Abstract
Various kinds of nanomaterials have been described in recent years that represent stable and low-cost alternatives to biomolecules (such as enzymes) for use in (bio)analytical methods. The materials typically include, metal/metal oxides, metal complexes, nanocomposites, porphyrins, phthalocyanines, smart polymers, and carbonaceous nanomaterials. Due to their biomimetic and other properties, such nano-materials may replace natural enzymes in chemical sensors, biosensors, and in various kinds of bioassays. This overview (with 252 references) highlights the analytical potential of such nanomaterials. It is divided into sections on (a) the types of nanomaterials according to their intrinsic nature, (b) non-enzymatic sensor designs (including electrochemical, colorimetric, fluorescent and chemiluminescent methods), and (c), applications of non-enzymatic sensors in the biomedical, environmental and food analysis fields. We finally address current challenges and future directions.

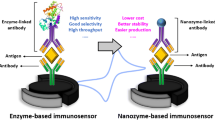
This review discusses different types of nanomaterials, which are explored as a potential biomimetic material to replace the natural enzyme in the field of biosensors, and have found widespread applications in biomedical, food and environmental analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biosensors and bioassays can replace many of the classical analytical methods such as chromatography, photometry, or mass spectrometry. The most commonly used bio-receptor elements in the design of biosensors are enzymes, antibodies and DNA aptamers [1]. However, some novel bio-recognition elements such as modified enzymes, nanobodies, peptides, affibody, protein scaffolds and DNAzymes have also been recently integrated in the design of biosensors [2]. Despite much progress, increased interest in exploration of novel bio-receptors applicable in sensing methodologies to fulfill the necessity of control specific and cost effective assay still exists. In this context, significant work has been done in exploration of enzyme mimetics to replace natural enzymes in the construction of enzymatic assays. Some level of success has been achieved in this direction with the help of chemically synthesized alternatives. Among enzyme mimetics, non-natural ribosomes and deoxyribosomes have been developed through in vitro selection processes [3]. Other enzyme mimetics including cytochrome P450 mimetic [4], serine proteases mimetics [5], dioxygenase [6], phosphodiesterase [7], ligase [8], nuclease [9] and methanogenesis [10] mimetics have been explored. Alternative to natural and chemical bio-recognition element, nano-receptors have been emerged as a promising receptor material in detection systems for laboratory and field-based applications with the advent of nanotechnology. Recently, nanomaterials have attracted tremendous interest in enzyme mimetic research because of their several distinct properties, such as high surface area to volume ratio, abundance of reactive groups on their surface, unique optical properties and fascinating catalytic activity. To date, catalase-, oxidase-, peroxidase-like activities have been demonstrated for various types of nanomaterials [11]. These nanomaterial-based enzyme mimetics offer advantages of low cost, high stability and sustained catalytic activities. As a consequence, biosensors based on these nanomaterials have been extensively employed for many applications in the bioassay, biotechnology and biomedical field.
With the advent of novel nanostructures and new interface materials, these nano-recognition elements will be major players in future sensors development. Similarly, important advances have been achieved with utilization of nanomaterials in the design of electrochemical sensors to improve their analytical performance. Electrochemical biosensors have been traditionally based on bio-receptor elements for highly specific and selective detection of various analytes, while direct electrochemical detection of electroactive analytes is also possible on transducer surface. However, direct electrochemistry based on redox properties of analyte has poor selectivity, sensitivity and accuracy. To overcome these limitations, analyte specific nanomaterials are attracting increasing interest, thanks to their low cost, simple operation, high sensitivity and fast response [12]. The analyte specific nanomaterials permit a good orientation of moieties onto the electrode surface, decreases distance between electro-active species and transducer surface, thus improving electron transfer rate. Keeping in view the important role of nanomaterials in (bio) sensors design, this review paper will focus on the analytical potential of biomimetic and analyte specific nanomaterials. We will discuss different types of nanomaterials according to their intrinsic nature, detection methodologies employed in non-enzymatic assays and applications of non-enzymatic sensors in the biomedical, environmental and food analysis fields.
Biomimetic nanomaterials
In this section, we will review numerous biomimetic nanomaterials that can replace natural enzymes for sensing applications. Among them, cerium oxide (ceria) nanoparticles are famous for its high catalytic performance in several applications owing to the presence of both Ce+3 and Ce+4 oxidation states and in the presence of oxygen vacancies [11, 13, 14]. The presence of this redox couple and switching of ceria oxidation states (between Ce3+ and Ce4+) makes it a promising material for the fabrication of amperometric biosensors [15]. Nanoceria have been found a promising material for enzyme free detection of different analytes [12]. Wang et al. [16] studied the determination of hydrogen peroxide, glucose and cholesterol by using the respective oxidase and hydrogen peroxide formation with Cu2(OH)3Cl-CeO2 nanocomposite. Iron oxide nanomaterials have also found many applications such as in hyperthermia treatment of cancer [17], targeted drug delivery [18], contrast agents in magnetic resonance imaging [19], magnetic storage media magnetic inks, capture and separation of analysts, biosensing applications etc. [20]. They have a potential to be used in developing different point of care (bio) sensors. Gao et al. [21] unexpectedly discovered the peroxidase like properties of iron oxide magnetic nanoparticles. Since then, a lot of work has been done to explore the biomimetic nature of these iron oxide magnetic nanoparticles. Peroxides play an important role in defending against pathogens and detoxification of reactive oxygen species by catalyzing the oxidation of substrates with peroxides. They used nanoparticles of different sizes to study the effect of particles size on activity of these nanoparticles, and concluded that lower sized nanoparticles showed better response than larger ones. Similarly, manganese dioxide materials with different structural morphologies such as nanowires, nanosheets, nanospheres etc. have been used for their enzyme like properties [22]. Soundappan et al. [23] fabricated manganese oxide electrochemically on indium tin oxide glass electrode for the detection of hydrogen peroxide [24]. Other metal oxides such as zinc oxide [25], copper oxide [26], vanadium oxide [27], AgVO3 [28], cobalt oxide [29] nanomaterials have been reported for showing intrinsic enzyme like properties. Furthermore, metal sulfides such as magnetic Fe3S4 nanoparticles and molybdenum nanosheets have shown peroxidase like activity for the detection of glucose and mercury ions respectively [30, 31].
In addition to metal oxide nanoparticles, metal nanoparticles also have been investigated extensively for their inherent enzyme mimic actions. Gold nanoparticles (AuNPs) have been widely studied for catalytic and biomedical applications due to their excellent biocompatibility, unique chemical, physical, electronic properties, high density and high surface to volume ratio [11]. Comotti et al. [32] studied citrate-coated gold nanoparticles catalyzed aerobic oxidation of glucose. Their proposed mechanism was similar to natural enzymatic activity i.e. glucose oxidase. Li et al. [33] used mercury coated gold nanoparticles as peroxidase mimic for the determination of metallothioneins. Likewise, some other metal nanoparticles including platinum [34] and silver [11] have also been studied as enzyme mimics.
Carbon based nanomaterials, such as graphene [35], fullerene [36], carbon nanotubes [37] have received considerable attention as enzyme mimetic in biosensors. The significant attention towards the application of carbon nanomaterials for biosensors is due to their ability to improve electron-transfer kinetics, high surface-to-volume ratios and biocompatibility associated with their unique morphology. Fullerenes and their derivatives, since their discovery, have been used in various applications such as drug delivery, nanomedicines diagnostics etc., due to their distinctive chemical reactivity and being water insoluble nature. Fullerene has been modified with different water soluble functional groups to make it soluble in water and to be able to use for biomedical applications and exploring enzyme like activities [38]. Similarly, the intrinsic enzyme like activity of single walled carbon nanotubes and multi-walled carbon nanotubes has been reported by many groups [39–42]. The activity of these single walled and multiple walled carbon nanotubes depends upon their surface area, pH, and temperature. Graphene oxide as a promising carbon material was also found to retain intrinsic enzyme like properties as reported by Qu et al. [43]. Chen et al. [44] used reduced graphene oxide nanosheets, functionalized with poly(styrene sulfonate) and used for the colorimetric determination of ascorbic acid. In the same context, next section will focus on the analyte specific nano-materials for various analytes.
Generally, metals are good conductors and hence have low limit of detection. However, they are less stable because of the surface poising due to surface oxidation. Also, metals like platinum, gold, silver etc. are very expensive and the synthesis of their nanoparticles is a complicated procedure. Metal oxide nanoparticles are emerging in the field of biosensors during recent years due to their less complicated synthesis, more stability, less expensive and easy availability [45]. However, they are not very sensitive. To increase their sensitivity, they are to be doped with other metal and non-metal dopants. Carbon materials on the other hand are being non-metallic in nature have high conductivity. The study of their biosensing properties is hot topic in biosensors due to their ease of functionalization, less expensive, highly stable and non-metallic nature which is less poisonous as compared to metals and metal oxides.
Substantial developments have been achieved in synthesis procedures in producing diverse nanomaterials with highly controllable shape, size, surface charge and physicochemical properties [46–48]. The introduction of such nanomaterials has greatly enhanced the sensitivity and selectivity for the direct detection of various analytes, without requirement of any additional dye. Over the past few decades, researchers have synthesized a huge number of nano-materials which have been employed for direct recognition of various analytes on transducer platform.
For example, carbon nanomaterials (CNTs) exhibit numerous fascinating features, which make them suitable for developing electrode materials [49–52]. Peng and co-workers [53] highlighted the functionalization of carbon nanotubes with polyethylenimine for electrochemical detection of chromium (VI) in the concentration range from 0.002–20 μmol L−1, with a detection limit of 0.0006 μmol L−1. CNTs have also been used for electrochemical detection of guanine [54] heavy metals [55], fungicide [56] pharmaceutical formulations [57, 58], phenolic compounds [59, 60] hydrazine [61], nitric oxide [62] and explosives [63]. Similarly, graphene and its derivatives such as graphene oxide, functionalized and doped graphene materials, have also been widely employed for direct recognition of different analytes, as an alternative to direct detection based on the natural enzyme recognition. These materials show unique electronic properties, high chemical stability, large surface-to-volume ratio, good mechanical strength, wide electrochemical window, and excellent support substrate. Until now, graphene have extensively been explored as electrochemical sensors for the direct detection of glucose [64–67], hydrogen peroxide [68–71], neurotransmitters [72] and estriol [73]. Single-walled carbon nanohorns (SWCNHs) is yet another emerging class of carbon-based nanomaterials. They possess a conical shape with a tubular length of ~50 nm and base diameter of ~5 nm. SWCNHs provided good results when employed for the electrochemical assays of different analytes such as hydrazine [74, 75], hydrogen peroxide [75] and bisphenol A [76].
Silica-based materials have strong inorganic solids backbone having large surface area and a three-dimensional structure constitute of highly interconnected open spaces, resulting to generate highly porous structure materials. A variety of possible chemical modifications can be performed on its surface due to its porous nature, which leads to a variety of materials with diverse functionalities and properties [77–79]. Rahim et al. [80] reported in situ synthesis of transition metal phthalocyanines (MPcs) in pores of SiO2/C mesoporous materials. These hybrid materials SiO2/C/MPcs were used as pressed disks to construct carbon ceramic electrode (working electrode) and tested as electrochemical sensor for analyte specific detection of nitrite, dissolved oxygen [81, 82], dopamine [83], ascorbic acid [84], 4-aminophenole [85], oxalic acid [86], and fenitrothion pesticide [87]. Silica based materials were also modified with organic groups, ionic liquids, metal and metal oxides, tested as analytes specific materials for detection of NADH [88, 89], nitrite [90], phenolic compounds [91], dopamine [92] and hydrogen peroxide [93]. These hybrid nanomaterials exhibited excellent activity towards specific detection of target analytes as compared to those based on single component nanomaterial. The composite materials enhance the fast electron transfer involved during redox process. A brief overview on different types of biomimetic nano-materials is provided in the Table 1.
The next section of the review paper will discuss the methodologies to integrate these biomimetic nanomaterials in the development of enzyme mimicking optical or electrochemical assays.
Electrochemical and optical detection using enzyme mimicking nanomaterials
Electrochemical detection
Electrochemical transducers are most widely employed in developing bio/chemo-sensors for various analytes because of its simple fabrication, low-cost, sensitivity, portability, and easy-to-use features [116]. In electrochemical sensors, electrochemical reactions are monitored by measuring a change in current (amperometry), change in potential (potentiometry), change in conduction between electrodes (conductometry), or both resistance and reactance (electrochemical impedance spectroscopy) [46, 117]. Thus, electrochemical sensor devices are commonly divided into three main classes based on the parameter to be measured such as current, potential, and impedance. The detection device based on electrochemical principle, which demonstrates three prime components: (1) analyte, (2) transducer, and (3) signal processing unit.
