Abstract
The authors describe a method for the determination of the activity of alkaline phosphatase (ALP) that utilizes dsDNA-templated copper nanoparticles (CuNPs) coupled to enzymatic amplification via λ exonuclease. A hybrid of a DNA modified with a phosphate moiety at the 5′-end (P-DNA) and a P-DNA complementary sequence (cP-DNA) is employed as the dsDNA substrate for ALP. In the absence of ALP, the dsDNA is cleaved by the λ exonuclease, which hinders the formation of CuNPs which display fluorescence with excitation/emission peaks at 340/565 nm. However, ALP-mediated hydrolysis of the 5′-phosphoryl end impedes the cleavage of dsDNA by the λ exonuclease, and this promotes the formation of fluorescent dsDNA-templated CuNPs via ascorbate-mediated reduction. Under the optimized experimental conditions, this method exhibits a high specificity to ALP and has a 0.1 U⋅L−1 limit of detection. The strategy also provides the basis for a screening platform for inhibitors of ALP.

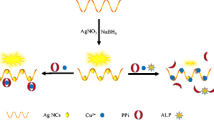
Schematic presentation of a fluorescence assay for the detection of alkaline phosphatase based on dsDNA-templated copper nanoparticles and exonuclease based amplification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkaline phosphatase (ALP) is found in almost all living organisms and in a variety of tissues (liver, bone, intestine, kidney, and placenta) [1]. Since ALP plays a pivotal role in cell cycle, growth, apoptosis, and signal transduction pathways, it is commonly used as a biomarker for the development of phosphorylation-related biochemical behaviors and clinical laboratory tests [2]. Studies have shown that abnormal serum levels of ALP are closely related to a variety of human diseases, especially those of the liver and bone [3]. Currently, serum ALP level is routinely used as a diagnostic index for several human diseases such as bone disorders (Paget’s disease and osteomalacia), cancer (breast cancer and prostatic cancer), diabetes, and liver dysfunction and the normal concentration of ALP in human serum is in the range of 20–140 U/L [4–6]. Considering the significance of ALP levels in diagnosis of human diseases, the development of a simple, sensitive, yet inexpensive method for detection of ALP activity and its inhibition is required.
Several novel analytical strategies have been developed for detection and quantitation of ALP activity, such as colorimetric assays [7–9], electrochemical detection assays [10–12], and assays based on surface enhanced resonance Raman scattering (SERRS) assay [13]. Although these novel analytical strategies are usually highly sensitive and require small sample volume, they are expensive and require tedious sample preparation compared to the fluorescence-based methods. So far, some facile, low-cost, and sensitive fluorescence-based strategies have been used to assay the activity of ALP via its ability to remove phosphate groups from different types of substrates such as nucleic acids, proteins and small molecules [14–29]. Among these substrates, nucleic acids have been extensively used to set up fluorescence-based methods for detection of ALP activity. For example, Wang et al. reported a promising new fluorescence-based sensing strategy for ALP detection that utilizes the polycation-induced noncovalent perylene DNA probe self-assembly [21]. However, this approach involves complicated procedures for probe synthesis. Two fluorescence assays that use the graphene oxide platform and nucleic acid substrates for ALP detection have also been reported [18, 25]. However, the synthesis of graphene oxide is time-consuming and expensive. Du and co-works reported a new method for detection of ALP activity based on a DNA hairpin and thioflavin T (ThT) probes [14]. Although this method is low-cost and facile, and has poor sensitivity, which impedes its application. Thus, development of a simple, sensitive, cost-effective, and robust method to assay ALP activity is required.
DNA-templated metal nanoparticle—a new fluorescent probe—has attracted interest in the field of analytical sciences due to its inherent advantages such as superior optical properties, low toxicity, and high sensitivity [30]. Compared to the DNA-templated silver nanoparticles, the DNA-templated copper nanoparticles (CuNPs) possess the inherent advantage of being inexpensive and simple to synthesize. The DNA-templated CuNPs have been widely used for biomarker detection owing to their specificity towards the DNA templates and generation of relatively low background from biomolecules such as DNA, microRNA, small molecules, metal ions, enzyme and proteins in biological systems [31–35]. For example, two groups reported the detection of ALP activity based on the formation of fluorescent CuNPs employing DNA as a template [36, 37]. In this system, ALP hydrolyzes pyrophosphate and releases free Cu2+ ions, which effectively form DNA-templated fluorescent CuNPs.
