graphene and its derivatives such as graphene oxide, possess large surface-to-volume ratio and high chemical stability. These advantages have contributed to the detection of biological macromolecules [27, 28]. It would be of great interest to explore them in multicomponent systems for synergistic properties. Graphene oxide was chosen to reduce the chances for particles agglomeration and resulted in maximum enzymatic activity of NiCo2S4 for colorimetric detection. The composite materials enhance the fast electron transfer involved during redox process [29]. In comparison, the method described by Chen et al. has low detection limit [20], but the detection of our method covering a wider range. It was reported that silver-based nanozymes (peroxidase-like) hybrid with other metals (Au, Pt, and Pd) were explored for monitoring ascorbic acid concentration [30]. For comparison, the synthesis processing of our method has the advantages of easily available raw materials and high cost-effectiveness. Moreover, the hydrothermal method used in this study is inexpensive, feasible and not easy to be contaminated.
Characterization of the anocomposite
As shown in Scheme 1, NiCo2S4-rGO composite was synthesized by a two-step approach. Firstly, under the precipitation of hexamethylenetetramine, with the increase of pH value of the reaction solution, Ni-Co precursor were formed from a reaction of Co2+ and Ni2+ cations. In order to form the hierarchical structure, Ni-Co precursor was added into ultrasound-treated GO solution under whisking and then the mixture was transferred into an autoclave for reaction. In hydrothermal processing, the hydrophilic Ni-Co precursor was sulfonated to form NiCo2S4 through an anion-exchange reaction under the attraction of the functional groups on the GO surface [31]. With the reduction of oxygenated functional groups, the GO turned into rGO.
The interconnected NiCo2S4 particles exhibits a flower-like morphology formed by stacked nanosheets (Fig. 1a). The thickness of these particles ranges from hundreds of nanometers to several micrometers as shown in Fig. 1b. The surface of NiCo2S4 appears to be much coarser compared to the Ni-Co precursor, mainly due to the rapid ion exchange and the Kodak effect in the process of vulcanization [32]. The TEM image further confirms the rough surface (Fig. 1c). As expected, NiCo2S4-rGO composite showed a layered porous structure composed of rumpled rGO nanoscale (Fig. 1d, e). Results shown that there were flower-like structures, while the reduced graphene oxide limited the stacking process of NiCo2S4 nanosheets (Fig. S1). This architecture can provide enough active sites for oxidation reduction. By comparing with Fig. 1c, the TEM image of NiCo2S4-rGO shown in Fig. 1f shows that the crumpled rGO nanosheets wrap around NiCo2S4 nanomaterials.
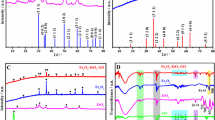
XRD patterns (Fig. S2a) shows that the peaks for all the samples can be indexed to (220), (311), (400), (511), and (440) plane reflections of the NiCo2S4 (JCPDS no. 43–1477). Lattice stripes are caused by the following crystal planes including 0.54 nm (111), 0.33 nm (220) and 0.28 nm (311), which are similar to the XRD pattern we obtained, and proves the presence of NiCo2S4 in NiCo2S4-rGO composite. Because of the low temperature of the reaction, the Ni-Co precursor has poor crystallinity. The Ni-Co precursor can be matched to Ni0.75Co0.25(CO3)0.125(OH)2·0.38H2O (JCPDS No.40–0216), as shown in Fig. S3. In general, rGO peak should be located at around 23.5°, the cracks can be due to the collapse of some crystal surfaces. The EDS spectrum further proves the presence of Co, Ni, S and C in the NiCo2S4-rGO composite (Fig. S2b). It should be noted that the signals of Pt and Cl in the EDS spectrum are attributed to the instrument itself. The above results indicate that NiCo2S4 has been successfully attached onto the surface of rGO nanosheets.
The analysis of X-ray photoelectron spectroscopy (XPS) is detailed in the Electronic Supporting Material (ESM).
