Abstract
A colorimetric detection scheme is introduced for the determination of alkaline phosphatase (ALP) activity based on Cu(II)-modulated G-quadruplex-based DNAzymes. It is exploiting the strong affinity of Cu(II) for pyrophosphate (PPi) upon which the cofactor PPi is trapped by Cu(II). Hence, the activity of the DNAzyme is inhibited. ALP catalyzes the hydrolysis of PPi, causing the release of Cu(II). DNAzyme, in turn, is activated and catalyzes the cleavage of the DNA probe substrate. The released G-rich sequence folds into the G-quadruplex, which can bind hemin and catalyze the oxidation of 2,2′-azinobis (3-ethylbenzothiozoline)-6-sulfonate (ABTS), and this leads to an increase in absorbance at 420 nm. Absorbance increases linearly with increasing ALP activity in 0.07 to 300 U.L−1 range, with a 70 mU.L−1 detection limit. The method was applied in ALP inhibition tests and to the determination of ALP activity in spiked serum samples where it gave satisfactory results.

A colorimetric method has been developed for the detection of alkaline phosphatase based on the use of Cu(II)-modulated G-quadruplex-based DNAzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkaline phosphatase (ALP), a widely distributed enzyme in mammalian tissues, is responsible for dephosphorylation in metabolic pathways [1]. Because the level of serum ALP is linked closely to an extensive range of diseases, including osteopathy, hepatopathy, breast and prostatic carcinoma, cardiac diseases, and diabetes [2, 3], it has been developed to become an important biomarker to help in early diagnosis of hardly-recognized diseases. Beside its high potential in clinical field, it is also advantageous to forensic investigations [4]. Hence, progressive exploration of a strategy for ALP detection that has high velocity, sensitivity, facility, and adjustable dynamic range is undoubtedly in considerably high demand.
Over the past decades, various methods have been put forward to detect ALP activity, and some of these have made some progress in their high sensitivities based on techniques, such as fluorometry [5,6,7,8,9,10,11,12], electrochemistry [13,14,15,16,17], chromatography [18], colorimetry [19,20,21], and surface-enhanced Raman spectroscopy [22]. Despite some progress, some of these methods have disadvantages: they are time-consuming, high-cost, and incapable of convenient and/or real-time measurement. It is worth mentioning that among these various methods, the fluorometric methods, in which different types of fluorescence probes are mainly utilized, has its advantage and is of many researchers’ interests owing to its rapid response, relatively high sensitivity, and convenience. Thousands of papers have established numerous fluorescence probes, and a large portion of which, probes are designed to detect ALP activity. These probes can include conjugated polyelectrolytes, small molecule organic probes, metal nanoclusters, nanosheets, DNA-templated nanoparticles, and quantum dots (QDs) [5,6,7,8,9,10]. Although these fluorescent strategies have indeed made an unignorable contribution to ALP detection, their disadvantages which cannot be ignored can include poor photostability and water solubility of organic fluorescent dyes, complex synthesis and purification processes of conjugated polyelectrolytes, and high toxicity of QDs. Recently, some late-model methods have been established for the detection of ALP activity, in which carbon dots were used as fluorescent signal [23, 24]. Nevertheless, these fluorescence quenching-based enzymatic assays can possibly produce false positive signals when interfered by environmental stimulus. Qu et al. have developed a novel and sensitive turn-on fluorescence method via carbon dots and MnO2 nanosheets [10]. Its popularity is, however, restricted by its expensive and time-consuming synthesis of MnO2 nanosheets. In addition, Sun and co-workers have advanced the fluorescence-based ALP detection with highly fluorescent dots. In contrast to these unfavorable features, a unique visual detection technique that can directly observe such signals without any highly complex and/or costly apparatus has gained increasing attention. A commercial kit has been the dominate detection method for detecting of ALP [25]. However, the sensitivity is sometimes unsatisfactory. Therefore, a facile, sensitive, and low-cost method for the detection of ALP is desired.
Herein, we report a colorimetric assay system based on Cu2+-modulated G-Quadruplex-based DNAzymes for the detection of ALP activity [26]. Owing to the advantage of strong binding ability of Cu2+ and pyrophosphate (PPi) [27,28,29], cofactor Cu2+ can be trapped and lead to effective inhibition of DNAzyme activity. Because ALP can catalyze the hydrolysis of PPi, ALP can cause the release of Cu2+, and in turn activate DNAzyme, which catalyzes the cleavage of DNA probe substrate. These events result in increased absorbance of 2,2′-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid)-H2O2 system [30,31,32].