Here, a fluorescence-based strategy is prepared and applied in ALP activity detecting that utilizes the specificity of the fluorescent CuNP probes towards dsDNA templates and the λ exonuclease (λ exo) cleavage reaction. λ exo specifically degrades dsDNA strands with a 5′-phosphoryl termini, generating single-stranded DNA (ssDNA) and mononucleotides. A dsDNA, constructed by hybridizing a P-DNA (modified with a phosphate moiety at 5′-end) and a P-DNA complementary sequence (cP-DNA), was used as the substrate for ALP in our method. In addition, the dsDNA used in this assay was used as templates for synthesizing fluorescent dsDNA-templated CuNPs. In the absence of ALP, the dsDNA is digested by λ exo, which prevents formation of the dsDNA-templated CuNPs. Once the 5′-phosphoryl end is hydrolyzed by ALP, the λ exo cleavage reaction is impeded and the system displays strong fluorescence due to the formation of the dsDNA-templated CuNPs. This new approach provides a low-cost and sensitive platform for detection of ALP and its inhibitors. Furthermore, our method can be also used for detection of ALP in human serum.
Experimental section
Materials and reagents
Alkaline phosphatase from E. coli C75 was procured from Takara Biotechnology Co., Ltd. (DaLian, China, http://www.takara.com.cn,). λ exonuclease was purchased from New England Biolabs (Beverly, MA, USA, http://www.neb.sg). Glucose oxidase (GOx) was purchased from MP Biomedicals (Santa Ana, California, USA, http://www.mpbio.com). Thrombin was purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA, http://www.sigmaaldrich.com). Bovine serum albumin (BSA) was purchased from Solarbio (Beijing, China, http://www.solarbio.com). 3′-(N-morpholino) propanesulfonic acid (MOPS), copper sulfate (CuSO
4
), and sodium ascorbate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, http://www.shreagent.lookchem.com). Inorganic phosphate (Pi), which exists in a buffered equilibrium mixture of Na2HPO4 and NaH2PO4 (pH 7.4) was used in this assay. All other reagents were of analytical reagent grade. Ultrapure water (18.2 MΩ.cm) from a Milli-Q water purification system (Millipore Corp, Bedford, MA, USA) was used in all the experiments. The two oligonucleotides, P-DNA and cP-DNA, were purchased from Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China) and purified using high performance liquid chromatography. The DNA sequences are as follows. P-DNA: 5′-p-CGTGATGAACGTATGAGCGTAT-3′, cP-DNA: 5′-ATACGCTCATACGTTCA TCACG-3′. The buffer used are as follows: MOPS buffer (10 mM MOPS, 150 mM NaCl and 10 mM MgCl2, pH 7.5).
Apparatus
All fluorescence measurements were performed on an F-2700 fluorescence spectrophotometer (Hitachi, Japan) with excitation at 340 nm and emission at 500–650 nm for the dsDNA-templated CuNPs. The excitation slits and emission slits were set for 5.0 and 10.0 nm, respectively. Each experiment was carried out in a final volume of 100 μL. All the error bars in this paper were estimated from three replicate measurements.
Determination of ALP
In order to quantitatively measure ALP activity, 500 nM each of P-DNA and cP-DNA were mixed in 100 μL MOPS buffer (10 mM MOPS, 150 mM NaCl and 10 mM MgCl2) and incubated for 10 min at 37 °C to form the dsDNA. Next, different concentrations of ALP were added to the substrate and incubated for 10 min at 37 °C. 45 U⋅mL−1 λ exo was subsequently added to the reaction solution, and incubated for 20 min at 37 °C. 140 μM Cu2+ and 1 mM sodium ascorbate were added to the mixture and incubated for 10 min at room temperature before measurement.
For detection of ALP in biological fluids, spiked samples were prepared by addition of different concentrations of ALP to 1% (v/v) human serum.
Inhibition of ALP activity
In this assay, Pi, the inhibitor of ALP, was incubated at various concentrations ranging from 0 to 50 μM with 500 nM dsDNA (P-DNA/cP-DNA) in 100 μL MOPS buffer. Then, 50 U⋅L−1 of ALP was added to the substrate and incubated for 10 min at 37 °C. Other experimental procedures were the same as mentioned before.