Electrochemical detection based analytical methods have wedged various fields, comprising environmental analysis, diagnostics, food sciences, pharmacology and enzymatic kinetics [118–120]. Recently, the exploration of more effective transfer of electron between the enzyme active site and the surface of electrode helps to deduce more sensitivity to the biosensors. Furthermore, the enzyme presented low stability with time, pH and temperature has given motivation to explore modifications in the electrochemical biosensors designs. The new substitute is based on to utilize artificial enzymes [121–124] and biomimetic materials [125–127] that attempts to imitate natural enzymes with the same efficiency and selectivity, which can be used to fabricate electrochemical sensors and biosensors with higher sensitivity. In such devices, the electrode surface will be modified by the immobilization of redox substance which act as an enzyme active site that catalyzes the substrate conversion in the same manner. This idea has been described in the literature [83, 92, 128–131], however, still exists a great area to be explored.
Non-enzymatic electrocatalysts exist in various forms, pure metals, alloys and bimetallic system, metal metal-oxide heterogeneous catalyst, metal phthalocyanine and porphyrin complexes, carbon-based materials and boron diamond. All these materials except the last two rely on the transition metal. The process of electrocatalysis usually occur via the adsorption of the analyte to the surface of electrode, which probably encompasses the formation of appropriate bonds between the analyte and the d-electrons and the d-orbitals of the metal substrate [132]. This bond formation and breaking is essential in the midst of catalytic process, the bond of intermediate strength would be ideal and so as not limited to adsorption or hamper adsorption at any instant. The strength of bond is dependent on the Gibbs free energy of adsorption. Otherwise a change in bond strength may be execute an alteration in the metal oxidation state, which may change the metal-adsorbate interaction and boost desorption of the product [132].
Burke [133] suggested a model known as ‘Incipient Hydrous Oxide Adatom Mediator’ (IHOAM) for the electrooxidation of different molecules on metal surface. This model depends on the surveillance that ‘active’ metal surface atoms underwent a pre-monolayer oxidation phase that forms an incipient hydrous oxide layer of reactive OHads, which arbitrate oxidation and hinder reduction of kinetically sluggish electrode reactions. The active sites on the electrode surface have low lattice coordination value and lattice stabilization energy.
Owing to the low stability they are highly reactive, which go through pre-monolayer oxidation at a lower potential than thermodynamic surface oxidation products. The catalytic significance of the active OHads layer was well known as compared to small organic compound oxidation, and the emergence of the hydrous species was identified as a fast, pre-oxidation step succeeding chemisorption of the glucose molecule. The hydrous pre-monolayer then mediates oxidation of the adsorbed species at a lower potential. Agnihotri et al. [134] fabricated non-enzymatic sensor for cholesterol using β-cyclodextrin functionalized graphene. Graphene wrapped Cu2O nanocubes were synthesized by Wei et al. [135] can mimic both oxidase and peroxidase like activity and used for electrochemical detection of glucose and hydrogen peroxide with detection limit of 3.3 μM and 20.8 μM, respectively. Dashtestani et al. [136] fabricated amperometric sensor for superoxide anions based on nanocomposite consisting of gold nanoparticles and the copper (II) complex of cysteine (GNP/Cu-Cys) which mimic the enzyme superoxide dismutase (SOD), with a linear range from 3.1 to 326 μM, a detection limit of 2.8 μM and a sensitivity of 0.018 μA μM cm−2.
Metal nanomaterials such as gold [110, 137, 138], platinum [139, 140], nickle [141–143], palladium [144, 145], and a variety of bimetal nanomaterials [146–149] are potential candidates to mimic natural enzymes. Many metal oxide-based nanomaterials such as cobalt oxide [150], cerium oxide [151, 152], copper oxide [97, 153, 154], manganese oxide [155, 156], vanadium pentoxide [27], and iron oxide [157] have also been explored to imitate natural enzyme. Some metal complexes such as metallophthalocyanines [83, 84] and metal Schiff-base complexes [92] were also reported to exhibit tremendous electrocatalytic activities toward oxidation of dopamine which mimetize dopamine monooxygenase enzyme. The electrochemical detection methods have advantages over the other existing techniques due to its sensitivity, fast response, negligible interference effect and robustness. Different nanomaterials were used as an enzyme mimics for electrochemical assays of different analytes are summarized Table 2. In the upcoming section we will discuss the colorimetric detection techniques by using various nano-materials as enzyme mimetics.
Colorimetric detection
Optical sensing methods have gathered great interest due to their intrinsic high sensitivity and simplicity. Compared to other analytical techniques for the detection of analytes, the colorimetric methods are much simpler and their response can be detected with bare eyes or by photometry [162].
Metal nanoparticles produce an intense absorption due to the movement of conduction band electron when these are excited with electromagnetic waves. In noble metals these localized electrons results in high absorption and scattering which lies in UV-Visible region making them perfect candidate for the colorimetric detections and applications. Also, aggregation of the metal nanoparticles leads to significant change in the color as compared to their color in dispersed state. Most of the metal based colorimetric biosensors are based on mechanism of changing color because of coupling phenomenon due to aggregation of nanoparticles. The change in color may also be due to oxidation/reduction reaction between nanoparticles and analyte [163]. Zhao et al. [164] synthesized modified molybdenum sulphide nanoparticles and fabricated a biosensor for colorimetric detection of hydrogen peroxide and glucose. They presented that cation surfactant modified molybdenum sulphide nanoparticles showed better affinity with positively charged 3,3,5,5, tetramethylbenzidine (TMB). The intensity of the absorption of TMB was increased at 652 nm as concentration of hydrogen peroxide increased in the sample. Similarly, concentration of glucose also changed color intensity of TMB in presence of surfactant modified molybdenum nanoparticles. Cao et al. [165] prepared horseradish peroxidase (HRP) functionalized gold nanoclusters for the colorimetric detection of melamine triggered by visible light at very low concentrations. Similarly, Wang et al. [166] synthesized magnetic mesoporous silica nanoparticles for detection of hydrogen peroxide and glucose calorimetrically through TMB. The magnetic silica nanoparticles oxidized glucose by oxygen to gluconic acid and hydrogen peroxide. This hydrogen peroxide further reacted with TMB which results in color change of solution.
Fluorescent detection
Recently, fluorescent based systems sparked tremendous interests in biosensing field, which in general consist of a recognition module for the target analyte and a fluorescent transducer (normally a fluorescent dye) that produces intensity or wavelength changes of the fluorescent signals on interaction of analyte with recognition element [167]. Recent advancements in the field of nanomaterials have resolved the problem of lower quantum yield and extinction coefficient, by developing nanomaterials having unique optical properties. Variety of nanomaterials, as synthetic fluorophores, have been used as powerful alternative transducers (enzyme mimics) in fluorescent biosensors with an emphasis on improved signal strength and detection limits. Hu et al. reported catalyzed decomposition of hydrogen peroxide by cupric oxide performing peroxidase like activity with a highly fluorescent product [98]. Moreover, glucose or L-lactate were also catalyzed for their detection by cupric oxide nanoparticles with improved detection limits. Shi et al. developed Fe3O4 magnetic microspheres based fluorescent system for the detection of Glucose and p-nitrophenol in human serum and water samples, respectively to mimic peroxidase like activity [168]. The analytes were detected via quantitative measurement of fluorescence production with lower detection limit high sensitivity, good selectivity. The developed system had peachy potential in the sensing and biosensing of variety of H2O2 based analytes. Similarly, Qiu’s group utilized cerium coordinated polymer nanoparticles as artificial peroxidase for selective and sensitive detection of hydrogen peroxide [169]. Free radical scavenging property of these cerium compounds improved the sensitivity and selectivity where H2O2 headed the oxidation of fluorescent cerium nanoparticles to non-fluorescent products which was ultimately quantitatively detected by fluorescence measurements with very low detection limit.
In addition to these fluorescent based biosensors working on the principal of fluorescence ON/OFF switching by nanomaterials, pronounced efforts have been made in this field with composite materials and modified nanomaterials. Moreover, quantitative measurement of fluorescence quenching phenomenon is also one of the effective tools of modern fluorescent biosensors. In this direction numerous materials have been used for biosensing applications such as, bovine serum albumin (BSA) protected Au nanoparticles were used to detect salicylaldehyde and Zinc ions [170]. Gold nanoparticles (AuNP) are the most widely studied material in the ever expanding world of nanomaterials. Chang et al. [171] used protected gold nanoparticles for hydrogen peroxide and cholesterol detection via fluorescent detection. Similarly, Huang et al. [172] used core-shell structured magnetic nanoparticles to detect cholesterol, use of nanoparticles improved the limit of detection and sensitivity of these fluorescence based biosensors. Overall, number of nanomaterials and composites have been used so far, as fluorescent and chemiluminescent probes mimicking the biomolecules as have been detailed in the Table 3.
Chemiluminescence detection
Furthermore, a newer technique chemiluminescence (CL) is becoming important due to its higher sensitivity, rapidness, easier protocol and cost effective detection. Shi et al. used Co doped magnetic nanoparticles (CoFe2O4) for the first time for non-enzymatic chemiluminescence based sensitive detection of hydrogen peroxide and glucose [177]. The nanomaterial was more stable and sensitive as compared to the enzymes, which opened newer ways for the researchers and dragged several groups to work on similar lines.
Chen et al. employed cupric oxide nanoparticles with luminol-H2O2 system for the highly sensitive detection of hydrogen peroxide [100]. The CuO NPs enhanced the peroxidase mimicking of the nanocomposite 400 hundred times, due to its peroxidase like activity to decompose hydrogen peroxide into hydroxyl radicals. Similarly, another group utilized CoFe2O4 magnetic nanoparticles for luminol-H2O2 chemiluminescence system and found that the nanocomposite performed 10 times better catalytic activity, which was quenched on addition of trace levels of Ag (I). This phenomenon was further utilized for sensitive detection of Ag (I) from water samples with a detection limit of 0.15 ng mL−1.
Chemiluminescence has been utilized for determination of glucose using glucose oxidase which produces gluconic acid and hydrogen peroxide. The hydrogen peroxide can produce chemiluminescent signals on reacting with luminescent materials like luminol, rhodamine derivatives etc. which ultimately can be quantized in terms of concentration of glucose [179, 180].
Similarly, luminol assisted cupric oxide nanoparticles-based chemiluminescence sensor have been fabricated for cholesterol monitoring. Peroxidase-like activity of cupric oxide nanoparticles were used to oxidize luminol by hydrogen peroxide produced by cholesterol oxidase. Combination of these reactions resulted in chemiluminescence of luminol, the intensity of which was directly proportional to the cholesterol concentration, as have been demonstrated in the following Fig. 1 [76, 102].