Experimental
Materials and methods
Alkaline phosphatase (ALP), Uracil DNA glycosylase (UDG), 8-hydroxy guanine DNA glycosidase (hoGG1), bovine serum albumin (BSA), T4 polynucleotide kinase (T4 PNK),and Lambda exonuclease (λ Exo) were purchased from Takara Biotechnology Co., Ltd. (http://www.takarabiomed.com.cn/) (DaLian, China). HPLC-purified DNA probe(agc ttc ttt cta ata cgg tgg gta ggg cgg gtt ggg cta ccc acc tgg gcc tct ttc ttt tta aga aag aac)was obtained from Sangon Biotechnology Co., Ltd. (http://www.sangon.com/) (Shanghai, China). HeEPES, free acid, Sodium chloride (NaCl), Tris (Tris-(hydroxymethyl) aminomethane), hydrochloric acid (HCl), copper sulfate (CuSO4), pyrophosphate (PPi), and hydrogen peroxide (H2O2) were purchased from Sinopharm Chemical Reagent Co., Ltd. (http://en.reagent.com.cn/) (Shanghai, China). Hemin and 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) were purchased from Yuanye (http://www.shyuanye.com/) (Shanghai, China). Inorganic phosphate (Pi) was obtained from an equilibrium buffer solution of Na2HPO4 and NaH2PO4 (pH = 7.4). All other reagents were of analytical-reagent grades. Ultrapure water (18.2 MΩ cm−1) was used in all experiments. All DNA sequences were prepared in TE buffer and stored at −20 °C.
The absorbance was recorded on an Enspire® multimode plate readers (Perkin Elmer, USA) using a 96-well plate. The absorption spectra of the solution were measured at wavelengths from 400 to 470 nm. The absorbance was obtained at 420 nm.
Investigation of feasibility
To investigate feasibility of the method in assaying ALP activity, two types of samples (mixtures A and B) were prepared. In the first sample (mixture A; 35 μL), 60 nM DNA Probe, 10.5 mM NaCl, and 3.5 mM HEPES were first added into 29.5 μL of ultrapure water. The mixture was then heated to 80 °C for 2 min and cooled down to room temperature for 30 min. The second sample (mixture B; 35 μL) was prepared by mixing 4 × 103 U.L−1 ALP, 700 μM.L−1 PPi, 400 μM.L−1 Cu2+ in 31 μL tris buffer, and was then incubated at 37 °C for about 30 min. After that, mixtures A and B were mixed and incubated at 25 °C for 15 min, and 1 μL of 100 μM hemin was subsequently added and incubated at 25 °C for 30 min to allow the DNA probe to properly fold and form G-quadruplex/hemin complex. Finally, 15 μL each of 20 mM H2O2 and 20 mM ABTS was added into the mixture. After 10 min, the absorption spectra from 400 to 470 nm was measured. Samples, in which ALP were not added, were done in parallel for comparison.
Optimization of analysis conditions
Various concentrations of DNA probe (10–200 nM), PPi (100–800 μM), and Cu2+ (100–1000 μM) were tested in order to find an optimal condition.
ALP activity assay
Twelve samples were prepared in the assay of ALP activity under an optimized condition. Mixture A in a reaction buffer (29.5 μL H2O, 3.5 mM HEPES, 10.5 mM NaCl) containing 60 nM DNA probe, and mixture B in a reaction buffer (10 mM Tris-HCl, 700 μM.L−1 PPi, 400 μM.L−1 Cu2+, pH 7.5) containing various amounts of ALP were first mixed, and 1 μL of 100 μM, 15 μL of 20 mM H2O2, and 15 μL of 20 mM ABTS were then added. The detailed reaction conditions were the same as that used in the investigation of feasibility.
Determination of ALP activity in human serum samples
ALP activity was assayed under the optimal experimental conditions. Human serum samples (1%) and different ALP activities (in the working range of this method) were thoroughly mixed, and the reaction was allowed to take place at room temperature for 3 min. While mixture A was prepared according to that for the ALP activity assay, mixture B was in 10 mM Tris-HCl (pH 7.5), 700 μM.L−1 PPi, 400 μM.L−1 Cu2+, and different activities of ALP. Subsequent procedures and absorbance measurements were carried out following the ALP activity assay. Recoveries of ALP from the serum samples were calculated by the regression equation using the absorbance and the activity of ALP.