Results and discussion
Verification of the feasibility of ALP detection using dsDNA-templated CuNPs
The principle of the detection method is illustrated in Scheme 1. Firstly, we designed a P-DNA modified with a phosphate moiety at the 5′-end and a complementary P-DNA sequence (cP-DNA) without any modification. In the absence of ALP, the P-DNA/cP-DNA complexes can be cleaved by λ exo, which hinders the formation of the dsDNA-templated CuNPs. In the presence of ALP, the 5′-phosphate of P-DNA is hydrolyzed to a hydroxyl group, and the λ exo cleavage reaction is impeded. Upon addition of Cu2+ and ascorbate to the reaction solution, dsDNA-templated CuNPs were formed that exhibited a strong fluorescence response. ALP levels were subsequently determined by monitoring the variation in fluorescence intensity.
Next, we assessed the feasibility of the proposed method. We observed low levels of fluorescence at 340 nm excitation when single stranded P-DNA or cP-DNA was used to synthesize the CuNPs, respectively (Fig. 1, Curve A and Curve B). However, high fluorescence signal was detected when dsDNA (P-DNA/cP-DNA complexes) was used to synthesize the CuNPs (Fig. 1, Curve C). The fluorescence of the CuNPs decreased with addition of λ exo to the dsDNA owing to the λ exo-mediated cleavage of the dsDNA template (Fig. 1, Curve D). Once the 5′-phosphoryl end is hydrolyzed by ALP, the λ exo cleavage reaction is impeded and the system displays strong fluorescence due to the formation of the dsDNA-templated CuNPs (Fig. 1, Curve E). In summary, the results of this experiment demonstrated the feasibility of the proposed strategy for detection of ALP activity.
The feasibility of the proposed method (emission peaks at 565 nm). (A) Synthesis of CuNPs with 500 nM P-DNA; (B) Synthesis of CuNPs with 500 nM cP-DNA; (C) Synthesis of CuNPs with 500 nM dsDNA (P-DNA/cP-DNA complexes); (D) 500 nM dsDNA and 45 U⋅mL−1 λ exo were incubated for 10 min at 37 °C and then used for CuNP synthesis; (E) 500 nM dsDNA and 45 U⋅mL−1 λ exo were incubated for 10 min at 37 °C after pre-incubation of the dsDNA with 200 U⋅L−1 ALP for 10 min at 37 °C, and then used for CuNP synthesis
Optimization of experimental conditions
The following parameters were optimized: concentrations of dsDNA, Cu2+, and λ exo; the reaction time of ALP with the dsDNA; the reaction time between the Cu2+/ascorbate mixture with the dsDNA; and the λ exo cleavage reaction time on the fluorescence signal output. Corresponding data appear in the Electronic Supporting Material (Fig. S1-Fig. S6). We found the following experimental conditions gave best results: (1) 500 nM dsDNA; (2) 140 μM Cu2+; (3) 10 min reaction time between the Cu2+/ascorbate mixture with the dsDNA; (4) 10 min reaction time between ALP and its substrate; (5) 45 U⋅mL−1 λ exo; (6) 20 min λ exo cleavage reaction time. These were used throughout the experiment.
Quantitative measurement of ALP activity
To evaluate the sensitivity of the proposed method, a series of ALP concentrations ranging from 0 to 150 U⋅L−1 (0, 0.1, 0.5, 1, 3, 5, 8, 15, 30, 50, 150 U⋅L−1) were used under the optimal conditions described above. As shown in Fig. 2a, the fluorescence intensity at 565 nm dynamically increased with increase in the concentration of ALP. Figure 3b shows the relationship between fluorescence intensity and the concentration of ALP. The inset of Fig. 2b shows that the fluorescence intensity had a linear relationship (R2 = 0.9711) with ALP concentration in the concentration range of 0–8 U⋅L−1 and the regression equation was F = 59.606C + 102.13 (C, U⋅L−1), where F was the fluorescence intensity at 565 nm and C was the ALP concentration. The limit of detection (LOD) of the proposed strategy was estimated to be 0.1 U⋅L−1, which was lower than those of previously reported ALP detection methods (Table 1). Furthermore, the total reaction time in our method only required 40 min, which was much shorter than most existing methods (Table 1). Therefore, the method we developed can detect ALP with high sensitivity and simplicity.
a Fluorescence emission spectra of dsDNA-CuNP at 565 nm in the presence of increasing amounts of ALP (0, 0.1, 0.5, 1, 3, 5, 8, 15, 30, 50, 150 U⋅L−1). Optimized assay conditions were used. b Graph depicting the changes in fluorescence output at 565 nm as a function of ALP concentration. Inset: Linear relationship between fluorescence intensity and low ALP concentrations. Error bars were estimated from three replicate measurements
Selectivity of the ALP activity assay
In order to investigate the selectivity of the proposed assay, several proteins such as GOx, thrombin and BSA were tested under the optimized reaction conditions. Figure 3 shows that other than ALP, none of these proteins triggers an increase in fluorescence intensity. These results demonstrated that the proposed assay was specific for ALP, thereby highlighting its diagnostic potential for detecting ALP activity in biological samples.
