Abstract
Purpose
While laparoscopic pelvic exenteration reduces intraoperative blood loss, dorsal venous complex bleeding during this procedure causes issues. We previously introduced a method to transect the dorsal venous complex and urethra using a linear stapler during cooperative laparoscopic and transperineal endoscopic (two-team) pelvic exenteration. The present study assessed its effectiveness in reducing intraoperative blood loss by comparing it with conventional laparoscopic pelvic exenteration.
Methods
This retrospective cohort study was conducted at a Japanese tertiary referral center. Eleven cases of two-team laparoscopic pelvic exenteration with staple transection of the dorsal venous complex (T-PE group) were compared to 25 cases of conventional laparoscopic pelvic exenteration (C-PE group). The primary outcome measure was intraoperative blood loss.
Results
There were no significant between-group differences in patient background. The mean intraoperative blood loss was significantly lower in the T-PE group than in the C-PE group (200 vs. 850 mL, p = 0.01). The respective mean operation time, postoperative complication rate, and R0 resection rate were similar between the T-PE and C-PE groups (636 min vs. 688 min, p = 0.36; 36% vs. 44%, p = 0.65; 100% vs. 100%, p = 1.00).
Conclusions
Two-team laparoscopic pelvic exenteration with staple transection of the dorsal venous complex reduced intraoperative blood loss from the dorsal venous complex in a technically safe and oncologically feasible manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the development of preventive medicine, approximately 10% of rectal cancer patients are still diagnosed with adjacent organ involvement on initial imaging examinations [1]. In some cases, pelvic exenteration (PE) is the only procedure through which a cure for such advanced tumors can be expected. However, technical difficulties, such as high intraoperative blood loss and a high rate of perioperative complications, pose a problem.
Laparoscopic surgery is well-known to reduce intraoperative blood loss compared to open surgery due to the magnified surgical field and pneumoperitoneum pressure. Recently, some articles have reported less blood loss and reasonably oncological feasibility of laparoscopic PE compared to open PE [2, 3]. However, the division of the dorsal venous complex (DVC) in laparoscopic PE is an unfamiliar technique for colorectal surgeons, and it is sometimes difficult to achieve hemostasis despite using the traditional bunching method, soft coagulation, or vessel-sealing devices.
To reduce bleeding from the DVC, we previously reported a technique that transects the DVC and urethra using a linear stapler inserted through the perineal port in cooperative laparoscopic and transperineal endoscopic (two-team) PE [4].
The present study evaluated the impact of this method on intraoperative blood loss reduction compared with that of conventional laparoscopic PE.
Methods
Patient selection
This single-center retrospective study was approved by the ethics committee of the Cancer Institute Hospital (approval number: 2018-GA-1109). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
In our institution, laparoscopic PE was introduced in 2013, and since then, we have performed all PE procedures for primary colorectal cancer laparoscopically. Between January 2013 and December 2021, 44 patients with primary colorectal cancer underwent PE at the Cancer Institute Hospital. Three women and 5 with PE and sacrectomy were excluded, leaving 36 patients enrolled in this study. These patients were divided into two groups: a group receiving two-team laparoscopic PE with transection of the DVC and urethra using a linear stapler (T-PE, n = 11) and group receiving conventional laparoscopic PE (C-PE, n = 25) (Fig. 1).
In the present study, PE included both total pelvic exenteration (TPE) and anterior pelvic exenteration (APE). According to a previous systematic review, TPE was defined as the en bloc removal of the anus, levator ani muscle, rectum, bladder, distal ureter, and internal reproductive organs with tumor, and APE was defined as the removal of the rectum, bladder, distal ureter, and internal reproductive organs while preserving the anal sphincter through coloanal anastomosis or the double staple technique [5]. Neoadjuvant chemotherapy or chemoradiotherapy was administered to patients with locally advanced low rectal cancer. However, for patients with symptoms of bowel obstruction, neoadjuvant treatment is introduced after colostomy, and for patients with uncontrollable infections due to tumor invasion, upfront surgery is considered. Bilateral pelvic lymph node dissection was performed when the tumor was located in the lower rectum. The final decision regarding these treatment plans was made during our team conferences.
Surgical procedure of two-team laparoscopic PE
The transperineal and laparoscopic approaches were started synchronously. The laparoscopic approach was performed using the conventional five-trocar pneumoperitoneum technique. The inferior mesenteric artery was dissected at its origin, and adequate lymphoidectomy was performed. After mobilization of the sigmoid mesocolon, posterior dissection of the rectum was continued to expose the levator ani muscle. The bilateral pelvic lymph nodes of the internal iliac and obturator areas were dissected en bloc with the tumor. The ureters were mobilized and transected at the ureterovesical junction.