Oxidase-like activity and steady-state kinetic of NiCo2S4-rGO
TMB was used as the substrate to evaluate the catalytic performance of NiCo2S4-rGO. The activity of oxidase-activity of the rGO, single NiCo2S4 were compared with NiCo2S4-rGO nanocomposite. As shown in Fig. 2a, rGO and single NiCo2S4 show weak catalytic activity for TMB oxidation. NiCo2S4-rGO nanocomposite exhibited good catalytic activity, and the increase of catalytic activity can be attributed to the hierarchical porous structure of NiCo2S4 nanometer and the larger specific surface area of rGO. The significant improvement on catalytic performance of NiCo2S4-rGO is attributed to the synergistic effect of NiCo2S4 and rGO.
a UV-Vis absorption spectra and visual color changes of TMB, TMB + rGO, TMB + NiCo2S4-rGO, TMB+ NiCo2S4, respectively. Conditions: pH 4.0 acetate buffer, TMB 0.6 mM, catalyst 1 mg·mL−1, 25 °C for 35 min incubation. b NiCo2S4-rGO composite material catalyzing the oxidation of various substrates to produce different colors
To further confirm the oxidase-like catalytic activity of NiCo2S4-rGO composite, the oxidation reactions of two typical oxidase substrates of TMB and OPD were investigated, showing blue and light yellow in the process of oxidation. Their oxidized products have the maximum absorbance peaks at 652 and 450 nm, respectively (Fig. 2b). These phenomena prove that the intrinsic oxidase-like property of NiCo2S4-rGO composite. The dependency of the catalytic activity of the NiCo2S4-rGO composite material on TMB concentration and interaction time were also studied (Fig. S5a-b). The catalytic activity of NiCo2S4-rGO was evaluated under different temperatures. As shown in Fig. S5c, NiCo2S4-rGO showed higher oxidase activity in a wide temperature range, particularly near room temperature. It has been reported that the catalytic activity of rGO/Cu8S5/PPy depends largely on pH value, for determination of H2O2 and phenol [33]. The influence of pH values on NiCo2S4-rGO was evaluated. The data of Fig. S5d show that the catalytic activity of NiCo2S4-rGO was dependent on pH, and the absorbance reaches the maximum value at pH = 4. Accordingly, pH and temperature was set as 4.0 and 25 °C, respectively, as the optimum conditions. Moreover, over 90% of the catalytic capability of the NiCo2S4-rGO is still retained even after 20 days (Fig. S6), indicating good stability.
In order to study the oxidase activity of NiCo2S4-rGO, TMB was used as a single variable for steady-state kinetic analysis under the mechanism of Michaelis-Menten kinetics (Fig. S7). By applying the molar attenuation coefficient of 39,000 M−1 cm−1, the absorbance value was converted to the blue product concentrations. According to the Michaelis-Menten equation: 1/v = (Km/Vmax) (1/[S]) + 1/Vmax, the Michaelis constant (Km) represents the intensity of intermolecular binding affinity, thus the Km value was calculated with the Line-weaver-Burk dual reciprocal graph. The results are summarized in Table S1.
Possible mechanism of oxidase-like activity
To find out the possible mechanism of oxidase-like activity of NiCo2S4-rGO, the O2-dependent catalytic oxidation experiment of TMB was performed. As shown in Fig. 3a, the catalytic activity increased significantly under O2-saturaed condition. However, the catalytic activity of NiCo2S4-rGO was inhibited under N2-staturated condition, indicating the importance of dissolved oxygen in the reaction system. The catalytic mechanism of this mimic nano-oxidase may be due to the capability for activation of O2 to generate reactive oxygen species (ROS) in the TMB oxidation reaction [34]. As shown in Fig. 3b, the presence of TBA had no obvious effect on the reaction, indicating a few of •OH was produced. SOD, PBQ were used to scavenge superoxide radicals (O2•−), and NaN3 was selected to trap singlet molecular oxygen atoms (1O2). After adding these scavengers, the absorbance intensity significantly decreased (Fig. 3b, c). The results indicate generation of 1O2 and O2•− in the catalytic process. In a word, with NiCo2S4-rGO acting as catalysts to activate oxygen molecules, generating reactive oxygen intermediates (1O2 and O2•−). The produced ROS trap the electrons supplied by substrates, and then oxidized the substrates [35, 36]. During the entire process, the function of NiCo2S4-rGO composites is similar to that of oxidases.
a UV-Vis spectrum of NiCo2S4-rGO-TMB system in N2/O2 saturated system under optimal conditions. b Inhibition of different scavenger for NiCo2S4-rGO-TMB system. c Effect of SOD with different concentration for NiCo2S4-rGO-TMB system. Reaction conditions: 0.6 mM TMB; 45 μg·L−1 NiCo2S4-rGO; 15 min reaction time; pH 4.0; room temperature
Performance of NiCo2 S 4 -rGO oxidase mimic for dopamine detection
The presence of dopamine inhibits oxidase activity of NiCo2S4-rGO nano-enzyme, resulting in discoloration and decreased absorbance. Based on the NiCo2S4-rGO, a reliable and low cost colorimetric method was developed for dopamine detection. Clearly, the absorbance increases with the increase of dopamine concentration (Fig. 4a). It shows good linear relationship in the range of 0.5–100 μM, and the linear regression equation can be expressed as ΔA = 0.01685 + 0.00507 Cdopamine (R2 = 0.99431, n = 16), as shown in Fig. 4b. The detection limit is as low as 0.42 μM, which was calculated through the equation LOD = 3S0/K, where S0 means the standard deviation of blank measurements and K represents the slope of calibration line. Compared with other reported approaches (Table 1), the present method demonstrates high sensitivity for dopamine detection.