Schematic representation of cupric oxide nanoparticles-based chemiluminescence sensor for cholesterol detection. Peroxidase-like activity of cupric oxide nanoparticles was used to oxidize luminol by hydrogen peroxide produced by cholesterol oxidase. Combination of these reactions resulted in chemiluminescence of luminol, the intensity of which was directly proportional to the cholesterol concentration [102]
Applications of non-enzymatic assays
Biomedical assays
A brief overview regarding the use of these enzyme mimetic materials in fabricating biomedical assays for various analytes is given below:
Hydrogen peroxide and glucose detection
The detection of hydrogen peroxide is very much important in biological, clinical, environmental, and food industry. Over the past few years, several enzyme mimetics have been synthesized and subsequently utilized in developing sensor devices for hydrogen peroxide and glucose detection. AgVO3 nanobelts exhibit peroxidase like activity and was used for detecting H2O2 [28]. The production of CoFe2O4 nanotubes from electrospinning technique and subsequent functionalization with PdNPs enhances their peroxidase mimetic activity for sensitive detection of H2O2 [181]. A peroxidase mimetic layered double hydroxide (LDH) was prepared by electrostatic interaction between negatively charged carbon-dots and positively charged NiAl-LDH nanoplates [182]. A colorimetric sensor device for hydrogen peroxide was also fabricated by growing prussian blue (PB) on microporous MOFs having peroxidase like activity [183]. Magnetic nanoparticles of iron oxide also exhibit peroxidase-like activity. Other peroxidase like nanozymes including nanostructured layered double hydroxide [184], polyoxometalate/carboxyl-modified mesoporous polymers [185], and copper sulphide (concave structures) were used in sensing H2O2 and glucose [186]. FeS nano-sheets or nano needles furnish high surface area in comparison to nanoparticles [187, 188]. These nanostructures were used to fabricate electrochemical sensor for H2O2 based on their peroxidase like activity. More peroxidase mimics of ferrites were explored by doping Bi and Eu in FeO3 and Co, Zn, Mn in Fe2O4. Other peroxidase mimics were also explored such as PtPd porous nanorods [189], carbon nanodots (CDs), and PtPd nanodendrites on graphene nanosheets [190]. One dimensional PtPd porous nanorods exhibits lower detection limit of 8.6 nM for analyzing H2O2 released from living cells in comparison to all other nanostructures fabricated for detecting H2O2 [189]. PtNPs conjugated to apoferritin can mimic both catalase and peroxidase like activity depending on pH and temperature. Gold nanomaterials showed oxidase like properties and can serve as glucose oxidase (GOx) mimic and can convert adsorbed glucose to gluconic acid and H2O2. Enzyme mimic of AuNPs with other metals were also explored. Peroxidase like activity of Bi-AuNPs was used to develop fluorescent sensor for thrombin. Silver-based nanozymes (peroxidase-like) hybrid with other metals (Au, Pt, and Pd) were explored for monitoring ascorbic acid concentration [191]. In hybrid nanozymes, the polarization effects were induced in Fe3O4 by doping gold which enhances reduction property of Fe3O4 MNPs. Similarly, enhanced sensing capacity of Fe3O4 towards H2O2 was achieved by adding AgNPs [192]. Pronounced activity was also observed in FeTe nanorods in comparison to Fe3O4 MNPs due to its large surface area [193]. Peroxidase like activities were also observed in different forms of carbon materials such as carbon nanotubes (CNTs), graphene, carbon-nanodots, and carbon nanoparticles. Activities of CNTs are dependent on pH, temperature, H2O2, Fe contents and as well as its shape. The helical shape CNTs are more catalytically active than MWCNTs and expresses greater affinity to H2O2 than MNPs. Higher activity of graphene was achieved by combining it with hemin to detect DNA. However, peroxidase like activity of gold-modified graphene was inhibited due to adsorption of DNA which later on restored after releasing or cleavage of DNA resulting in developing a sensor for DNA. On the basis of this phenomenon, an insulin sensor was fabricated based on affinities of anti-insulin and insulin-aptamer complex [11]. A composite of V2O3 and mesoporous carbon also exhibits peroxidase like activity and utilized for colorimetric detection of H2O2 and glucose [194]. Other peroxidase mimics used for analyzing H2O2 and glucose are: VO2 nanobelts [105], MnSe-loaded graphene carbon nitride [195], graphene dots [196], and N-doped graphene QDs [197]. Hydrothermal synthesis of monocrystalline VO2 nanobelts possess an outstanding catalytic activity and exhibits very low detection limit of 0.65 μM towards glucose in comparison to all other nanomaterials used for glucose detection [105].
Cholesterol detection
Graphene shows higher affinity to targeted analytes than natural enzymes due to high surface area. Graphene-hemin hybrid nanosheets (nanozyme) express higher response to H2O2 than HRP. Graphene or graphene oxide based hybrid materials as nanozymes shows higher enzyme mimetic activities due to synergic effect. A synergic effect in peroxidase like properties was also observed in nanoribbons based on MWCNTS-rGO. This synergic effect is attributed to the higher electron-transfer rate in this heterostructure which is 15.9 folds higher than MWCNTs alone. This MWCNTs-rGO heterostructure was utilized to develop a selective colorimetric sensor system for cholesterol [198]. The incorporation of ZnO NPs on CNTs surface exhibit a synergistic peroxidase mimetic property for fabricating a sensor device for colorimetric detection of cholesterol with a detection limit of 0.2 nM and concentration range of 0.5–500 nM in comparison to all other sensors fabricated for cholesterol detection [199]. Hong et al. fabricated a chemiluminescent sensor for cholesterol based on peroxidase mimetic cupric oxide nanoparticles which shows LOD of 0.17 μM [102].
Other analytes
Different nanozymes based on metals, bi & tri-metals, metal oxides, metal-graphene hybrids have also been used in fabricating biomedical assays for detecting various analytes. Nanozymes based on AuNPs as peroxidase mimetics were utilized to detect alanine [200], and IgG [201], whereas, xanthine [202], choline and acetylcholine [203] was monitored by using nitrogen supported sulfur doped PdNPs and PtNPs in BSA scaffold. Moreover, bimetals (Pt/Au NPs) and rod-shaped nanostructures of trimetals (Au/Pt/Cu) were synthesized to analyze E-coli [204] and IgG [205], respectively. Enzyme mimetic hybrids of CuS and gold with graphene were used to detect respiratory syncytial virus [206], dopamine [207], DNA [208], and miRNA-21 [209]. Nanozymes that were used in developing biomedical assays for various analytes are summarized in the Table 4.
Environmental monitoring
In this part of review, environmental application of enzyme mimics would be slightly discussed and summarized in tabular form.
Chai et al. synthesized silver nanoparticles on silver phosphate microcubes (Ag@Ag3PO4MCs) microcubes by reacting [Ag (NH3)2]+ and Na2HPO4 under UV light for detecting Hg2+ ions selectively [216]. These microcubes exhibit oxidase-like activity and capable to oxidize 3,3′,5,5′-tetramethylbenzidine (TMB) and o-phenylenediamine (OPD) in slightly acidic media and dissolved oxygen. The oxidase-like properties of Ag@Ag3PO4 microcubes greatly enhanced by adding AgNPs (silver nanoparticles) in it which results in quicker oxidation of TMB and OPD. Mercury ions act as inhibitor for enzyme activity due to a very specific interaction with Ag and thus, on the basis of this inhibition property a colorimetric sensor for mercury ions were fabricated with LOD of 0.253 nM. Zhang et al. synthesized a very efficient hybrid material (Au/Fe3O4/GO) with enhanced nanozyme activity for ultrasensitive colorimetric detection of Hg2+ in aqueous solution with a detection limit as low as 0.15 nM [217]. The hybrid material was prepared by attaching gold and Fe3O4 nanoparticles on graphene oxide (GO). This ternary hybrid (Au/Fe3O4/GO) offers very lower detection limit in comparison to all other nanozymes used for detecting Hg2+. The graphene oxide in ternary hybrid furnishes large surface area for anchoring gold and Fe3O4 nanoparticles which reduces the chances for particle agglomeration and results in maximum enzymatic activity of gold for colorimetric detection of mercury ions. However, maximum detection ranges of 0–100 μM towards mercury ions was observed by using magnetic nanoparticles (MNPs) attached with target specific single-stranded DNA (ssDNA). Peroxidase mimetics of Pt were synthesized by using bovine serum albumin (BSA) as nucleation template for colorimetric detection of Hg2+ [218]. This BSA stabilized Pt nanozyme possess excellent peroxidase activity towards TMB and Hg2+ was selectively detected via Hg2+-Pt0 interaction by suppressing enzymatic activity of PtNPs. The lowest detection limit of 602 pM towards Pb2+ was achieved in case of colorimetric aptasensor triple-helix molecular switch (THMS) and enzyme mimetic AuNPs. However, maximum detection range for lead ions was observed in a nanozyme based on catechin-synthesized AuNPs.
Nanoceria has been synthesized to develop a colorimetric assay for the detection of toxic substance catechol with detection limit of 0.2 μM [219]. Peroxidase like activity of Cu2+ was utilized to develop a colorimetric sensor for detecting a very toxic pollutant glyphosate by using TMB as substrate [113]. Peroxidase mimetic magnetic nanoparticles were used for colorimetric detection of organophosphorus pesticides and nerve agents. Whereas, these magnetic nanoparticles were also utilized for selective detection of ethoprophos (pesticide) via chemiluminescence (CL) method [114, 115]. Highest detection range (0.1 nM - 100 μM) and lowest detection limit of 0.1 nM was observed towards ethoprophos while using enzyme mimetic magnetic nanoparticles. Different scientific approaches to tune nanozyme activities towards metal ions, pesticides, and environment pollutants are summarized in Table 5.
Food analysis
In recent years, considerable attention has been focused on the food safety and food quality because of nutritional awareness, rapidly changing food choices and growing food consumption. As the food stuff and dietary products are directly associated with public health concern, therefore any contamination can cause a serious threat to living beings. Hence, the development of highly sensitive and rapid screening methods is of substantial interest to deal with food related health issues. Some interesting applications of non-enzymatic nanomaterial based sensors in the food analysis will be summarized in the following sections:
Pesticides
Extensive use of pesticides in agriculture, their high water solubility and high toxicity level in living beings demands the highly sensitive, rapid and accurate methods for the detection of these pollutants. A biomimetic sensor for the highly selective and sensitive determination of carbofuran pesticide was fabricated on carbon paste electrode immobilized with a composite of graphene oxide and hemin complex by Ademar et al. [230]. The efficiency of the sensor was scrutinized by employing cyclic voltammetry and square wave voltammetry. Fabricated assembly exhibited linearity between 5.0 × 10−6-9.5 × 10−5 mol. L−1 with a sensitivity of 1.1 × 105 μA.mol−1 and a limit of detection of 9 × 10−9 mol. L−1. A non-enzymatic simultaneous electrochemical detection of carbaryl (CBR) and carbofuran (CBF) pesticide was demonstrated by Wang et al. [231] based on the reduced graphene oxide film decorated with cobalt (II) oxide. CoO/rGO sensor exhibited a good response towards both pesticides in terms of two well-separated and well behaved DPV peaks for a mixed solution. The fabricated electrode showed a linear response over a wide range of concentrations i.e. 0.5–200 μM for CBR and 0.2–70 μM for CBF (S/N = 3) with a limit of detection of 7.5 and 4.2 μg. L−1 for CBR and CBF, respectively. This method was applied for the successful detection of the above mentioned pesticides in vegetable and fruit samples.
Antioxidants
Antioxidants in food are receiving much attention in recent years owing to their ability to improve health and prevent various diseases such as ageing, cardiac diseases and some neurodegenerative ailments e.g. Alzheimer’s and Parkinson’s disease. Keeping in view the physiological roles in maintaining the health, there is a growing interest to accurately access and evaluates the antioxidant capacity of dietary products. Vanillin, an antioxidant, was selectively determined by Lei’s group through cyclic voltammetry [232]. The electrochemical sensor for this purpose was fabricated by electrodepositing the Au-Pd alloy nanoparticles on reduced graphene oxide film. The hybrid film showed a linear electrocatalytic response towards vanillin ranges from 0.1–7 μM under optimal conditions with a detection limit of 20 nM and a sensitivity of 1.6 mA.mM−1.cm−2. The modified electrode was employed successfully for the determination of vanillin in vanilla biscuit, vanilla tea and vanilla beans. Lulia et al. [233] presented the voltammetry based quantitative determination of caffeic acid, a polyphenolic antioxidant, in three different tea types i.e. Turkish green, black and white teas by employing a disposable pencil graphite electrode. The sensor response was observed in terms of differential pulse voltammogram which exhibited a linear pattern with varying concentration of the analyte over a range 1 × 10−7-3 × 10−3 M with a detection limit of 8.83 × 10−8 M. The total phenolic contents for three tea types calculated from DPV were 35.81 (green), 34.59 (white) and 31.21 mg (black) caffeic acid equivalent/g tea. These values were found to be in accordance with those evaluated by Folin-Ciocalteu method, though well-known but more time consuming spectrophotometric methodology. A portable colorimetric sensor based on immobilized ceria nanoparticles was reported by Erica and group for detection of some antioxidants namely vanillic acid, ascorbic acid, quercetin, gallic acid, epigallocatechin gallate and caffeic acid [234]. The colorimetric response was found to be concentration dependent with a limit of detection over a wide concentration range i.e. from 20 to 400 μM depending upon the type of antioxidant involved. The fabricated sensor was applied successfully for evaluating the antioxidant contents in real samples i.e. medicinal mushrooms and teas. Owing to the multidimensional characteristics of ceria nanoparticles, this sensor was found to have many advantages over other systems which involve commercial oxidant kits, sensitive enzymes and conventional colorimetric dyes, in terms of fast response, cost effectiveness, portability, easy handling, high sensitivity and stability. The summaries of some non-enzymatic sensors used for various analytes associated with the food safety and quality assurance are described in Table 6.