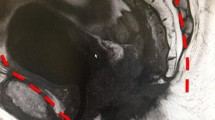
In the case of TPE, a multi-access port (GelPoint Path, Applied Medical, Rancho Santa Margarita, CA, USA) was set at the incision of the perianal skin. Under a continuous pneumo-circulation system (AirSeal Intelligent Flow System, SurgiQuest, Milford, CT, USA), the ischiorectal fat was dissected until it reached the levator ani muscle. With transection of the pubococcygeal and rectococcygeal muscles just caudal to the coccyx, the transperineal approach was communicated to the laparoscopic approach. The lateral and anterolateral parts of the iliococcygeal muscle were dissected along the ischiopubic rami, and the puborectal muscle was dissected along the prostate to minimize bleeding under pneumoperitoneum (Fig. 2a). After adequate dissection of bilateral puboprostatic ligaments from the abdominal side and dissection of the sphincter muscle of the urethra from the tranperineal side, the DVC and urethra were completely exposed. A linear stapler (ECHELON FLEX Powered ENDOPATH stapler, with a blue 60-mm cartridge from Ethicon, Cincinnati, OH, USA; or Signia stapler, with a camel 60-mm cartridge from Covidien, Norwalk, CT, USA) was inserted through the transperineal port along the dorsal side of the pubic body, and then the DVC and urethra were carefully compressed and transected (Fig. 3). After tumor extraction through the perineal wound, an ileal conduit and a permanent colostomy were created.
In the case of APE, a multi-access port was inserted into the anal canal, and the rectum was closed with 2 clockwise sutures around the rectal lumen using a barbed suture (STRATAFIX from Ethicon or V-Loc from Covidien) 10 mm from the distal edge of the tumor. A full-thickness incision of the rectal wall and dissection of the intersphincteric space was performed with reference to the contraction of the external anal sphincter and the levator ani muscle. The transperineal approach was communicated to the laparoscopic approach with the transection of the rectococcygeal muscle (Fig. 2b). After dissection of the puborectal muscle and puboprostatic ligaments, the DVC and urethra were dissected in the same manner as for TPE (Fig. 3). The tumor was removed through a small incision in the umbilicus, hand-sewn coloanal anastomosis with diverting ileostomy was performed, and an ileal conduit was created (Fig. 2).
Data collection
We collected the patient's background and surgical and pathological data from our database. Patient background data included the age, American Society of Anesthesiologists physical status, body mass index (BMI), tumor location, tumor size, carcinoembryonic antigen level, clinical TNM stage, and presence or absence of preoperative therapy. Surgical and pathological data included the surgical procedure, operation time, estimated blood loss, blood transfusion, conversion to open surgery, mortality, postoperative complications, postoperative hospital stay, rate of positive resection margins, and pathological TNM stage. The data were compared between the T-PE and C-PE groups.
a After the puboprostatic ligaments and puborectal muscle were dissected, the dorsal venous complex (DVC) and urethra were exposed. b, c A linear stapler was inserted through the perineal port, and the DVC and urethra were carefully compressed. d After the transection of the DVC and urethra, slight bleeding occurred at the staple line
Statistical analyses
Data are expressed as the median and interquartile range (IQR). The Mann–Whitney U test was used to analyze continuous variables, and the chi-square test and Fisher’s exact test were used to analyze categorical variables. A logistic regression analysis was used to clarify the factors affecting intraoperative blood loss. Results with a p value of < 0.05 were considered significant. All analyses were performed using R, version 3.5.2. (R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 1 shows the patients’ characteristics. All T-PE procedures were performed in the latter period of the present study. The median BMI tended to be higher in the C-PE group (20.5 vs 22.0, p = 0.07), while the tumor size tended to be larger in the T-PE group (85 mm vs. 60 mm, p = 0.09). There were no significant differences in the age, tumor location, clinical TNM stage, serum CEA level, or preoperative treatment between the groups. All but one patient underwent complete transection using a linear stapler.
Table 2 shows the postoperative short-term outcomes. Two patients from the C-PE group required conversion to open surgery for a bulky tumor, while no conversion was observed in the T-PE group. The mean operative time was similar in both groups (636 vs. 688 min in the T-PE and C-PE groups, respectively, p = 0.36), while the mean estimated blood loss was smaller in the T-PE group than in the C-PE group (200 mL vs. 850 mL in the T-PE and C-PE groups, respectively, p = 0.01). The rate and amount of blood transfusion were similar between the groups. Although the postoperative complication rate (36% vs. 44% in the T-PE and C-PE groups, respectively, p = 0.65) was not significantly different between the groups, a pelvic abscess was observed only in the C-PE group. There was no postoperative mortality, and postoperative hospital stays (32 days vs. 32 days in the T-PE and C-PE groups, respectively, p = 1.00) were similar between the groups.