Selectivity
To determine the effects of interfering substances on dopamine detection in human serum, selectivity analyses were performed. The typical interferents, including CO32−, NH4+, Zn2+, Cl−, oxalate, phenylalanine (Phe), L-histidine (His), lysine (Lys) glycine (Gly), glucose (Glu), L-tyrosine (Tyr), L-cysteine (L-cys), bovine serum albumin (BSA), uric acid (UA), ascorbic acid (AA) and glutathione (GSH) were tested. As shown in Fig. 5, these compounds did not cause significant color changes except for GSH and L-cys. In practical analysis, the interference of biothiols can be resolved by N-ethylmaleimide as the masking reagent for thiols [43]. Overall, the system shows good selectivity in the determination of dopamine.
Detection of dopamine in real samples
Human serum samples were used to evaluate the determination of dopamine. The samples (5 mL) were obtained from the hospital, and the coagulant was added rapidly. These serum samples were pretreated by high speed refrigerated centrifuge. Then the supernatant was separated and stored and stored at 4 °C. It should be noted that in order to ensure the dopamine concentrations were in the linear range and reduce the effects of interference, the serum samples were diluted with ultrapure water. The calibration plot was obtained by spiking the serum samples with standard dopamine solution. The absorbance changes of the serum samples at 652 nm was recorded, then the diluted concentration of dopamine was calculated by calibration plot. In order to evaluate the accuracy of the method, standards of dopamine in various amounts were added in diluted human serum samples, and recoveries were calculated. The recoveries in human serum sample were in the range of 102.9 and 107.9% (Table 2). Thus, this method is reliable for the analysis of complex biological samples.
Conclusion
In conclusion, NiCo2S4 nanomaterials on porous reduced graphene oxide sheets were synthesized by a hydrothermal method. The NiCo2S4-rGO nanocomposites were used as an oxidase mimic system to detect dopamine. The hierarchical NiCo2S4-rGO nanocomposites exhibited superior oxidase-like activity compared to individual NiCo2S4 nanomaterials and rGO nanosheets, indicating a synergistic effect among the components in the composites. In addition, dopamine can significantly inhibit the oxidase-like activity of the NiCo2S4-rGO composite. Based on these results, a reliable and low-cost colorimetric detection method for quantitative determination of dopamine is proposed. However, the catalytic activity of this material needs to be further improved. It is not suitable for detecting substances with strong reducibility in human serum. In order to avoid excessive effects on colorimetric detection, the pre-treatment of samples is typically required. Overall, this study presents the fabrication of a new type of oxidase-like nanozyme and provides a reliable colorimetric method for dopamine detection in human serum samples.