Conclusions and perspectives
Nano receptors based methodologies offer a novel and attractive paradigm in terms of new and augmented functionality that encompasses a wide variety of application in the analytical domain. This review provided a brief survey of the different types of nanomaterials, which are employed as a potential receptors element to replace natural enzyme in the field of biosensors, and have found widespread applications for biomedical, food and environmental analysis. These nano receptors are characterized with various advantages which included but not limited low cost, facile preparation, large scale synthesis, high stability and sustained catalytic activities. Nano receptor materials do not have real enzyme-like properties and it is not possible to regenerate nanomaterial surface in most of the cases for subsequent measurement, limiting their applications in amperometric biosensors or for repeated assays. Some attempts were made to regenerate the nano receptor surface for repeated cycles [249], but a decrease in the catalytic efficiency was observed after 8 cycles [250]. Moreover, controlling the reactivity of nanomaterials against certain interfering molecules is a very difficult task which may results in the generation of nonspecific signals, thus affecting the assays selectivity and specificity. The reactivity of nanomaterials is mainly related to the functional groups of the analytes, and closely related interfering molecules share a very similar structure to the analyte of interest and have possibility to react with the nanomaterials. This reactivity may result in generation of signals even in the absence of analyte and produce false positive results. To replace enzymes for biosensing applications, it is highly desirable to design selective and specific nanomaterials to overcome the matrix interferences. Moreover, future research may focus on the methods to regenerate the nano-surface to increase the reusability of the nano-sensors. Although, much progress has been seen in the use of these novel receptors to replace the natural enzyme, but there are few report on the integration of these materials in the design of affinity based sensors such as those based on the antibody or aptamer. For example, biomimetic nanomaterials have been employed to replace the common enzyme labels in the construction of immunosensors and aptasensors in some recent reports. We may envision the combination of the catalytic activity of nanomaterials and specificity of the antibody or aptamer molecules to construct novel designs of affinity based assays. Additionally, there are some points that need to be addressed in the design of non-enzymatic assays; 1) nanomaterial must be able to retain its catalytic activity under complex biological systems and under varying physiological conditions, as per requirement for most of the biological assays; 2) it would be of great interest to tune the nanomaterial’s surface in a controllable fashion for simultaneous measurements of various targets; 3) although, there is literature on the hybridization of various nanomaterials to design multicomponent systems, but most of the nanomaterials combinations are yet to explore in multicomponent systems for synergistic properties; 4) methods to overcome the nanomaterials aggregation under complex biological system should be focused and resolved.
References
Omrani NM, Hayat A, Korri-Youssoufi H, Marty JL (2016) Electrochemical biosensors for food security: mycotoxins detection. In: Biosensors for security and bioterrorism applications. Springer, pp 469–490. doi:10.1007/978-3-319-28926-7_22
Lafleur JP, Jönsson A, Senkbeil S, Kutter JP (2016) Recent advances in lab-on-a-chip for biosensing applications. Biosens Bioelectron 76:213–233
Fiammengo R, Jaschke A (2005) Nucleic acid enzymes. Curr Opin Biotechnol 16(6):614–621. doi:10.1016/j.copbio.2005.10.006
Volz H, Holzbecher M (1997) A bridged Porphyrinato (thiolato) iron (III) complex as a model of the active Center of the Cytochrome P‐450 Isozyme. Angew Chem Int Ed Engl 36(13–14):1442–1445
Ghosh M, Conroy JL, Seto CT (1999) Hydrolysis of amides catalyzed by 4‐Heterocyclohexanones: small molecule mimics of serine proteases. Angew Chem Int Ed 38(4):514–516
Funabiki T, Yamazaki T, Fukui A, Tanaka T, Yoshida S (1998) Oxygenative cleavage of Chlorocatechols with molecular oxygen catalyzed by non‐Heme iron (III) complexes and its relevance to Chlorocatechol Dioxygenases. Angew Chem Int Ed 37(4):513–515
Molenveld P, Engbersen JF, Reinhoudt DN (1999) Specific RNA dinucleotide cleavage by a synthetic calix [4] arene‐based Trinuclear Metallo (II)‐phosphodiesterase. Angew Chem Int Ed 38(21):3189–3192
Han MJ, Yoo KS, Chang JY, Ha TK (2000) 5‐(β‐Cyclodextrinylamino)‐5‐Deoxy‐α‐D‐Riboses as models for nuclease, ligase, phosphatase, and Phosphorylase. Angew Chem Int Ed 39(2):347–349
Muche MS, Göbel MW (1996) Bis (guanidinium) alcohols as models of staphylococcal nuclease: substrate binding through ion pair complexes and fast phosphoryl transfer reactions. Angew Chem Int Ed Engl 35(18):2126–2129
Signor L, Knuppe C, Hug R, Schweizer B, Pfaltz A, Jaun B (2000) Methane formation by reaction of a methyl Thioether with a photo‐excited nickel Thiolate—a process mimicking methanogenesis in Archaea. Chemistry–A European Journal 6(19):3508–3516
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42(14):6060–6093. doi:10.1039/C3CS35486E
Hayat A, Catanante G, Marty JL (2014) Current trends in nanomaterial-based amperometric biosensors. Sensors 14(12):23439–23461
Lim C, Alavijeh AS, Lauritzen M, Kolodziej J, Knights S, Kjeang E (2015) Fuel cell durability enhancement with cerium oxide under combined chemical and mechanical membrane degradation. ECS Electrochemistry Letters 4(4):F29–F31
Ansari AA, Solanki PR, Malhotra B (2008) Sol-gel derived nanostructured cerium oxide film for glucose sensor. Appl Phys Lett 92(26):263901
Luo X, Morrin A, Killard AJ, Smyth MR (2006) Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 18(4):319–326
Wang N, Sun J, Chen L, Fan H, Ai S (2015) A Cu2(OH)3Cl-CeO2 nanocomposite with peroxidase-like activity, and its application to the determination of hydrogen peroxide, glucose and cholesterol. Microchim Acta 182(9):1733–1738. doi:10.1007/s00604-015-1506-8
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021
Dobson J (2006) Magnetic nanoparticles for drug delivery. Drug Dev Res 67(1):55–60
Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC (2008) Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 29(4):487–496
Xu Y, Wang E (2012) Electrochemical biosensors based on magnetic micro/nano particles. Electrochim Acta 84:62–73
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583
Li L, Du Z, Liu S, Hao Q, Wang Y, Li Q, Wang T (2010) A novel nonenzymatic hydrogen peroxide sensor based on MnO 2/graphene oxide nanocomposite. Talanta 82(5):1637–1641
Thiagarajan S, Tsai TH, Chen S-M (2011) Electrochemical fabrication of nano manganese oxide modified electrode for the detection of H2O2. Int J Electrochem Sci 6:2235–2245
Yang X, Chen X, Zhang X, Yang W, Evans DG (2008) Intercalation of methylene blue into layered manganese oxide and application of the resulting material in a reagentless hydrogen peroxide biosensor. Sensors Actuators B Chem 129(2):784–789
Wang J, Sun XW, Wei A, Lei Y, Cai X, Li CM, Dong ZL (2006) Zinc oxide nanocomb biosensor for glucose detection. Appl Phys Lett 88(23):3106
Wang X, Liu E, Zhang X (2014) Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution. Electrochim Acta 130:253–260
André R, Natálio F, Humanes M, Leppin J, Heinze K, Wever R, Schröder HC, Müller WE, Tremel W (2011) V2O5 nanowires with an intrinsic peroxidase‐like activity. Adv Funct Mater 21(3):501–509
Xiang Z, Wang Y, Ju P, Zhang D (2016) Optical determination of hydrogen peroxide by exploiting the peroxidase-like activity of AgVO3 nanobelts. Microchim Acta 183(1):457–463. doi:10.1007/s00604-015-1670-x
Lang X-Y, Fu H-Y, Hou C, Han G-F, Yang P, Liu Y-B, Jiang Q (2013) Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors. Nat Commun 4:2169–2177. doi:10.1038/ncomms3169
Ding C, Yan Y, Xiang D, Zhang C, Xian Y (2016) Magnetic Fe3S4 nanoparticles with peroxidase-like activity, and their use in a photometric enzymatic glucose assay. Microchim Acta 183(2):625–631. doi:10.1007/s00604-015-1690-6
Lu Y, Yu J, Ye W, Yao X, Zhou P, Zhang H, Zhao S, Jia L (2016) Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Microchim Acta 183(8):2481–2489. doi:10.1007/s00604-016-1886-4
Comotti M, Della Pina C, Matarrese R, Rossi M (2004) The catalytic activity of “naked” gold particles. Angew Chem Int Ed 43(43):5812–5815
Li X-J, Wang Y-S, Yang S-Y, Tang X, Liu L, Zhou B, Wang X-F, Zhu Y-F, Huang Y-Q, He S-Z (2016) Determination of metallothioneins based on the enhanced peroxidase-like activity of mercury-coated gold nanoparticles aggregated by metallothioneins. Microchim Acta 183(7):2123–2129. doi:10.1007/s00604-016-1828-1
Yang M, Yang Y, Liu Y, Shen G, Yu R (2006) Platinum nanoparticles-doped sol–gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens Bioelectron 21(7):1125–1131
Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26(12):4637–4648
Gavalas VG, Chaniotakis NA (2000) [60] Fullerene-mediated amperometric biosensors. Anal Chim Acta 409(1):131–135
Lanzellotto C, Favero G, Antonelli M, Tortolini C, Cannistraro S, Coppari E, Mazzei F (2014) Nanostructured enzymatic biosensor based on fullerene and gold nanoparticles: preparation, characterization and analytical applications. Biosens Bioelectron 55:430–437
Partha R, Conyers JL (2009) Biomedical applications of functionalized fullerene-based nanomaterials. Int J Nanomedicine 4:261
Wohlstadter JN, Wilbur JL, Sigal GB, Biebuyck HA, Billadeau MA, Dong L, Fischer AB, Gudibande SR, Jameison SH, Kenten JH (2003) Carbon nanotube‐based biosensor. Adv Mater 15(14):1184–1187
Atashbar MZ, Bejcek B, Singamaneni S, Santucci S (2004) Carbon nanotube based biosensors. In: Sensors, 2004. Proceedings of IEEE, IEEE, pp 1048–1051
Chen RJ, Bangsaruntip S, Drouvalakis KA, Kam NWS, Shim M, Li Y, Kim W, Utz PJ, Dai H (2003) Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc Natl Acad Sci 100(9):4984–4989
Yang N, Chen X, Ren T, Zhang P, Yang D (2015) Carbon nanotube based biosensors. Sensors Actuators B Chem 207:690–715
Song Y, Wang X, Zhao C, Qu K, Ren J, Qu X (2010) Label‐free colorimetric detection of single nucleotide polymorphism by using single‐walled carbon nanotube intrinsic peroxidase‐like activity. Chemistry–A European Journal 16(12):3617–3621
Chen J, Ge J, Zhang L, Li Z, Li J, Sun Y, Qu L (2016) Reduced graphene oxide nanosheets functionalized with poly(styrene sulfonate) as a peroxidase mimetic in a colorimetric assay for ascorbic acid. Microchim Acta 183(6):1847–1853. doi:10.1007/s00604-016-1826-3