Table 3 demonstrates the pathological result. Pathological T4b was identified in 45% of patients from the T-PE group and 36% from the C-PE group. Pathological complete regression was seen in one case from the C-PE group. The R0 resection rate was 100% in both groups. There was no significant difference in the pathological N and TNM stages between the groups.
Table 4 details the factors that affected intraoperative blood loss (blood loss < 560 ml vs. ≥ 560 ml in the T-PE and C-PE groups, respectively; dichotomized with a clinically relevant cutoff point). A univariate analysis revealed that a BMI ≥ 21, two-team approach, and operation time ≥ 600 min were significant factors associated with a low amount of blood loss. In a multivariate analysis, only a two-team approach remained an independent factor associated with a low amount of blood loss.
Discussion
This study revealed that transecting the DVC and urethra using a linear stapler in two-team laparoscopic PE dramatically reduced intraoperative blood loss compared to conventional laparoscopic PE. The operation time, postoperative complications, and R0 resection rates were similar between the two groups. To our knowledge, this is the first report to assess the impact of staple transection of the DVC and urethra on intraoperative blood loss during laparoscopic PE.
Since Brunschwig first reported pelvic exenteration in 1948, a deep understanding of pelvic anatomy, advances in anesthesia, and the development of surgical devices have enabled surgeons to perform PE more safely in terms of improvement of morbidity, mortality, and oncological outcomes. For instance, the postoperative mortality rate ranged from 17 to 23% in the 1940s to 1970s [6, 7], improving by 1–4% in the 1990s to 2000s [8, 9]. In contrast, intraoperative blood loss, which is known to be a risk factor for increased postoperative mortality as well as worsened long-term outcomes in colorectal cancer [10,11,12,13], has remained high in PE, ranging between 800 and 2700 mL even in the 2000s [14,15,16]. In the era of minimally invasive surgery, the incidence of laparoscopic PE has increased, and a recent meta-analysis revealed that laparoscopic PE is associated with reduced intraoperative blood loss compared to open surgery, with a median intraoperative blood loss of 550 mL in the laparoscopic group and 2300 mL in the open group [3]. In the present study, the mean intraoperative blood loss of the C-PE group was 850 mL, which was similar to that in previous reports, while that of the T-PE group was 200 mL, which was dramatically smaller than that reported previously. According to a Japanese multicenter cohort study, the mean blood loss of laparoscopic total mesorectal excision with lateral lymph node dissection was 193 mL [17], which is similar to the T-PE group in the present study. Therefore, staple transection may completely control bleeding around the DVC.
In the field of urology, there are some reports, wherein only the DVC was transected using an endovascular stapler in radical prostatectomy; however, its benefit in reducing intraoperative blood loss is unclear. Wu et al. noted that staple ligation of the DVC in robot-assisted laparoscopic radical prostatectomy (RARP) resulted in reduced blood loss compared with suturing [18]. In contrast, a randomized comparison study of endoscopic stapling with cut and suture ligation or suture ligation of the DVC in RARP revealed no marked differences in intraoperative blood loss [19]. In contrast to urological operations, not only the DVC but also the puborectal muscle is transected in PE, and colorectal surgeons often struggle with the problem of bleeding from branches of the internal pudendal vein that runs through the puborectal muscle and flows into the DVC. In the present study, we ligated and transected the DVC en bloc with part of the puborectal muscle using a linear stapler, which might reduce bleeding around the DVC.
To enable the insertion of the linear stapler through the perineal port while maintaining pneumoperitoneum and a clear surgical view, we introduced a two-team approach. When transecting the DVC in conventional PE, the handling of forceps or a linear stapler inserted through the abdominal port is limited by the pubic body or the tumor itself, which may result in dissection of the DVC being impossible or a positive resection margin if the tumor invades the distal prostate or membranous urethra. Because the DVC and urethra are located near the perineal port, a linear stapler is inserted directly along the pubic body, and the tip of the linear stapler is visible from the abdominal side. The DVC and urethra are easily transected, even at the bulbous portion, with a secure negative resection margin.