ALP activity inhibition assay
Since inhibition of ALP activity is closely associated with therapy of human diseases, we tested the validity of the proposed assay in evaluating the inhibition of ALP activity. Pi, which can inhibit ALP activity by binding competitively to the ALP active site, was chosen as a model inhibitor in our assay. Addition of Pi up to a concentration of 2.5 mM had no effect on the formation of CuNPs and the activity of λ exo, which suggested that the decrease in fluorescence intensity must therefore be caused by the inhibitory effect of Pi on ALP activity (Fig. S7 and S8). We found that the fluorescence intensity at 565 nm and the relative activity of ALP dynamically decreased with an increase in Pi concentration (Fig. S9) with an IC50 value of 3 μM. These results indicate that our method can be potentially used for ALP inhibitor screening.
Application of the proposed assay in biological systems
To evaluate the practical use of the proposed sensing platform, we attempted to detect ALP in 1% human serum [26]. 1 U⋅L−1, 5 U⋅L−1, and 8 U⋅L−1ALP were added to 1% (v/v) human serum, respectively, and detected by the proposed assay. The results are shown in Table 2; the recovery rates of different concentrations of ALP in 1% human serum were 97.7% for 1 U⋅L−1, 103.3% for 5 U⋅L−1, and 98% for 8 U⋅L−1. The results show that the method may have practical applications for ALP detection in biological systems.
Conclusions
In summary, we have successfully demonstrated a turn-on fluorescence method for ALP detection based on dsDNA-templated copper nanoparticles coupled with λ exonuclease cleavage. The advantage of the method is low detection limit (0.1 U⋅L−1), fast response time (40 min), good selectivity and excellent repeatability. Besides, this method can be used in practical applications involving real biological systems and screening for inhibitors of ALP. We envision that our fluorescence-based strategy for detection of ALP activity may find applications in clinical diagnostics and drug screening.
References
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct 21:441–483. doi:10.1146/annurev.bb.21.060192.002301
Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H (2015) Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res 27:413–418. doi:10.1007/s40520-014-0296-x
Fishman WH (1987) Clinical and biological significance of an isozyme tumor marker-PLAP. Clin Biochem 20:387–392. doi:10.1016/0009-9120(87)90003-8
Kinoshita Y, Arai M, Ito N, Takashi Y, Makita N, Nangaku M, Shinoda Y, Fukumoto S (2016) High serum ALP level is associated with increased risk of denosumab-related hypocalcemia in patients with bone metastases from solid tumors. Endocr J 63:479–484. doi:10.1507/endocrj.EJ16-0003
Shi D, Sun Y, Lin L, Shi C, Wang G, Zhang X (2016) Naked-eye sensitive detection of alkaline phosphatase (ALP) and pyrophosphate (PPi) based on a horseradish peroxidase catalytic colorimetric system with Cu(ii). Analyst 141:5549–5554. doi:10.1039/c6an01124a
Molina-Delgado MÁ, Aguilar-Caballos MP, Gómez-Hens A (2016) Simultaneous photometric microplate assay for free and total thiamine using gold nanoparticles and alkaline phosphatase. Microchim Acta 183:1385–1390. doi:10.1007/s00604-016-1767-x
Deng J, Jiang Q, Wang Y, Yang L, Yu P, Mao L (2013) Real-time colorimetric assay of inorganic pyrophosphatase activity based on reversibly competitive coordination of Cu2+ between cysteine and pyrophosphate ion. Anal Chem 85:9409–9415. doi:10.1021/ac402524e
Li C, Zhen S, Wang J, Li Y, Huang C (2013) A gold nanoparticles-based colorimetric assay for alkaline phosphatase detection with tunable dynamic range. Biosens Bioelectron 43:366–371. doi:10.1016/j.bios.2012.12.015