Shi X, Gu W, Li B, Chen N, Zhao K, Xian Y (2014) Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim Acta 181(1):1–22. doi:10.1007/s00604-013-1069-5
Govindhan M, Adhikari B-R, Chen A (2014) Nanomaterials-based electrochemical detection of chemical contaminants. RSC Adv 4(109):63741–63760
Zhu C, Yang G, Li H, Du D, Lin Y (2014) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87(1):230–249
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 182(1–2):1–41
Shah B, Lafleur T, Chen A (2013) Carbon nanotube based electrochemical sensor for the sensitive detection of valacyclovir. Faraday Discuss 164:135–146
Chatterjee S, Wen J, Chen A (2013) Electrochemical determination of methylglyoxal as a biomarker in humanplasma. Biosens Bioelectron 42:349–354
Chatterjee S, Chen A (2012) Functionalization of carbon buckypaper for the sensitive determination of hydrogen peroxide in human urine. Biosens Bioelectron 35(1):302–307
Wanekaya AK (2011) Applications of nanoscale carbon-based materials in heavy metal sensing and detection. Analyst 136(21):4383–4391
Peng Y, Wang Y, Li Y, He X (2016) Polyethylenimine Functionalized Multi‐walled Carbon Nanotubes for Electrochemical Detection of Chromium (VI). Electroanalysis
Balan I, David IG, David V, Stoica A-I, Mihailciuc C, Stamatin I, Ciucu AA (2011) Electrocatalytic voltammetric determination of guanine at a cobalt phthalocyanine modified carbon nanotubes paste electrode. J Electroanal Chem 654(1):8–12
Yao L, Teng J, Zhu M, Zheng L, Zhong Y, Liu G, Xue F, Chen W (2016) MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens Bioelectron 85:331–336
Razzino CA, Sgobbi LF, Canevari TC, Cancino J, Machado SA (2015) Sensitive determination of carbendazim in orange juice by electrode modified with hybrid material. Food Chem 170:360–365
Kalambate PK, Srivastava AK (2016) Simultaneous voltammetric determination of paracetamol, cetirizine and phenylephrine using a multiwalled carbon nanotube-platinum nanoparticles nanocomposite modified carbon paste electrode. Sensors Actuators B Chem 233:237–248
Karimian N, Gholivand M, Malekzadeh G (2016) Cefixime detection by a novel electrochemical sensor based on glassy carbon electrode modified with surface imprinted polymer/multiwall carbon nanotubes. J Electroanal Chem 771:64–72
Umar A, Kim S, Kumar R, Algarni H, Al-Assiri M (2016) Platinum nanoparticles decorated carbon nanotubes for highly sensitive 2-nitrophenol chemical sensor. Ceram Int 42(7):9257–9263
Yao Y, Liu Y, Yang Z (2016) A novel electrochemical sensor based on a glassy carbon electrode modified with Cu–MWCNT nanocomposites for determination of hydroquinone. Anal Methods 8(12):2568–2575
Heydari H, Gholivand MB, Abdolmaleki A (2016) Cyclic voltammetry deposition of copper nanostructure on MWCNTs modified pencil graphite electrode: an ultra-sensitive hydrazine sensor. Mater Sci Eng C 66:16–24
Maluta JR, Canevari TC, Machado SA (2014) Sensitive determination of nitric oxide using an electrochemical sensor based on MWCNTs decorated with spherical Au nanoparticles. J Solid State Electrochem 18(9):2497–2504
Fifield LS, Grate JW (2010) Hydrogen-bond acidic functionalized carbon nanotubes (CNTs) with covalently-bound hexafluoroisopropanol groups. Carbon 48(7):2085–2088
Yang Z, Yan X, Li Z, Zheng X, Zheng J (2016) Synthesis of Cu2O on AlOOH/reduced graphene oxide for non-enzymatic amperometric glucose sensing. Anal Methods 8(7):1527–1531. doi:10.1039/C5AY03258J
Deng H, Liu C, Yang S, Xiao S, Zhou Z-K, Wang Q-Q (2008) Additive-mediated splitting of lanthanide orthovanadate nanocrystals in water: morphological evolution from rods to sheaves and to spherulites. Cryst Growth Des 8(12):4432–4439
Chang G, Shu H, Huang Q, Oyama M, Ji K, Liu X, He Y (2015) Synthesis of highly dispersed Pt nanoclusters anchored graphene composites and their application for non-enzymatic glucose sensing. Electrochim Acta 157:149–157. doi:10.1016/j.electacta.2015.01.085
Zhang H, Xu X, Yin Y, Wu P, Cai C (2013) Nonenzymatic electrochemical detection of glucose based on Pd 1 Pt 3–graphene nanomaterials. J Electroanal Chem 690:19–24
Thanh TD, Balamurugan J, Lee SH, Kim NH, Lee JH (2016) Novel porous gold-palladium nanoalloy network-supported graphene as an advanced catalyst for non-enzymatic hydrogen peroxide sensing. Biosens Bioelectron 85:669–678
Lv Y, Wang F, Zhu H, Zou X, Tao C-a, Wang J (2016) Electrochemically Reduced Graphene Oxide-nafion/Au Nanoparticle Modified Electrode for Hydrogen Peroxide Sensing
Wang S, Han Z, Li Y, Peng R, Feng B (2015) An electrochemical sensor based on reduced graphene oxide and copper sulfide hollow nanospheres. RSC Adv 5(130):107318–107325. doi:10.1039/C5RA20926A
Xu X, Fang B, Zhang F, Tian Y, Wu J (2010) Influence of rare earth co-dopant on the photocatalytic property of TiO2 nano-particles. Journal of Wuhan University of Technology--Materials Science Edition 25(3):370–374
Cincotto FH, Canevari TC, Campos AM, Landers R, Machado SA (2014) Simultaneous determination of epinephrine and dopamine by electrochemical reduction on the hybrid material SiO 2/graphene oxide decorated with Ag nanoparticles. Analyst 139(18):4634–4640
Cincotto FH, Canevari TC, Machado SA, Sánchez A, Barrio MAR, Villalonga R, Pingarrón JM (2015) Reduced graphene oxide-Sb 2 O 5 hybrid nanomaterial for the design of a laccase-based amperometric biosensor for estriol. Electrochim Acta 174:332–339
Zhao S, Wang L, Wang T, Han Q, Xu S (2016) A high-performance hydrazine electrochemical sensor based on gold nanoparticles/single-walled carbon nanohorns composite film. Appl Surf Sci 369:36–42
Lu B, Zhang Z, Hao J, Xu G, Zhang B, Tang J (2012) Electrochemical sensing platform based on Schiff-base cobalt(ii)/single-walled carbon nanohorns complexes system. Anal Methods 4(11):3580–3585. doi:10.1039/C2AY25940K
Dai H, Gong L, Xu G, Zhang S, Lu S, Jiang Y, Lin Y, Guo L, Chen G (2013) An electrochemical sensing platform structured with carbon nanohorns for detecting some food borne contaminants. Electrochim Acta 111:57–63
Maroneze CM, Gushikem Y, Kubota LT (2016) Applications of MN4 macrocyclic metal complexes in electroanalysis. In: Electrochemistry of N4 macrocyclic metal complexes. Springer, pp 107–133. doi: 10.1007/978-3-319-31332-0_3
Walcarius A (2013) Mesoporous materials and electrochemistry. Chem Soc Rev 42(9):4098–4140
Walcarius A (2015) Mesoporous materials‐based electrochemical sensors. Electroanalysis 27(6):1303–1340
Rahim A, Santos LS, Barros S, Kubota LT, Landers R, Gushikem Y (2014) Electrochemical detection of nitrite in meat and water samples using a mesoporous carbon ceramic SiO2/C electrode modified with in situ generated manganese (II) phthalocyanine. Electroanalysis 26(3):541–547
Rahim A, Santos LS, Barros SB, Kubota LT, Gushikem Y (2013) Dissolved O 2 sensor based on cobalt (II) phthalocyanine immobilized in situ on electrically conducting carbon ceramic mesoporous SiO 2/C material. Sensors Actuators B Chem 177:231–238
Santos LS, Landers R, Gushikem Y (2011) Application of manganese (II) phthalocyanine synthesized in situ in the SiO 2/SnO 2 mixed oxide matrix for determination of dissolved oxygen by electrochemical techniques. Talanta 85(2):1213–1216
Rahim A, Barros SB, Kubota LT, Gushikem Y (2011) SiO 2/C/Cu (II) phthalocyanine as a biomimetic catalyst for dopamine monooxygenase in the development of an amperometric sensor. Electrochim Acta 56(27):10116–10121
Barros SB, Rahim A, Tanaka AA, Arenas LT, Landers R, Gushikem Y (2013) In situ immobilization of nickel (II) phthalocyanine on mesoporous SiO 2/C carbon ceramic matrices prepared by the sol–gel method: use in the simultaneous voltammetric determination of ascorbic acid and dopamine. Electrochim Acta 87:140–147
Rahim A, Muhammad N, Nishan U, Khan US, Rehman F, Kubota LT, Gushikem Y (2015) Copper phthalocyanine modified SiO 2/C electrode as a biomimetic electrocatalyst for 4-aminophenol in the development of an amperometric sensor. RSC Adv 5(106):87043–87050
Rahim A, Barros SB, Arenas LT, Gushikem Y (2011) In situ immobilization of cobalt phthalocyanine on the mesoporous carbon ceramic SiO 2/C prepared by the sol–gel process. Evaluation as an electrochemical sensor for oxalic acid. Electrochim Acta 56(3):1256–1261
Canevari TC, Prado TM, Cincotto FH, Machado SA (2016) Immobilization of ruthenium phthalocyanine on silica-coated multi-wall partially oriented carbon nanotubes: electrochemical detection of fenitrothion pesticide. Mater Res Bull 76:41–47
Canevari TC, Vinhas RC, Landers R, Gushikem Y (2011) SiO 2/SnO 2/Sb 2 O 5 microporous ceramic material for immobilization of meldola’s blue: application as an electrochemical sensor for NADH. Biosens Bioelectron 26(5):2402–2406
Maroneze CM, Rahim A, Fattori N, da Costa LP, Sigoli FA, Mazali IO, Custodio R, Gushikem Y (2014) Electroactive properties of 1-propyl-3-methylimidazolium ionic liquid covalently bonded on mesoporous silica surface: development of an electrochemical sensor probed for NADH, dopamine and uric acid detection. Electrochim Acta 123:435–440
Tkachenko O, Rahim A, Baraban A, Sukhov R, Khristenko I, Gushikem Y, Kholin Y (2013) Hybrid silica-organic material with immobilized amino groups: surface probing and use for electrochemical determination of nitrite ions. J Sol-Gel Sci Technol 67(1):145–154
Canevari TC, Arenas LT, Landers R, Custodio R, Gushikem Y (2013) Simultaneous electroanalytical determination of hydroquinone and catechol in the presence of resorcinol at an SiO 2/C electrode spin-coated with a thin film of Nb 2 O 5. Analyst 138(1):315–324
dos Santos MP, Rahim A, Fattori N, Kubota LT, Gushikem Y (2012) Novel amperometric sensor based on mesoporous silica chemically modified with ensal copper complexes for selective and sensitive dopamine determination. Sensors Actuators B Chem 171:712–718
Maroneze CM, dos Santos GP, de Moraes VB, da Costa LP, Kubota LT (2016) Multifunctional catalytic platform for peroxidase mimicking, enzyme immobilization and biosensing. Biosens Bioelectron 77:746–751
Weerathunge P, Ramanathan R, Shukla R, Sharma TK, Bansal V (2014) Aptamer-controlled reversible inhibition of gold nanozyme activity for pesticide sensing. Anal Chem 86(24):11937–11941
Zhang Z, Zhu H, Wang X, Yang X (2011) Sensitive electrochemical sensor for hydrogen peroxide using Fe3O4 magnetic nanoparticles as a mimic for peroxidase. Microchim Acta 174(1):183–189. doi:10.1007/s00604-011-0600-9
Wang J, X-j C, Liao K-m, Wang G-h, Han M (2015) Pd nanoparticle-modified electrodes for nonenzymatic hydrogen peroxide detection. Nanoscale Res Lett 10(1):1–6. doi:10.1186/s11671-015-1021-1
Li Z, Chen Y, Xin Y, Zhang Z (2015) Sensitive electrochemical nonenzymatic glucose sensing based on anodized CuO nanowires on three-dimensional porous copper foam. Sci Rep 5:16115–16123