Results and discussion
Experimental principles
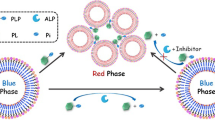
Principle of the proposed detection method is illustrated in Fig. 1b, and the structure of the DNA porbe is shown in Fig. 1a. The DNA probe consists of three main components: domain III represents the DNA-cleaving Cu2+-dependent DNAzyme; domain II contains the sequence of HRP-mimicking DNAzyme, which can give colorimetric signal readout; and domain I is the substrate of DNA-DNAzyme. In the absence of ALP, PPi can strongly chelate and form stable complex with Cu2+ ion. As a result, the activity of DNAzyme, which catalyzes the cleavage of DNA probe substrate, is inhibited, leading to a low absorbance background in the assay system. On the other hand, when APL is present, it catalyzes the hydrolysis of PPi into phosphate, causing the release of free Cu2+ cofactor, which thus activates DNAzyme to catalyze the cleavage of DNA probe substrate. The released G-rich sequence (domain II) folds into a G-quadruplex that can intercalate hemin and form catalytically active HRP-mimicking DNAzyme, resulting in an increased absorbance of the ABTS-H2O2 system, in which ALP levels can subsequently be determined by the variations of absorbance intensity.
Feasibility of the proposed strategy
To verify whether or not the proposed strategy is feasible, two samples were prepared: sample A containing DNA probe, Cu2+, and PPi; and sample B containing DNA probe, Cu2+, PPi, and ALP. Fig. 2 shows the absorption spectra of the detection system in the presence (curve b) and absence (curve a) of ALP. The data showed that when ALP is absent (curve a), the absorbance is markedly lower than that when ALP is present, suggesting that Cu2+ may be reduced by PPi and no G-quadruplexes are formed. As displayed in Fig. 2b, absorbance is significant increased in the presence of ALP (4 × 103 U.L−1) compared with that in the absence of ALP, indicating that ALP blocks PPi from chelating Cu2+, and in turn G-quadruplexes are formed and led to such enhancement of colorimetric signal. Finally, these results demonstrated that the proposed ALP detection strategy was feasible.
Optimization of method
The following parameters were optimized: (a) DNA probe concentration; (b) Cu2+ concentration; (c) PPi concentration. Respective data and figures are given in the Fig. S1. The following experimental conditions are found to give best results: (a) A DNA probe concentration of 60 nM; (b) A Cu2+ concentration of 400 μM; (c) A PPi concentration of 700 μM.
Quantification of ALP activity
Under the chosen optimal conditions, performance of the proposed strategy, in terms of ALP activity detection, was systematically investigated, in which the activities of ALP are varied from 0 to 3000 U.L−1 (0.07, 1, 5, 30, 50, 70, 100, 300, 500, 1200, 2000, and 3000 U.L−1). As shown in Fig. S2, the absorbance increased with increasing activity of ALP and plateaued at 2000 U/L. The inset of Fig. 3 showed that the absorbance has a linear correlation (R2 = 0.9946) with ALP activity ranges from 0.07 to 300 U.L−1. The detection limit is estimated to be 0.07 U.L−1 according to the 3σ rule, which is comparable to or better than those of other methods (Table 1).
Selectivity
A series of enzymes, including UDG, hoGG1, Lambda Exo, and PNK, each at a concentration of 2 × 103 U.L−1, were tested. Some other molecules, such as lysozyme, BSA, streptavidin (SA), glycine, alanine, arginine, serine, tryptophane, glutamic acid, L-histidine, C6H12O6 and ATP, each at a concentration of 0.5 μM, were also tested. Fig. 4 shows that none of these proteins led to increased absorbance, in contrast to ALP. The results demonstrate that the method has good selectivity.
ALP activity inhibition assays
The inhibition of ALP is closely associated with drug screening and disease therapy; validity of the proposed assay in evaluation of ALP inhibition was thus investigated. Na3VO4 as a common ALP inhibitor was employed for inhibiting assays [35]. Fig. 5 shows that the relative activity of ALP drastically decreased with increasing Na3VO4 concentration with an IC50 value of 0.9 mM. This result shows that the proposed strategy is suitable for the identification and characterization of enzymatic inhibitors.
ALP activity assays in biological samples
To investigate practical use of the method, ALP was tested in 1% human serum. Table 2 shows the recovery amount of ALP detected by the proposed assay when ALP of 10, 20, and 50 U.L−1 were added into the biological sample. The recovery rates were 100.6%, 102.5%, and 91% from sample added with 10, 20, and 50 U.L−1 of ALP, respectively. These results show that the proposed method is highly potential for practical detection in biological systems.