Yang J, Zheng L, Wang Y, Li W, Zhang J, Gu J, Fu Y (2016) Guanine-rich DNA-based peroxidase mimetics for colorimetric assays of alkaline phosphatase. Biosens Bioelectron 77:549–556. doi:10.1016/j.bios.2015.10.003
Shen C, Li X, Rasooly A, Guo L, Zhang K, Yang M (2016) A single electrochemical biosensor for detecting the activity and inhibition of both protein kinase and alkaline phosphatase based on phosphate ions induced deposition of redox precipitates. Biosens Bioelectron 85:220–225. doi:10.1016/j.bios.2016.05.025
Zhang L, Hou T, Li H, Li F (2015) A highly sensitive homogeneous electrochemical assay for alkaline phosphatase activity based on single molecular beacon-initiated T7 exonuclease-mediated signal amplification. Analyst 140:4030–4036. doi:10.1039/c5an00516g
Shen B, Li J, Cheng W, Yan Y, Tang R, Li Y (2015) Electrochemical aptasensor for highly sensitive determination of cocaine using a supramolecular aptamer and rolling circle amplification. Microchim Acta 182:361–367. doi:10.1007/s00604-014-1333-3
Dong J, Li Y, Zhang M, Yan T, Qian W (2014) Ultrasensitive surface-enhanced Raman scattering detection of alkaline phosphatase. Anal Methods 6:9168–9172. doi:10.1039/C4AY01885K
Du J, Xiong L, Ma C, Liu H, Wang J, Wang K (2016) Label-free DNA hairpin probe for real-time monitoring of alkaline phosphatase activity. Anal Methods 8:5095–5100. doi:10.1039/C6AY00989A
Kong R, Fu T, Sun NN, Qu FL, Zhang SF, Zhang XB (2013) Pyrophosphate-regulated Zn2+-dependent DNAzyme activity: an amplified fluorescence sensing strategy for alkaline phosphatase. Biosens Bioelectron 50:351–355. doi:10.1016/j.bios.2013.06.064
Li Y, Li YN, Liu Z, Su X (2014) Sensitive fluorometric detection of alkaline phosphatase using a water-soluble conjugated polymer. RSC Adv 4:42825–42830. doi:10.1039/C4RA05844E
Liu S, Pang S, Na W, Su X (2014) Near-infrared fluorescence probe for the determination of alkaline phosphatase. Biosens Bioelectron 55:249–254. doi:10.1016/j.bios.2013.12.023
Liu XG, Xing XJ, Li B, Guo YM, Zhang YZ, Yang Y, Zhang LF (2016) Fluorescent assay for alkaline phosphatase activity based on graphene oxide integrating with λ exonuclease. Biosens Bioelectron 81:460–464. doi:10.1016/j.bios.2016.03.030
Park KS, Lee CY, Park HG (2014) A sensitive dual colorimetric and fluorescence system for assaying the activity of alkaline phosphatase that relies on pyrophosphate inhibition of the peroxidase activity of copper ions. Analyst 139:4691–4695. doi:10.1039/c4an00778f
Tang C, Qian Z, Huang Y, Xu J, Ao H, Zhao M, Zhou J, Chen J, Feng H (2016) A fluorometric assay for alkaline phosphatase activity based on β-cyclodextrin-modified carbon quantum dots through host-guest recognition. Biosens Bioelectron 83:274–280. doi:10.1016/j.bios.2016.04.047
Wang Y, Chen J, Jiao H, Chen Y, Li W, Zhang Q, Yu C (2013) Polymer templated perylene-probe noncovalent self-assembly: a new strategy for label-free ultrasensitive fluorescence turn-on biosensing. Chem Eur J 19:12846–12852. doi:10.1002/chem.201203998
Wang FY, Li YX, Li WY, Zhang QF, Chen J (2014) A facile method for detection of alkaline phosphatase activity based on the turn-on fluorescence of resorufin. Anal Methods 6:6105–6109. doi:10.1039/C4AY00634H
Xiang MH, Liu JW, Li N, Tang H, Yu RQ, Jiang JH (2016) A fluorescent graphitic carbon nitride nanosheet biosensor for highly sensitive, label-free detection of alkaline phosphatase. Nano 8:4727–4732. doi:10.1039/c5nr08278a
Wolfbeis OS, Koller E (1985) Photometric and fluorimetric assay of alkaline phosphatase with new coumarin-derived substrates. Microchim Acta 85:389–395. doi:10.1007/BF01201534
Zhu WP, Zhao ZW, Li Z, Jiang JH, Shen GL, Yu RQ (2013) A graphene oxide platform for the assay of DNA 3- phosphatases and their inhibitors based on hairpin primer and polymerase elongation. J Mater Chem B 1:361–367. doi:10.1039/C2TB00109H