Hu A-L, Liu Y-H, Deng H-H, Hong G-L, Liu A-L, Lin X-H, Xia X-H, Chen W (2014) Fluorescent hydrogen peroxide sensor based on cupric oxide nanoparticles and its application for glucose and l-lactate detection. Biosens Bioelectron 61:374–378. doi:10.1016/j.bios.2014.05.048
Shi Y, Su P, Wang Y, Yang Y (2014) Fe3O4 peroxidase mimetics as a general strategy for the fluorescent detection of H2O2-involved systems. Talanta 130:259–264. doi:10.1016/j.talanta.2014.06.053
Chen W, Hong L, Liu A-L, Liu J-Q, Lin X-H, Xia X-H (2012) Enhanced chemiluminescence of the luminol-hydrogen peroxide system by colloidal cupric oxide nanoparticles as peroxidase mimic. Talanta 99:643–648. doi:10.1016/j.talanta.2012.06.061
Abdolmohammad-Zadeh H, Rahimpour E (2015) A novel chemosensor for Ag(I) ion based on its inhibitory effect on the luminol–H2O2 chemiluminescence response improved by CoFe2O4 nano-particles. Sensors Actuators B Chem 209:496–504. doi:10.1016/j.snb.2014.11.096
Hong L, Liu A-L, Li G-W, Chen W, Lin X-H (2013) Chemiluminescent cholesterol sensor based on peroxidase-like activity of cupric oxide nanoparticles. Biosens Bioelectron 43:1–5. doi:10.1016/j.bios.2012.11.031
Zhu W, Zhang J, Jiang Z, Wang W, Liu X (2014) High-quality carbon dots: synthesis, peroxidase-like activity and their application in the detection of H2O2, Ag + and Fe3+. RSC Adv 4(33):17387–17392. doi:10.1039/c3ra47593j
Qiao F, Chen L, Li X, Li L, Ai S (2014) Peroxidase-like activity of manganese selenide nanoparticles and its analytical application for visual detection of hydrogen peroxide and glucose. Sensors Actuators B Chem 193:255–262. doi:10.1016/j.snb.2013.11.108
Nie G, Zhang L, Lei J, Yang L, Zhang Z, Lu X, Wang C (2014) Monocrystalline VO2 (B) nanobelts: large-scale synthesis, intrinsic peroxidase-like activity and application in biosensing. J Mater Chem A 2(9):2910–2914. doi:10.1039/c3ta15051h
Ray C, Dutta S, Sarkar S, Sahoo R, Roy A, Pal T (2014) Intrinsic peroxidase-like activity of mesoporous nickel oxide for selective cysteine sensing. Journal of Materials Chemistry B 2(36):6097–6105. doi:10.1039/c4tb00968a
Mu J, Zhang L, Zhao M, Wang Y (2014) Catalase mimic property of Co3O4 nanomaterials with different morphology and its application as a calcium sensor. ACS Appl Mater Interfaces 6(10):7090–7098
Deng H-H, Li G-W, Hong L, Liu A-L, Chen W, Lin X-H, Xia X-H (2014) Colorimetric sensor based on dual-functional gold nanoparticles: analyte-recognition and peroxidase-like activity. Food Chem 147:257–261
Yang H, Xiong Y, Zhang P, Su L, Ye F (2015) Colorimetric detection of mercury ions using MnO2 nanorods as enzyme mimics. Anal Methods 7(11):4596–4601. doi:10.1039/c5ay00633c
Long YJ, Li YF, Liu Y, Zheng JJ, Tang J, Huang CZ (2011) Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem Commun 47(43):11939–11941. doi:10.1039/c1cc14294a
Hu J, Zhuang Q, Wang Y, Ni Y (2016) Label-free fluorescent catalytic biosensor for highly sensitive and selective detection of the ferrous ion in water samples using a layered molybdenum disulfide nanozyme coupled with an advanced chemometric model. Analyst 141(5):1822–1829. doi:10.1039/c5an02457a
Chen C, Lu L, Zheng Y, Zhao D, Yang F, Yang X (2015) A new colorimetric protocol for selective detection of phosphate based on the inhibition of peroxidase-like activity of magnetite nanoparticles. Anal Methods 7(1):161–167. doi:10.1039/c4ay02461c
Chang Y, Zhang Z, Hao J, Yang W, Tang J (2016) A simple label free colorimetric method for glyphosate detection based on the inhibition of peroxidase-like activity of Cu(II). Sensors Actuators B Chem 228:410–415. doi:10.1016/j.snb.2016.01.048
Liang M, Fan K, Pan Y, Jiang H, Wang F, Yang D, Lu D, Feng J, Zhao J, Yang L, Yan X (2013) Fe3O4 magnetic nanoparticle peroxidase mimetic-based colorimetric assay for the rapid detection of organophosphorus pesticide and nerve agent. Anal Chem 85(1):308–312. doi:10.1021/ac302781r
Guan G, Yang L, Mei Q, Zhang K, Zhang Z, Han M-Y (2012) Chemiluminescence switching on peroxidase-like Fe3O4 nanoparticles for selective detection and simultaneous determination of various pesticides. Anal Chem 84(21):9492–9497. doi:10.1021/ac302341b
Wang J (2006) Analytical electrochemistry, 3rd edn. John Wiley & Sons, New York, pp 1–250
Ambrosi A, Chua CK, Bonanni A, Pumera M (2014) Electrochemistry of graphene and related materials. Chem Rev 114(14):7150–7188
Raj MA, John SA (2014) Electrochemical determination of xanthine oxidase inhibitor drug in urate lowering therapy using graphene nanosheets modified electrode. Electrochim Acta 117:360–366
Toh HS, Tschulik K, Batchelor-McAuley C, Compton RG (2014) Electrochemical quantification of iodide ions in synthetic urine using silver nanoparticles: a proof-of-concept. Analyst 139(16):3986–3990
Metters JP, Kadara RO, Banks CE (2011) New directions in screen printed electroanalytical sensors: an overview of recent developments. Analyst 136(6):1067–1076
Aiba Y, Sumaoka J, Komiyama M (2011) Artificial DNA cutters for DNA manipulation and genome engineering. Chem Soc Rev 40(12):5657–5668
Huang X, Liu X, Luo Q, Liu J, Shen J (2011) Artificial selenoenzymes: designed and redesigned. Chem Soc Rev 40(3):1171–1184
Gn W, Liu J (2011) Design of biomimetic catalysts by molecular imprinting in synthetic polymers: the role of transition state stabilization. Acc Chem Res 45(2):239–247
Dhakshinamoorthy A, Navalon S, Alvaro M, Garcia H (2012) Metal nanoparticles as heterogeneous Fenton catalysts. ChemSusChem 5(1):46–64
Pietrzyk A, Suriyanarayanan S, Kutner W, Maligaspe E, Zandler ME, D’Souza F (2010) Molecularly imprinted poly[bis(2,2′-bithienyl)methane] film with built-in molecular recognition sites for a piezoelectric microgravimetry chemosensor for selective determination of dopamine. Bioelectrochemistry 80(1):62–72. doi:10.1016/j.bioelechem.2010.03.004
Schirhagl R, Latif U, Podlipna D, Blumenstock H, Dickert FL (2012) Natural and biomimetic materials for the detection of insulin. Anal Chem 84(9):3908–3913
Moreira FTC, Dutra RAF, Noronha JPC, Sales MGF (2013) Electrochemical biosensor based on biomimetic material for myoglobin detection. Electrochim Acta 107:481–487. doi:10.1016/j.electacta.2013.06.061
Ruy MR, Figueira EC, Sotomayor MD (2015) Biomimetic sensor for detection of hydrochlorothiazide employing amperometric detection and Chemometrics for application in doping in sports. J Braz Chem Soc 26(10):2069–2076
Molaakbari E, Mostafavi A, Beitollahi H, Alizadeh R (2014) Synthesis of ZnO nanorods and their application in the construction of a nanostructure-based electrochemical sensor for determination of levodopa in the presence of carbidopa. Analyst 139(17):4356–4364. doi:10.1039/C4AN00138A
dos Santos Ruy MR, Figueira EC, Sotomayor MDPT (2014) Electroanalytical determination of bumetanide employing a biomimetic sensor for detection of doping in sports. Anal Methods 6(15):5792–5798
Boni AC, Sotomayor MD, Lanza MR, Tanaka SMC, Tanaka AA (2010) Application of a biomimetic sensor based on iron phthalocyanine chloride: 4-methylbenzylidene-camphor detection. J Braz Chem Soc 21(7):1377–1383
Pletcher D (1984) Electrocatalysis: present and future. J Appl Electrochem 14(4):403–415
Burke L (1994) Premonolayer oxidation and its role in electrocatalysis. Electrochim Acta 39(11):1841–1848
Agnihotri N, Chowdhury AD, De A (2015) Non-enzymatic electrochemical detection of cholesterol using β-cyclodextrin functionalized graphene. Biosens Bioelectron 63:212–217. doi:10.1016/j.bios.2014.07.037
Liu M, Liu R, Chen W (2013) Graphene wrapped Cu 2 O nanocubes: non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron 45:206–212
Dashtestani F, Ghourchian H, Eskandari K, Rafiee-Pour H-A (2015) A superoxide dismutase mimic nanocomposite for amperometric sensing of superoxide anions. Microchim Acta 182(5):1045–1053. doi:10.1007/s00604-014-1424-1
Zheng X, Liu Q, Jing C, Li Y, Li D, Luo W, Wen Y, He Y, Huang Q, Long YT (2011) Catalytic gold nanoparticles for nanoplasmonic detection of DNA hybridization. Angew Chem Int Ed 50(50):11994–11998
Çiftçi H, Tamer U (2012) Functional gold nanorod particles on conducting polymer poly (3-octylthiophene) as non-enzymatic glucose sensor. React Funct Polym 72(2):127–132
Ma M, Zhang Y, Gu N (2011) Peroxidase-like catalytic activity of cubic Pt nanocrystals. Colloids Surf A Physicochem Eng Asp 373(1):6–10
McLamore E, Shi J, Jaroch D, Claussen J, Uchida A, Jiang Y, Zhang W, Donkin SS, Banks M, Buhman K (2011) A self referencing platinum nanoparticle decorated enzyme-based microbiosensor for real time measurement of physiological glucose transport. Biosens Bioelectron 26(5):2237–2245
Zhu J, Jiang J, Liu J, Ding R, Li Y, Ding H, Feng Y, Wei G, Huang X (2011) CNT-network modified Ni nanostructured arrays for high performance non-enzymatic glucose sensors. RSC Adv 1(6):1020–1025
Zhang X, Wang G, Huang Y, Yu L, Fang B (2012) Non-enzymatic glucose detection using Ni/multi-walled carbon nanotubes composite. Micro & Nano Letters, IET 7(2):168–170
Nie H, Yao Z, Zhou X, Yang Z, Huang S (2011) Nonenzymatic electrochemical detection of glucose using well-distributed nickel nanoparticles on straight multi-walled carbon nanotubes. Biosens Bioelectron 30(1):28–34
Qu F, Sun H, Zhang Y, Lu H, Yang M (2012) Electrochemically deposited Pd nanorod array/sol–gel silica thin film for the fabrication of electrochemical sensors. Sensors Actuators B Chem 166:837–841
Wang Q, Cui X, Chen J, Zheng X, Liu C, Xue T, Wang H, Jin Z, Qiao L, Zheng W (2012) Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv 2(15):6245–6249
Mahshid SS, Mahshid S, Dolati A, Ghorbani M, Yang L, Luo S, Cai Q (2011) Template-based electrodeposition of Pt/Ni nanowires and its catalytic activity towards glucose oxidation. Electrochim Acta 58:551–555
Zhang K, Hu X, Liu J, Yin J-J, Hou S, Wen T, He W, Ji Y, Guo Y, Wang Q (2011) Formation of PdPt alloy nanodots on gold nanorods: tuning oxidase-like activities via composition. Langmuir 27(6):2796–2803
Liu J, Hu X, Hou S, Wen T, Liu W, Zhu X, Yin J-J, Wu X (2012) Au@ Pt core/shell nanorods with peroxidase-and ascorbate oxidase-like activities for improved detection of glucose. Sensors Actuators B Chem 166:708–714
Lien C-W, Huang C-C, Chang H-T (2012) Peroxidase-mimic bismuth–gold nanoparticles for determining the activity of thrombin and drug screening. Chem Commun 48(64):7952–7954
Yin J, Cao H, Lu Y (2012) Self-assembly into magnetic Co 3 O 4 complex nanostructures as peroxidase. J Mater Chem 22(2):527–534
Asati A, Kaittanis C, Santra S, Perez JM (2011) pH-tunable oxidase-like activity of cerium oxide nanoparticles achieving sensitive fluorigenic detection of cancer biomarkers at neutral pH. Anal Chem 83(7):2547–2553