Conclusions
In summary, a convenient colorimetric method based on Cu2+-modulated G-Quadruplex-based DNAzymes, is successfully developed and applied for ALP activity detection. The method exhibites high sensitivity to ALP with a detection limit of 0.07 U.L−1 under optimal conditions. Moreover, the proposed method is highly selective and successfully applied in quantitative determination of ALP in human serum samples with satisfactory result. This method does not require complicated synthesis and/or modification of the probes. Thus, the present strategy may have potential applications for ALP detection in analytical practice.
References
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Annual. Annu Rev Biophys Biomol Struct 21:441–483
Liu H, Ma C, Wang J, Wang K, Wu K (2017) A turn-on fluorescent method for determination of the activity of alkaline phosphatase based on dsDNA-templated copper nanoparticles and exonuclease based amplification. Microchim Acta 184:2483–2487
Ndrepepa G, Xhepa E, Braun S, Cassese S, Fusaro M, Schunkert H, Kastrati A (2017) Alkaline phosphatase and prognosis in patients with coronary artery disease. Eur J Clin Investig 47:378–387
Agudelo J, Halámková L, Brunelle E, Rodrigues R, Huynh C, Halámek J (2016) Ages at a crime scene: simultaneous estimation of the time since deposition and age of its originator. Anal Chem 88:6479–6484
Kang W, Ding Y, Zhou H, Liao QY, Yang X, Yang YG, Jiang JS, Yang M (2015) Monitoring the activity and inhibition of alkaline phosphatase via, quenching and restoration of the fluorescence of carbon dots. Microchim Acta 182:1161–1167
Guo L, Chen D, Yang M (2017) DNA-templated silver nanoclusters for fluorometric determination of the activity and inhibition of alkaline phosphatase. Microchim Acta 184:2165–2170
Zhang W, Gao Y, Li Y, Zhang Q, Hu Z, Zhang Y, Hussain E, Yang X, Yu D, Yu C (2017) Polyphosphoric acid-induced perylene probe self-assembly and label-free fluorescence turn-on detection of alkaline phosphatase. Anal Bioanal Chem 409:1031–1036
Halawa MI, Gao W, Saqib M, Kitte SA, Wu F, Xu G (2017) Sensitive detection of alkaline phosphatase by switching on gold nanoclusters fluorescence quenched by pyridoxal phosphate. Biosens Bioelectron 95:8–14
Xiang MH, Liu JW, Li N, Tang H, RQ Y, Jiang JH (2016) A fluorescent graphitic carbon nitride nanosheet biosensor for highly sensitive, label-free detection of alkaline phosphatase. Nano 8:4727–4732
Qu F, Pei H, Kong R, Zhu S, Xia L Novel turn-on fluorescent detection of alkaline phosphatase based on green synthesized carbondots and MnO2 nanosheets. Talanta 165:136–142
Li J, Si L, Bao J, Wang Z, Dai Z (2017) Fluorescence regulation of poly (thymine)-templated copper nanoparticles via an enzyme-triggered reaction towards sensitive and selective detection of alkaline phosphatase. Anal Chem 89:3681–3686
Hu Z, Chen J, Li Y, Wang Y, Zhang Q, Hussain E, Yang M, Shahzad SA, Yu D, Yu C (2017) Nucleic acid-controlled quantum dots aggregation: a label-free fluorescence turn-on strategy foralkaline phosphatase detection. Talanta 169:64–69
Liu Y, Xiong E, Li X, Li J, Zhang X, Chen J (2017) Sensitive electrochemical assay of alkaline phosphatase activity based on TdT-mediated hemin/G-quadruplex DNAzyme nanowires for signal amplification. Biosens Bioelectron 87:970–975
Lv JJ, Yang ZH, Zhuo Y, Yuan R, Chai YQ (2015) A novel aptasensor for thrombin detection based on alkaline phosphatase decorated ZnO/Ptnanoflowers as signal amplifiers. Analyst 140:8088–8091
Goggins S, Naz C, Marsh BJ, Frost CG (2015) Ratiometric electrochemical detection of alkaline phosphatase. Chem Commun 51:561–564
Peng J, Han XX, Zhang QC, Yao HQ, Gao ZN (2015) Copper sulfide nanoparticle-decorated graphene as a catalytic amplification platform forelectrochemical detection of alkaline phosphatase activity. Anal Chim Acta 878:87–94
Li X, Zhu L, Zhou Y, Yin H, Ai S (2017) Enhanced Photoelectrochemical method for sensitive detection of protein kinase a ActivityUsing TiO2/g-C3N4, PAMAM dendrimer, and alkaline phosphatase. Anal Chem 89:2369–2376