Kang W, Ding Y, Zhou H, Liao Q, Yang X, Yang Y, Jiang J, Yang M (2015) Monitoring the activity and inhibition of alkaline phosphatase via quenching and restoration of the fluorescence of carbon dots. Microchim Acta 182:1161–1167. doi:10.1007/s00604-014-1439-7
Zhao MM, Guo YJ, Wang LX, Luo F, Lin CY, Lin ZY, Chen GN (2016) A sensitive fluorescence biosensor for alkaline phosphatase activity based on the Cu(II)-dependent DNAzyme. Anal Chim Acta 948:98–103. doi:10.1016/j.aca.2016.10.033
Qian ZS, Chai LJ, Huang YY, Tang C, Shen JJ, Chen JR, Feng H (2015) A real-time fluorescent assay for the detection of alkaline phosphatase activity based on carbon quantum dots. Biosens Bioelectron 68:675–680. doi:10.1016/j.bios.2015.01.068
Schrenkhammer P, Rosnizeck IC, Duerkop A, Wolfbeis OS, Schäferling M (2008) Time-resolved fluorescence-based assay for the determination of alkaline phosphatase activity and application to the screening of its inhibitors. J Biomol Screen 13(1):9–16. doi:10.1177/1087057107312031
Guo YM, Cao FP, Lei XL, Mang LH, Cheng SJ, Song JT (2016) Fluorescent copper nanoparticles: recent advances in synthesis and applications for sensing metal ions. Nanoscale 8:4852–4863. doi:10.1039/c6nr00145a
Chen J, Liu J, Fang Z, Zeng L (2012) Random dsDNA-templated formation of copper nanoparticles as novel fluorescence probes for label-free lead ions detection. Chem Commun 48:1057–1059. doi:10.1039/c2cc16668b
Chen J, Ji X, Tinnefeld P, He Z (2016) Multifunctional dumbbell-shaped DNA-templated selective formation of fluorescent silver nanoclusters or copper nanoparticles for sensitive detection of biomolecules. ACS Appl Mater Interfaces 8:1786–1794. doi:10.1021/acsami.5b09678
Lai QQ, Liu MD, Gu CC, Nie HG, Xu XJ, Li Z, Yang Z, Huang SM (2016) A novel label-free fluorescence strategy for methyltransferase activity assay based on dsDNAtemplated copper nanoparticles coupled with an endonuclease-assisted signal transduction system. Analyst 141:1383–1389. doi:10.1039/c5an02123e
Park KW, Batule BS, Kang KS, Park KS, Park HG (2016) Rapid and ultrasensitive detection of microRNA by target-assisted isothermal exponential amplification coupled with poly (thymine)-templated fluorescent copper nanoparticles. Nanotechnology 27:425502–425509. doi:10.1088/0957-4484/27/42/425502
Yang L, Wang Y, Li B, Jin Y (2017) High-throughput identification of telomere-binding ligands based on the fluorescence regulation of DNA-copper nanoparticles. Biosens Bioelectron 87:915–917. doi:10.1016/j.bios.2016.09.055
Zhang L, Zhao J, Duan M, Zhang H, Jiang J, Yu R (2013) Inhibition of dsDNA-templated copper nanoparticles by pyrophosphate as a label-free fluorescent strategy for alkaline phosphatase assay. Anal Chem 85:3797–3801. doi:10.1021/ac4001942
Li J, Si L, Bao J, Wang Z, Dai Z (2017) Fluorescence regulation of poly(thymine)-templated copper nanoparticles via an enzyme-triggered reaction toward sensitive and selective detection of alkaline phosphatase. Anal Chem. doi:10.1021/acs.analchem.6b05112
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21205142, 31370104), The Research Innovation Program for Graduates of Central South University (2016zzts580).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 2667 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Ma, C., Wang, J. et al. A turn-on fluorescent method for determination of the activity of alkaline phosphatase based on dsDNA-templated copper nanoparticles and exonuclease based amplification. Microchim Acta 184, 2483–2488 (2017). https://doi.org/10.1007/s00604-017-2256-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2256-6