Zhu A, Sun K, Petty HR (2012) Titanium doping reduces superoxide dismutase activity, but not oxidase activity, of catalytic CeO 2 nanoparticles. Inorg Chem Commun 15:235–237
Chen W, Chen J, Liu AL, Wang LM, Li GW, Lin XH (2011) Peroxidase‐like activity of cupric oxide nanoparticle. ChemCatChem 3(7):1151–1154
Das G, Tran TQN, Yoon HH (2015) Spherulitic copper–copper oxide nanostructure-based highly sensitive nonenzymatic glucose sensor. Int J Nanomedicine 10:165
Qiu G, Huang H, Dharmarathna S, Benbow E, Stafford L, Suib SL (2011) Hydrothermal synthesis of manganese oxide nanomaterials and their catalytic and electrochemical properties. Chem Mater 23(17):3892–3901
Wan Y, Qi P, Zhang D, Wu J, Wang Y (2012) Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens Bioelectron 33(1):69–74
Cao X, Wang N (2011) A novel non-enzymatic glucose sensor modified with Fe2O3 nanowire arrays. Analyst 136(20):4241–4246
Luo J, Zhang H, Jiang S, Jiang J, Liu X (2012) Facile one-step electrochemical fabrication of a non-enzymatic glucose-selective glassy carbon electrode modified with copper nanoparticles and graphene. Microchim Acta 177(3):485–490. doi:10.1007/s00604-012-0795-4
Song J, Xu L, Xing R, Li Q, Zhou C, Liu D, Song H (2014) Synthesis of Au/graphene oxide composites for selective and sensitive electrochemical detection of ascorbic acid. Scientific reports 4:7515
Li Y, Zhai X, Wang H, Liu X, Guo L, Ji X, Wang L, Qiu H, Liu X (2015) Non-enzymatic sensing of uric acid using a carbon nanotube ionic-liquid paste electrode modified with poly(β-cyclodextrin). Microchim Acta 182(11):1877–1884. doi:10.1007/s00604-015-1522-8
Lee J-H, Hong H-G (2015) Nonenzymatic electrochemical sensing of hydrogen peroxide based on a polyaniline-MnO2 nanofiber-modified glassy carbon electrode. J Appl Electrochem 45(10):1153–1162. doi:10.1007/s10800-015-0881-5
Zhou L, Shen Q, Zhao P, Xiang B, Nie Z, Huang Y, Yao S (2013) Fluorescent detection of copper (II) based on DNA-templated click chemistry and graphene oxide. Methods 64(3):299–304
Zhang L, Li L (2016) Colorimetric thrombin assay using aptamer-functionalized gold nanoparticles acting as a peroxidase mimetic. Microchim Acta 183(1):485–490. doi:10.1007/s00604-015-1674-6
Zhao K, Gu W, Zheng S, Zhang C, Xian Y (2015) SDS–MoS 2 nanoparticles as highly-efficient peroxidase mimetics for colorimetric detection of H 2 O 2 and glucose. Talanta 141:47–52
Cao G-X, Wu X-M, Dong Y-M, Li Z-J, Wang G-L (2016) Colorimetric determination of melamine based on the reversal of the mercury(II) induced inhibition of the light-triggered oxidase-like activity of gold nanoclusters. Microchim Acta 183(1):441–448. doi:10.1007/s00604-015-1669-3
Wang Y, Zhou B, Wu S, Wang K, He X (2015) Colorimetric detection of hydrogen peroxide and glucose using the magnetic mesoporous silica nanoparticles. Talanta 134:712–717
Tamura T, Hamachi I (2014) Recent progress in Design of Protein-Based Fluorescent Biosensors and Their Cellular Applications. ACS Chem Biol 9(12):2708–2717. doi:10.1021/cb500661v
Deng H-H, Weng S-H, Huang S-L, Zhang L-N, Liu A-L, Lin X-H, Chen W (2014) Colorimetric detection of sulfide based on target-induced shielding against the peroxidase-like activity of gold nanoparticles. Anal Chim Acta 852:218–222
Liu F, He J, Zeng M, Hao J, Guo Q, Song Y, Wang L (2016) Cu–hemin metal-organic frameworks with peroxidase-like activity as peroxidase mimics for colorimetric sensing of glucose. J Nanopart Res 18(5):1–9. doi:10.1007/s11051-016-3416-z
Liu X, Fu C, Ren X, Liu H, Li L, Meng X (2015) Fluorescence switching method for cascade detection of salicylaldehyde and zinc(II) ion using protein protected gold nanoclusters. Biosens Bioelectron 74:322–328. doi:10.1016/j.bios.2015.06.034
Chang H-C, Ho J-aA (2015) Gold Nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal Chem 87(20):10362–10367. doi:10.1021/acs.analchem.5b02452
Huang J, Liu H, Zhang P, Zhang P, Li M, Ding L (2015) Immobilization of cholesterol oxidase on magnetic fluorescent core-shell-structured nanoparticles. Mater Sci Eng C 57:31–37. doi:10.1016/j.msec.2015.07.038
Hu XL, Wu XM, Fang X, Li ZJ, Wang GL (2016) Switchable fluorescence of gold nanoclusters for probing the activity of alkaline phosphatase and its application in immunoassay. Biosens Bioelectron 77:666–672. doi:10.1016/j.bios.2015.10.046
Zeng H-H, Qiu W-B, Zhang L, Liang R-P, Qiu J-D (2016) Lanthanide coordination polymer nanoparticles as an excellent artificial peroxidase for hydrogen peroxide detection. Anal Chem 88(12):6342–6348. doi:10.1021/acs.analchem.6b00630
Fan Y, Huang Y (2012) The effective peroxidase-like activity of chitosan-functionalized CoFe2O4 nanoparticles for chemiluminescence sensing of hydrogen peroxide and glucose. Analyst 137(5):1225–1231. doi:10.1039/c2an16105b
Iranifam M, Imani-Nabiyyi A, Khataee A, Kalantari J (2016) Enhanced luminol-O2 chemiluminescence reaction by CuO nanoparticles as oxidase mimics and its application for determination of ceftazidime. Anal Methods 8(18):3816–3823. doi:10.1039/C6AY00309E
Shi W, Zhang X, He S, Huang Y (2011) CoFe2O4 magnetic nanoparticles as a peroxidase mimic mediated chemiluminescence for hydrogen peroxide and glucose. Chem Commun (Camb) 47(38):10785–10787. doi:10.1039/c1cc14300j
Xie J, Cao H, Jiang H, Chen Y, Shi W, Zheng H, Huang Y (2013) Co3O4-reduced graphene oxide nanocomposite as an effective peroxidase mimetic and its application in visual biosensing of glucose. Anal Chim Acta 796:92–100. doi:10.1016/j.aca.2013.08.008
Yu J, Ge L, Dai P, Zhang C, Ge S, Huang J (2010) A novel glucose chemiluminescence biosensor based on a rhodanine derivative chemiluminescence system and multilayer-enzyme membrane. Turk J Chem 34(4):489–498
Čopra-Janićijević A, Tahirović A, Kalcher K (2012) One-shot chemiluminescence biosensor for determination of glucose in soft drinks. Glasnik hemičara i tehnologa Bosne i Hercegovine 39:1–6
Yang Z, Zhang Z, Jiang Y, Chi M, Nie G, Lu X, Wang C (2016) Palladium nanoparticles modified electrospun CoFe2O4 nanotubes with enhanced peroxidase-like activity for colorimetric detection of hydrogen peroxide. RSC Adv 6(40):33636–33642. doi:10.1039/c6ra01527a
Guo Y, Liu X, Wang X, Iqbal A, Yang C, Liu W, Qin W (2015) Carbon dot/NiAl-layered double hydroxide hybrid material: facile synthesis, intrinsic peroxidase-like catalytic activity and its application. RSC Adv 5(116):95495–95503. doi:10.1039/c5ra18087b
Cui F, Deng Q, Sun L (2015) Prussian blue modified metal-organic framework MIL-101(Fe) with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. RSC Adv 5(119):98215–98221. doi:10.1039/c5ra18589k
Zhang Y, Tian J, Liu S, Wang L, Qin X, Lu W, Chang G, Luo Y, Asiri AM, Al-Youbi AO (2012) Novel application of CoFe layered double hydroxide nanoplates for colorimetric detection of H 2 O 2 and glucose. Analyst 137(6):1325–1328
Liu S, Wang L, Zhai J, Luo Y, Sun X (2011) Carboxyl functionalized mesoporous polymer: a novel peroxidase-like catalyst for H 2 O 2 detection. Anal Methods 3(7):1475–1477
He W, Jia H, Li X, Lei Y, Li J, Zhao H, Mi L, Zhang L, Zheng Z (2012) Understanding the formation of CuS concave superstructures with peroxidase-like activity. Nanoscale 4(11):3501–3506
Dai Z, Liu S, Bao J, Ju H (2009) Nanostructured FeS as a mimic peroxidase for biocatalysis and biosensing. Chemistry–A European Journal 15(17):4321–4326
Dutta AK, Maji SK, Srivastava DN, Mondal A, Biswas P, Paul P, Adhikary B (2012) Synthesis of FeS and FeSe nanoparticles from a single source precursor: a study of their photocatalytic activity, peroxidase-like behavior, and electrochemical sensing of H2O2. ACS Appl Mater Interfaces 4(4):1919–1927
Ge S, Liu W, Liu H, Liu F, Yu J, Yan M, Huang J (2015) Colorimetric detection of the flux of hydrogen peroxide released from living cells based on the high peroxidase-like catalytic performance of porous PtPd nanorods. Biosens Bioelectron 71:456–462. doi:10.1016/j.bios.2015.04.055
Chen X, Su B, Cai Z, Chen X, Oyama M (2014) PtPd nanodendrites supported on graphene nanosheets: a peroxidase-like catalyst for colorimetric detection of H2O2. Sensors Actuators B Chem 201:286–292. doi:10.1016/j.snb.2014.04.067
He W, Wu X, Liu J, Hu X, Zhang K, Hou S, Zhou W, Xie S (2010) Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem Mater 22(9):2988–2994
Liu Z, Zhao B, Shi Y, Guo C, Yang H, Li Z (2010) Novel nonenzymatic hydrogen peroxide sensor based on iron oxide–silver hybrid submicrospheres. Talanta 81(4):1650–1654
Roy P, Lin Z-H, Liang C-T, Chang H-T (2012) Synthesis of enzyme mimics of iron telluride nanorods for the detection of glucose. Chem Commun 48(34):4079–4081
Han L, Zeng L, Wei M, Li CM, Liu A (2015) A V2O3-ordered mesoporous carbon composite with novel peroxidase-like activity towards the glucose colorimetric assay. Nanoscale 7(27):11678–11685. doi:10.1039/c5nr02694f
Qiao F, Qi Q, Wang Z, Xu K, Ai S (2016) MnSe-loaded g-C3N4 nanocomposite with synergistic peroxidase-like catalysis: synthesis and application toward colorimetric biosensing of H2O2 and glucose. Sensors Actuators B Chem 229:379–386. doi:10.1016/j.snb.2015.12.109
Zheng A-X, Cong Z-X, Wang J-R, Li J, Yang H-H, Chen G-N (2013) Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens Bioelectron 49:519–524. doi:10.1016/j.bios.2013.05.038
Lin L, Song X, Chen Y, Rong M, Zhao T, Wang Y, Jiang Y, Chen X (2015) Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal Chim Acta 869:89–95. doi:10.1016/j.aca.2015.02.024
Qian J, Yang X, Yang Z, Zhu G, Mao H, Wang K (2015) Multiwalled carbon nanotube@reduced graphene oxide nanoribbon heterostructure: synthesis, intrinsic peroxidase-like catalytic activity, and its application in colorimetric biosensing. Journal of Materials Chemistry B 3(8):1624–1632. doi:10.1039/c4tb01702a
Hayat A, Haider W, Raza Y, Marty JL (2015) Colorimetric cholesterol sensor based on peroxidase like activity of zinc oxide nanoparticles incorporated carbon nanotubes. Talanta 143:157–161. doi:10.1016/j.talanta.2015.05.051
Haider W, Hayat A, Raza Y, Anwar Chaudhry A, Rehman I-U, Marty JL (2015) Gold nanoparticle decorated single walled carbon nanotube nanocomposite with synergistic peroxidase like activity for d-alanine detection. RSC Adv 5(32):24853–24858. doi:10.1039/C5RA01258A
Wang S, Chen Z, Choo J, Chen L (2016) Naked-eye sensitive ELISA-like assay based on gold-enhanced peroxidase-like immunogold activity. Anal Bioanal Chem 408(4):1015–1022. doi:10.1007/s00216-015-9219-8
Shi W, Fan H, Ai S, Zhu L (2015) Pd nanoparticles supported on nitrogen, sulfur-doped three-dimensional hierarchical nanostructures as peroxidase-like catalysts for colorimetric detection of xanthine. RSC Adv 5(41):32183–32190. doi:10.1039/c5ra02312b
He S-B, Wu G-W, Deng H-H, Liu A-L, Lin X-H, Xia X-H, Chen W (2014) Choline and acetylcholine detection based on peroxidase-like activity and protein antifouling property of platinum nanoparticles in bovine serum albumin scaffold. Biosens Bioelectron 62:331–336. doi:10.1016/j.bios.2014.07.005