The two-team approach has the advantage of enabling dissection of the sphincter muscle of the urethra from the perineal side prior to dissection of the DVC. A previous report described a case of unsuccessful ligation of the DVC and urethra due to the thickness of the DVC [20]. This event is suspected to be due to inadequate dissection of the sphincter muscle of the urethra. Although it may not always be necessary, in the present study, the sphincter muscles of the urethra and puboprostatic ligament were adequately dissected on both the abdominal and perineal sides, and staple transection of the DVC and urethra was completed in almost all cases. However, severe tissue fibrosis around the DVC and puborectal muscle due to preoperative chemoradiotherapy were observed in one case, in which stapled transection was interrupted even after dissection of the puborectal muscle, puboprostatic ligament, and sphincter muscles of the urethra. In such cases, the DVC was coagulated and dissected using soft coagulation or a vessel-sealing system after the urethra had been ligated and divided.
Several articles have reported other advantages of two-team endoscopic extended surgery in terms of shorter operation time, less blood loss, and fewer complications [20,21,22,23]. In the present study, the operation time and postoperative complication rates were similar between the groups. This finding may be attributed to the inclusion criteria. Although no significant difference was seen in the tumor size, in the early period of introduction, two-team surgery was performed only for bulky tumors in which approaching the deep pelvis from the abdominal side was expected to be difficult. Therefore, more difficult cases tended to be included in the T-PE group rather than the C-PE group, resulting in no marked difference in the operation time or postoperative complication rate.
Several limitations associated with the present study warrant mention. First, it was a retrospective study. Second, there was difficulty in quantifying the correct amount of bleeding around the DVC. Although we assessed all operation videos in this study and concluded that the majority of the bleeding occurred around the DVC, it is difficult to distinguish the amount of bleeding from the DVC and that from other parts. Third, the possibility of selection bias was high. Although the patients’ background characteristics were similar between the two groups, all T-PE procedures were performed in the latter period of this study. Improved surgical performance with experience or advances in hemostasis devices may, therefore, have influenced our intraoperative blood loss results. Furthermore, the indication for two-team surgery was determined in a team conference with reference to the surgeon’s proficiency for transperineal surgery, the manpower required for two-team surgery, and the patient’s general condition. Therefore, a large-sample prospective study is warranted to confirm our findings. Fourth, the oncological feasibility of staple transection of the urethra is unclear. Staple transection of the ureter may cause local recurrence in cases in which the tumor is exposed on the bladder mucosa or in cases in which urine cytology is positive. However, the long-term outcome needs to be evaluated. Fifth, technical difficulties were encountered. Laparoscopic multi-visceral resection remains a challenging technique, and laparoscopic TPE is far from a standard procedure. Furthermore, transperineal endoscopic surgery requires a profound understanding of the surgical anatomy, and its oncological feasibility remains unclear. In the present study, all procedures were performed by experienced board-certified surgeons familiar with transperineal and laparoscopic surgery. We should note that the results of the present study are based on a procedure performed by a professional surgical team.
In conclusion, two-team endoscopic surgery and transection of the DVC using a linear stapler are safe and associated with a reduction in intraoperative blood loss in PE.
Availability of data and materials
The data sets generated and/or analyzed during the current study are not publicly available due to privacy and ethical concerns.
Code availability
Not applicable.
References
Ali SM, Antoniou A, Beynon J, Bhangu A, Bose P, Boyle K, et al. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg. 2013;100:E1–33. https://doi.org/10.1002/bjs.9192_1.
Kazi M, Kumar NAN, Rohila J, Sukumar V, Engineer R, Ankathi S, et al. Minimally invasive versus open pelvic exenterations for rectal cancer: a comparative analysis of perioperative and 3-year oncological outcomes. BJS Open. 2021;5:zrab074. https://doi.org/10.1093/bjsopen/zrab074.
Collaborative PelvEx. Minimally invasive surgery techniques in pelvic exenteration: a systematic and meta-analysis review. Surg Endosc. 2018;32:4707–15. https://doi.org/10.1007/s00464-018-6299-5.
Mukai T, Nagasaki T, Akiyoshi T, Yamaguchi T, Hiyoshi Y, Nagayama S, et al. Staple-transection of the dorsal venous complex and urethra in cooperative laparoscopic and transperineal endoscopic total pelvic exenteration for pelvic malignancies. Asian J Endosc Surg. 2021;14:816–20. https://doi.org/10.1111/ases.12932.
Yang TX, Morris DL, Chua TC. Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum. 2013;56:519–31. https://doi.org/10.1097/DCR.0b013e31827a7868.
Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948;1:177–83. https://doi.org/10.1002/1097-0142(194807)1:2%3c177::aid-cncr2820010203≥3.0.co;2-a.
Ketcham AS, Deckers PJ, Sugarbaker EV, Hoye RC, Thomas LB, Smith RR. Pelvic exenteration for carcinoma of the uterine cervix: a 15-year experience. Cancer. 1970;26:513–21. https://doi.org/10.1002/1097-0142(197009)26:3%3c513::aid-cncr2820260304≥3.0.co;2-6.