References
Chiu C, Moss CF (2007) The role of the external ear in vertical sound localization in the free flying bat, Eptesicus fuscus. J Acoust Soc Am 121(4):2227–2235
Leaf-nosed bat, in: Encyclopædia Britannica, Encyclopædia Britannica Online, 2009
Castro SL, Zigmond MJ (2001) Stress-induced increase in extracellular dopamine in striatum: role of glutamatergic action via N-methyl-D-aspartate receptors in substantia nigra. Brain Res 901(1–2):47–54
Vonk J, Shackelford TK (2012) The Oxford handbook of comparative evolutionary psychology. In: Nathan PE (ed) Oxford library of psychology. Oxford University Press, New York, p 574
Sajid M, Nazal MK, Mansha M, Alsharaa A, Jillani SMS, Basheer C (2016) Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: a review. Trends Anal Chem 76:15–29
Park SJ, Lee SH, Yang H, Park CS, Lee C-S, Kwon OS, Park TH, Jang J (2016) Human dopamine receptor-conjugated multidimensional conducting polymer nanofiber membrane for dopamine detection. Acs Appl Mater Inter 8(42):28897–28903
Shen J, Sun C, Wu X (2017) Silver nanoprisms-based Tb(III) fluorescence sensor for highly selective detection of dopamine. Talanta 165:369–376
Cheng Y, Wu J, Guo C, Li X-G, Ding B, Li Y (2017) A facile water-stable MOF-based "off-on" fluorescent switch for label-free detection of dopamine in biological fluid. J Mater Chem B 5(13):2524–2535
Xu B, Su Y, Li L, Liu R, Lv Y (2017) Thiol-functionalized single-layered MoS2 nanosheet as a photoluminescence sensing platform via charge transfer for dopamine detection. Sensor Actuat B-Chem 246:380–388
Diaz-Diestra D, Thapa B, Beltran-Huarac J, Weiner BR, Morell G (2017) L-cysteine capped ZnS:Mn quantum dots for room-temperature detection of dopamine with high sensitivity and selectivity. Biosens Bioelectron 87:693–700
Schumacher F, Chakraborty S, Kleuser B, Gulbins E, Schwerdtle T, Aschner M, Bornhorst J (2015) Highly sensitive isotope-dilution liquid-chromatography–electrospray ionization–tandem-mass spectrometry approach to study the drug-mediated modulation of dopamine and serotonin levels in Caenorhabditis elegans. Talanta 144:71–79
Cudjoe E, Pawliszyn J (2014) Optimization of solid phase microextraction coatings for liquid chromatography mass spectrometry determination of neurotransmitters. J Chromatogr A 1341:1–7
Wang H-H, Chen X-J, Li W-T, Zhou W-H, Guo X-C, Kang W-Y, Kou D-X, Zhou Z-J, Meng Y-N, Tian Q-W, Wu S-X (2018) ZnO nanotubes supported molecularly imprinted polymers arrays as sensing materials for electrochemical detection of dopamine. Talanta 176:573–581
Ping J, Wu J, Wang Y, Ying Y (2012) Simultaneous determination of ascorbic acid, dopamine and uric acid using high-performance screen-printed graphene electrode. Biosens Bioelectron 34(1):70–76
Mashhadizadeh MH, Yousefi T, Nozad Golikand A (2012) A nickel hexacyanoferrate and poly(1-naphthol) hybrid film modified electrode used in the selective electroanalysis of dopamine. Electrochim Acta 59:321–328
Li Y, Gu Y, Zheng B, Luo L, Li C, Yan X, Zhang T, Lu N, Zhang Z (2017) A novel electrochemical biomimetic sensor based on poly(cu-AMT) with reduced graphene oxide for ultrasensitive detection of dopamine. Talanta 162:80–89
Zhang L, Qv S, Wang Z, Cheng J (2003) Determination of dopamine in single rat pheochromocytoma cell by capillary electrophoresis with amperometric detection. Electrochim Acta 792(2):381–385
Wang X-X, Wu Q, Shan Z, Huang Q-M (2011) BSA-stabilized au clusters as peroxidase mimetics for use in xanthine detection. Biosens Bioelectron 26(8):3614–3619
Yang H, Yang R, Zhang P, Qin Y, Chen T, Ye F (2017) A bimetallic (co/2Fe) metal-organic framework with oxidase and peroxidase mimicking activity for colorimetric detection of hydrogen peroxide. Microchim Acta 184(12):4629–4635
Chen Q, Liang C, Zhang X, Huang Y (2018) High oxidase-mimic activity of Fe nanoparticles embedded in an N-rich porous carbon and their application for sensing of dopamine. Talanta 182:476–483
Hammes-Schiffer S, Benkovic SJ (2006) Relating protein motion to catalysis. Annu Rev Biochem 75(1):519–541
Xing M, Wang J (2016) Nanoscaled zero valent iron/graphene composite as an efficient adsorbent for co(II) removal from aqueous solution. J Colloid Interf Sci 474:119–128
Zhu Y, Yang Z, Chi M, Li M, Wang C, Lu X (2018) Synthesis of hierarchical Co3O4@NiO core-shell nanotubes with a synergistic catalytic activity for peroxidase mimicking and colorimetric detection of dopamine. Talanta 181:431–439