Jiang T, Song Y, Wei T, Li H, Du D, Zhu M-J, Lin Y (2016) Sensitive detection of Escherichia coli O157:H7 using Pt–Au bimetal nanoparticles with peroxidase-like amplification. Biosens Bioelectron 77:687–694. doi:10.1016/j.bios.2015.10.017
Hu X, Saran A, Hou S, Wen T, Ji Y, Liu W, Zhang H, Wu X (2014) Rod-shaped Au@PtCu nanostructures with enhanced peroxidase-like activity and their ELISA application. Chin Sci Bull 59(21):2588–2596. doi:10.1007/s11434-014-0316-4
Zhan L, Li CM, Wu WB, Huang CZ (2014) A colorimetric immunoassay for respiratory syncytial virus detection based on gold nanoparticles-graphene oxide hybrids with mercury-enhanced peroxidase-like activity. Chem Commun 50(78):11526–11528. doi:10.1039/c4cc05155f
Dutta S, Ray C, Mallick S, Sarkar S, Sahoo R, Negishi Y, Pal T (2015) A gel-based approach to design hierarchical CuS decorated reduced graphene oxide nanosheets for enhanced peroxidase-like activity leading to colorimetric detection of dopamine. J Phys Chem C 119(41):23790–23800. doi:10.1021/acs.jpcc.5b08421
Chen C, Li N, Lan J, Ji X, He Z (2016) A label-free colorimetric platform for DNA via target-catalyzed hairpin assembly and the peroxidase-like catalytic of graphene/Au-NPs hybrids. Anal Chim Acta 902:154–159. doi:10.1016/j.aca.2015.10.030
Zhao H, Qu Y, Yuan F, Quan X (2016) A visible and label-free colorimetric sensor for miRNA-21 detection based on peroxidase-like activity of graphene/gold-nanoparticle hybrids. Anal Methods 8(9):2005–2012. doi:10.1039/c5ay03296b
Liu Q, Ding Y, Yang Y, Zhang L, Sun L, Chen P, Gao C (2016) Enhanced peroxidase-like activity of porphyrin functionalized ceria nanorods for sensitive and selective colorimetric detection of glucose. Mater Sci Eng C 59:445–453. doi:10.1016/j.msec.2015.10.046
Mu J, He Y, Wang Y (2016) Copper-incorporated SBA-15 with peroxidase-like activity and its application for colorimetric detection of glucose in human serum. Talanta 148:22–28. doi:10.1016/j.talanta.2015.10.060
Ghosh AB, Saha N, Sarkar A, Dutta AK, Biswas P, Nag K, Adhikary B (2016) Morphological tuning of Eu2O2S nanoparticles, manifestation of peroxidase-like activity and glucose assay use. New J Chem 40(2):1595–1604. doi:10.1039/c5nj02705e
Lin X-Q, Deng H-H, Wu G-W, Peng H-P, Liu A-L, Lin X-H, Xia X-H, Chen W (2015) Platinum nanoparticles/graphene-oxide hybrid with excellent peroxidase-like activity and its application for cysteine detection. Analyst 140(15):5251–5256. doi:10.1039/c5an00809c
Wu X-Q, Xu Y, Chen Y-L, Zhao H, Cui H-J, Shen J-S, Zhang H-W (2014) Peroxidase-like activity of ferric ions and their application to cysteine detection. RSC Adv 4(110):64438–64442. doi:10.1039/c4ra11000e
Deng H-H, Hong G-L, Lin F-L, Liu A-L, Xia X-H, Chen W (2016) Colorimetric detection of urea, urease, and urease inhibitor based on the peroxidase-like activity of gold nanoparticles. Anal Chim Acta 915:74–80. doi:10.1016/j.aca.2016.02.008
Chai D-F, Ma Z, Qiu Y, Lv Y-G, Liu H, Song C-Y, Gao G-G (2016) Oxidase-like mimic of Ag@Ag3PO4 microcubes as a smart probe for ultrasensitive and selective Hg2+ detection. Dalton Trans 45(7):3048–3054. doi:10.1039/c5dt04192a
Zhang S, Li H, Wang Z, Liu J, Zhang H, Wang B, Yang Z (2015) A strongly coupled Au/Fe3O4/GO hybrid material with enhanced nanozyme activity for highly sensitive colorimetric detection, and rapid and efficient removal of Hg2+ in aqueous solutions. Nanoscale 7(18):8495–8502. doi:10.1039/c5nr00527b
Li W, Chen B, Zhang H, Sun Y, Wang J, Zhang J, Fu Y (2015) BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury(II) ions. Biosens Bioelectron 66:251–258. doi:10.1016/j.bios.2014.11.032
Hayat A, Cunningham J, Bulbul G, Andreescu S (2015) Evaluation of the oxidase like activity of nanoceria and its application in colorimetric assays. Anal Chim Acta 885:140–147
Huang X, Hao Y, Wu H, Guo Q, Guo L, Wang J, Zhong L, Lin T, Fu F, Chen G (2014) Magnetic beads based colorimetric detection of mercuric ion. Sensors Actuators B Chem 191:600–604
Mohammadpour Z, Safavi A, Shamsipur M (2014) A new label free colorimetric chemosensor for detection of mercury ion with tunable dynamic range using carbon nanodots as enzyme mimics. Chem Eng J 255:1–7
Zhu R, Zhou Y, Wang X-L, Liang L-P, Long Y-J, Wang Q-L, Zhang H-J, Huang X-X, Zheng H-Z (2013) Detection of Hg2+ based on the selective inhibition of peroxidase mimetic activity of BSA-Au clusters. Talanta 117:127–132. doi:10.1016/j.talanta.2013.08.053
Kim YS, Jurng J (2013) A simple colorimetric assay for the detection of metal ions based on the peroxidase-like activity of magnetic nanoparticles. Sensors Actuators B Chem 176:253–257. doi:10.1016/j.snb.2012.10.052
Tseng C-W, Chang H-Y, Chang J-Y, Huang C-C (2012) Detection of mercury ions based on mercury-induced switching of enzyme-like activity of platinum/gold nanoparticles. Nanoscale 4(21):6823–6830. doi:10.1039/c2nr31716h
Jia S-M, Liu X-F, Li P, Kong D-M, Shen H-X (2011) G-quadruplex DNAzyme-based Hg2+ and cysteine sensors utilizing Hg2 + −mediated oligonucleotide switching. Biosens Bioelectron 27(1):148–152. doi:10.1016/j.bios.2011.06.032
Taghdisi SM, Danesh NM, Lavaee P, Emrani AS, Ramezani M, Abnous K (2015) A novel colorimetric triple-helix molecular switch aptasensor based on peroxidase-like activity of gold nanoparticles for ultrasensitive detection of lead(ii). RSC Adv 5(54):43508–43514. doi:10.1039/c5ra06326d
Wu Y-S, Huang F-F, Lin Y-W (2013) Fluorescent detection of lead in environmental water and urine samples using enzyme mimics of catechin-synthesized Au nanoparticles. ACS Appl Mater Interfaces 5(4):1503–1509. doi:10.1021/am3030454
Zhao D, Chen C, Lu L, Yang F, Yang X (2015) A label-free colorimetric sensor for sulfate based on the inhibition of peroxidase-like activity of cysteamine-modified gold nanoparticles. Sensors Actuators B Chem 215:437–444. doi:10.1016/j.snb.2015.04.010
Sun R, Wang Y, Ni Y, Kokot S (2014) Spectrophotometric analysis of phenols, which involves a hemin–graphene hybrid nanoparticles with peroxidase-like activity. J Hazard Mater 266:60–67. doi:10.1016/j.jhazmat.2013.12.006
Wong A, Materon EM, Sotomayor MDPT (2014) Development of A biomimetic sensor modified with HEMIN and GRAPHENE oxide for monitoring of CARBOFURAN in food. Electrochim Acta 146:830–837. doi:10.1016/j.electacta.2014.09.091
Wang M, Huang J, Wang M, Zhang D, Chen J (2014) Electrochemical nonenzymatic sensor based on CoO decorated reduced graphene oxide for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. Food Chem 151:191–197
Shang L, Zhao F, Zeng B (2014) Sensitive voltammetric determination of vanillin with an AuPd nanoparticles − graphene composite modified electrode. Food Chem 151:53–57
David IG, Bizgan A-MC, Popa DE, Buleandra M, Moldovan Z, Badea IA, Tekiner TA, Basaga H, Ciucu AA (2015) Rapid determination of total polyphenolic content in tea samples based on caffeic acid voltammetric behaviour on a disposable graphite electrode. Food Chem 173:1059–1065
Sharpe E, Frasco T, Andreescu D, Andreescu S (2013) Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac). Analyst 138(1):249–262
Ping J, Wang Y, Wu J, Ying Y (2014) Development of an electrochemically reduced graphene oxide modified disposable bismuth film electrode and its application for stripping analysis of heavy metals in milk. Food Chem 151:65–71
Fei A, Liu Q, Huan J, Qian J, Dong X, Qiu B, Mao H, Wang K (2015) Label-free impedimetric aptasensor for detection of femtomole level acetamiprid using gold nanoparticles decorated multiwalled carbon nanotube-reduced graphene oxide nanoribbon composites. Biosens Bioelectron 70:122–129
Li W, Chen B, Zhang H, Sun Y, Wang J, Zhang J, Fu Y (2015) BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury (II) ions. Biosens Bioelectron 66:251–258
Zhang X, He S, Chen Z, Huang Y (2013) CoFe2O4 nanoparticles as oxidase mimic-mediated chemiluminescence of aqueous luminol for sulfite in white wines. J Agric Food Chem 61(4):840–847
Shu J, Qiu Z, Wei Q, Zhuang J, Tang D (2015) Cobalt-porphyrin-platinum-functionalized reduced graphene oxide hybrid nanostructures: a novel peroxidase mimetic system for improved electrochemical immunoassay. Sci Rep 5:15113–15124
Tan H, Ma C, Gao L, Li Q, Song Y, Xu F, Wang T, Wang L (2014) Metal–organic framework‐derived copper nanoparticle@ carbon nanocomposites as peroxidase mimics for colorimetric sensing of ascorbic acid. Chemistry–A European Journal 20(49):16377–16383
Wang Y, Hu J, Zhuang Q, Ni Y (2016) Enhancing sensitivity and selectivity in a label-free colorimetric sensor for detection of iron (II) ions with luminescent molybdenum disulfide nanosheet-based peroxidase mimetics. Biosens Bioelectron 80:111–117
Jiang T, Song Y, Wei T, Li H, Du D, Zhu M-J, Lin Y (2016) Sensitive detection of Escherichia coli O157: H7 using Pt–Au bimetal nanoparticles with peroxidase-like amplification. Biosens Bioelectron 77:687–694
Ding N, Yan N, Ren C, Chen X (2010) Colorimetric determination of melamine in dairy products by Fe3O4 magnetic nanoparticles − H2O2− ABTS detection system. Anal Chem 82(13):5897–5899
Thiramanas R, Jangpatarapongsa K, Tangboriboonrat P, Polpanich D (2013) Detection of vibrio cholerae using the intrinsic catalytic activity of a magnetic polymeric nanoparticle. Anal Chem 85(12):5996–6002
Gong J, Zhou T, Song D, Zhang L, Hu X (2009) Stripping voltammetric detection of mercury (II) based on a bimetallic Au − Pt inorganic − organic hybrid nanocomposite modified glassy carbon electrode. Anal Chem 82(2):567–573
Bulbul G, Hayat A, Andreescu S (2015) A generic amplification strategy for electrochemical aptasensors using a non-enzymatic nanoceria tag. Nanoscale 7(31):13230–13238
Zhang Z, Wang X, Yang X (2011) A sensitive choline biosensor using Fe3O4 magnetic nanoparticles as peroxidase mimics. Analyst 136(23):4960–4965
Cao G-X, Wu X-M, Dong Y-M, Li Z-J, Wang G-L (2016) Colorimetric determination of melamine based on the reversal of the mercury (II) induced inhibition of the light-triggered oxidase-like activity of gold nanoclusters. Microchim Acta 183(1):441–448
Kang X, Zhang J, Shang W, Wu T, Zhang P, Han B, Wu Z, Mo G, Xing X (2014) One-step synthesis of highly efficient Nanocatalysts on the supports with hierarchical pores using porous ionic liquid-water gel. J Am Chem Soc 136(10):3768–3771
Yang X, Cui H, Li Y, Qin J, Zhang R, Tang H (2013) Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance. ACS Catal 3(3):363–369. doi:10.1021/cs3008126
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Nasir, M., Nawaz, M.H., Latif, U. et al. An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta 184, 323–342 (2017). https://doi.org/10.1007/s00604-016-2036-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2036-8