Lakra S, Jadhav VJ, Garg SR (2016) Development of a chromatographic method for the determination of alkaline phosphatase activity in pasteurized milk. F Anal Methods 9:2002–2009
Jiao H, Chen J, Li W, Wang F, Zhou H, Li Y, Yu C (2014) Nucleic acid-regulated perylene probe-induced gold nanoparticle aggregation: a new strategy for colorimetric sensing of alkaline phosphatase activity and inhibitor screening. ACS Appl Mater Interfaces 6:1979–1985
Zhang Z, Chen Z, Wang S, Cheng F, Chen L (2015) Iodine-mediated etching of gold Nanorods for Plasmonic ELISA based on colorimetric detection of alkaline phosphatase. ACS Appl Mater Interfaces 7:27639–27645
Shi D, Sun Y, Lin L, Shi C, Wang G, Zhang X (2016) Naked-eye sensitive detection of alkaline phosphatase (ALP) and pyrophosphate (PPi) based on a horseradish peroxidase catalytic colorimetric system with Cu(ii). Analyst 141:5549–5554
Ruan C, Wang W, Gu B (2006) Detection of alkaline phosphatase using surface-enhanced Raman spectroscopy. Anal Chem 78:3379–3384
Qian ZS, Chai LJ, Huang YY, Tang C, Shen JJ, Chen JR, Feng H (2015) A real-time fluorescent assay for the detection of alkaline phosphatase activity based on carbon quantum dots. Biosens Bioelectron 68:675–680
Li G, Fu H, Chen X, Gong P, Chen G, Xia L, Wang H, You J, Wu Y (2016) Facile and sensitive fluorescence sensing of alkaline phosphatase activity with Photoluminescent carbon dots based on inner filter effect. Anal Chem 88:2720–2726
Tripathi G, Basu B (2012) A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram Int 38:41–349
Yin BC, Ye BC, Tan W, Wang H, Xie CC (2009) An allosteric dual-DNAzyme unimolecular probe for colorimetric detection of copper(II). J Am Chem Soc 131:14624–14625
Lin Y, Hu L, Li L, Wang KQ, Ji YF, Zou H (2015) Electrochemical determination of pyrophosphate at nanomolar levels using a gold electrode covered with a cysteine nanofilm and based on competitive coordination of Cu(II) ion to cysteine and pyrophosphate. Microchim Acta 182:2069–2075
Spangler C, Schaeferling M, Wolfbeis OS (2008) Fluorescent probes for microdetermination of inorganic phosphates and biophosphates. Microchim Acta 161:1–39
Luo L, Chen Y, Zhang L, Li YR, Zhang HQ, Tian Y (2017) SERS assay for pyrophosphate based on its competitive binding to Cu(II) ion on silver nanoparticles modified with cysteine and rhodamine 6G. Microchim Acta 184:595–601
Tang Y, Lai W, Zhang J, Tang D (2017) Competitive photometric and visual ELISA for aflatoxin B1 based on the inhibition of the oxidation of ABTS. Microchim Acta 184:2387–2394
Fang X, XM W, XL H, Li ZJ, Wang JL (2016) Native carbon nanodots as a fluorescent probe for assays based on the use of glucose oxidase or horseradish peroxidase. Microchim Acta 183:2761–2770
Xu H, Wu D, Li CQ, Lu Z, Liao XY, Huang J, ZS W (2017) Label-free colorimetric detection of cancer related gene based on two-step amplification of molecular machine. Biosens Bioelectron 90:314–320
Hu Q, He M, Mei Y, Feng W, Jing S, Kong J, Zhang X (2017) Sensitive and selective colorimetric assay of alkaline phosphatase activity with Cu(II)-phenanthroline complex. Talanta 163:146–152
Du J, Xiong L, Ma C, Liu H, Wang J (2016) Label-free DNA hairpin probe for real-time monitoring of alkaline phosphatase activity. Anal Methods 8:5095–5100
Zhao ZW, Zhu WP, Li Z, Jiang JH, Shen GL, Yu RQ (2012) Sensitive and selective label-free alkaline phosphatase detection based on DNA hairpin probe. Anal Sci 28:881–886
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21205142), The Research Innovation Program for Graduates of Central South University (2016zzts580, 2017zzts347).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 132 kb)
Rights and permissions
About this article
Cite this article
Tang, Z., Zhang, H., Ma, C. et al. Colorimetric determination of the activity of alkaline phosphatase based on the use of Cu(II)-modulated G-quadruplex-based DNAzymes. Microchim Acta 185, 109 (2018). https://doi.org/10.1007/s00604-017-2628-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2628-y