Yang Z, Ma F, Zhu Y, Chen S, Wang C, Lu X (2017) A facile synthesis of CuFe2O4/Cu9S8/PPy ternary nanotubes as peroxidase mimics for the sensitive colorimetric detection of H2O2 and dopamine. Dalton T 46(34):11171–11179
Dutta S, Ray C, Mallick S, Sarkar S, Sahoo R, Negishi Y, Pal T (2015) A gel-based approach to design hierarchical CuS decorated reduced graphene oxide nanosheets for enhanced peroxidase-like activity leading to colorimetric detection of dopamine. J Phys Chem C 119(41):23790–23800
Yang Z, Zhu Y, Chi M, Wang C, Wei Y, Lu X (2018) Fabrication of cobalt ferrite/cobalt sulfide hybrid nanotubes with enhanced peroxidase-like activity for colorimetric detection of dopamine. J Colloid Interf Sci 511:383–391
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42(14):6060–6093
Qian J, Yang X, Yang Z, Zhu G, Mao H, Wang K (2015) Multiwalled carbon nanotube@reduced graphene oxide nanoribbon heterostructure: synthesis, intrinsic peroxidase-like catalytic activity, and its application in colorimetric biosensing. J Mater Chem B 3(8):1624–1632
Nasir M, Nawaz MH, Latif U, Yaqub M, Hayat A, Rahim A (2017) An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta 184(2):323–342
He W, Wu X, Liu J, Hu X, Zhang K, Hou S, Zhou W, Xie S (2010) Design of AgM bimetallic alloy nanostructures (M = au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem Mater 22(9):2988–2994
Juan Y, Chang Y, Xiaoming F, Changtai Z, Jieshan Q (2015) Ultrafast self-assembly of graphene oxide-induced monolithic NiCo–carbonate hydroxide nanowire architectures with a superior volumetric capacitance for supercapacitors. Adv Funct Mater 25(14):2109–2116
Yang J, Duan X, Guo W, Li D, Zhang H, Zheng W (2014) Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors. Nano Energy 5:74–81
Yanzhou J, Yan G, Guangdi N, Maoqiang C, Zezhou Y, Ce W, Yen W, Xiaofeng L (2017) Synthesis of rGO/Cu8S5/PPy composite nanosheets with enhanced peroxidase-like activity for sensitive colorimetric detection of H2O2 and phenol. Part Part Syst Charact 34(3):1600233
He W, Liu Y, Yuan J, Yin J-J, Wu X, Hu X, Zhang K, Liu J, Chen C, Ji Y, Guo Y (2011) Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 32(4):1139–1147
Shen X, Liu W, Gao X, Lu Z, Wu X, Gao X (2015) Mechanisms of oxidase and superoxide Dismutation-like activities of gold, silver, platinum, and palladium, and their alloys: a general way to the activation of molecular oxygen. J Am Chem Soc 137(50):15882–15891
Tao Y, Ju E, Ren J, Qu X (2015) Bifunctionalized mesoporous silica-supported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv Mater 27(6):1097–1104
Teo PS, Rameshkumar P, Pandikumar A, Jiang Z-T, Altarawneh M, Huang NM (2017) Colorimetric and visual dopamine assay based on the use of gold nanorods. Microchim Acta 184(10):4125–4132
Leng Y, Xie K, Ye L, Li G, Lu Z, He J (2015) Gold-nanoparticle-based colorimetric array for detection of dopamine in urine and serum. Talanta 139:89–95
Chen X, Liu Q, Liu M, Zhang X, Lin S, Chen Y, Zhuang J, Yang D-P (2018) Protein-templated Fe2O3 microspheres for highly sensitive amperometric detection of dopamine. Microchim Acta 185(7):340
Zhao D, Yu G, Tian K, Xu C (2016) A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens Bioelectron 82:119–126
Wang B, M-m C, H-q Z, Wen W, Zhang X-h, S-f W (2017) A simple and sensitive fluorometric dopamine assay based on silica-coated CdTe quantum dots. Microchim Acta 184(9):3189–3196
Liu X, Zhang W, Huang L, Hu N, Liu W, Liu Y, Li S, Yang C, Suo Y, Wang J (2018) Fluorometric determination of dopamine by using molybdenum disulfide quantum dots. Microchim Acta 185(4):234
Xue Q, Cao X, Zhang C, Xian Y (2018) Polydopamine nanodots are viable probes for fluorometric determination of the activity of alkaline phosphatase via the in situ regulation of a redox reaction triggered by the enzyme. Microchim Acta 185(4):231
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (21305097) and the 2016 undergraduate innovation training program of Sichuan Agricultural University (04054674).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
The authors wish it to be known that, in their opinions, Yanying Wang, Li Yang and Yaqin Liu should be regarded as joint First Authors.
Electronic supplementary material
ESM 1
(DOC 7182 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Yang, L., Liu, Y. et al. Colorimetric determination of dopamine by exploiting the enhanced oxidase mimicking activity of hierarchical NiCo2S4-rGO composites. Microchim Acta 185, 496 (2018). https://doi.org/10.1007/s00604-018-3035-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3035-8