Robertson G, Lopes A, Beynon G, Monaghan JM. Pelvic exenteration: a review of the Gateshead experience 1974–1992. Br J Obstet Gynaecol. 1994;101:529–31. https://doi.org/10.1111/j.1471-0528.1994.tb13156.x.
Heriot AG, Byrne CM, Lee P, Dobbs B, Tilney H, Solomon MJ, et al. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284–91. https://doi.org/10.1007/s10350-007-9152-9.
Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–6. https://doi.org/10.1056/NEJM199305133281902.
Chiarugi M, Buccianti P, Disarli M, Galatioto C, Cavina E. Effect of blood transfusions on disease-free interval after rectal cancer surgery. Hepatogastroenterology. 2000;47:1002–5.
Tamagawa H, Numata M, Aoyama T, Kazama K, Atsumi Y, Iguchi K, et al. Impact of intraoperative blood loss on the survival of patients with stage II/III colorectal cancer: a multicenter retrospective study. In Vivo. 2021;35:3483–8. https://doi.org/10.21873/invivo.12649.
Hanna DN, Gamboa AC, Balch GC, Regenbogen SE, Holder-Murray J, Abdel-Misih SRZ, et al. Perioperative blood transfusions are associated with worse overall survival but not disease-free survival after curative rectal cancer resection: a propensity score-matched analysis. Dis Colon Rectum. 2021;64:946–54. https://doi.org/10.1097/DCR.0000000000002006.
Ishiguro S, Akasu T, Fujita S, Yamamoto S, Kusters M, Moriya Y. Pelvic exenteration for clinical T4 rectal cancer: oncologic outcome in 93 patients at a single institution over a 30-year period. Surgery. 2009;145:189–95. https://doi.org/10.1016/j.surg.2008.09.014.
Uehara K, Nakamura H, Yoshino Y, Arimoto A, Kato T, Yokoyama Y, et al. Initial experience of laparoscopic pelvic exenteration and comparison with conventional open surgery. Surg Endosc. 2016;30:132–8. https://doi.org/10.1007/s00464-015-4172-3.
Martínez A, Filleron T, Vitse L, Querleu D, Mery E, Balague G, et al. Laparoscopic pelvic exenteration for gynaecological malignancy: is there any advantage? Gynecol Oncol. 2011;120:374–9. https://doi.org/10.1016/j.ygyno.2010.11.032.
Yamaguchi T, Konishi T, Kinugasa Y, Yamamoto S, Akiyoshi T, Okamura R, et al. Laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer: a subgroup analysis of a large multicenter cohort study in Japan. Dis Colon Rectum. 2017;60:954–64. https://doi.org/10.1097/DCR.0000000000000843.
Wu SD, Meeks JJ, Cashy J, Perry KT, Nadler RB. Suture versus staple ligation of the dorsal venous complex during robot-assisted laparoscopic radical prostatectomy. BJU Int. 2010;106:385–90. https://doi.org/10.1111/j.1464-410X.2009.09146.x.
Feng T, Heulitt G, Lee JJ, Liao M, Li HF, Porter JR. Randomised comparison of techniques for control of the dorsal venous complex during robot-assisted laparoscopic radical prostatectomy. BJU Int. 2020;126:586–94. https://doi.org/10.1111/bju.15133.
Hayashi K, Kotake M, Kakiuchi D, Yamada S, Hada M, Kato Y, et al. Laparoscopic total pelvic exenteration using transanal minimal invasive surgery technique with en bloc bilateral lymph node dissection for advanced rectal cancer. Surg Case Rep. 2016;2:74. https://doi.org/10.1186/s40792-016-0198-6.
Hasegawa S, Kajitani R, Matsumoto Y, Ohmiya T, Nagano H, Komono A, et al. Combined laparoscopic and transperineal endoscopic total pelvic exenteration for local recurrence of rectal cancer. Tech Coloproctol. 2020;24:599–601. https://doi.org/10.1007/s10151-020-02187-9.
Nonaka T, Tominaga T, Akazawa Y, Sawai T, Nagayasu T. Feasibility of laparoscopic-assisted transanal pelvic exenteration in locally advanced rectal cancer with anterior invasion. Tech Coloproctol. 2021;25:69–74. https://doi.org/10.1007/s10151-020-02324-4.
Tominaga T, Nonaka T, Fukuda A, Shiraisi T, Hashimoto S, Araki M, et al. Combined transabdominal and transperineal endoscopic pelvic exenteration for colorectal cancer: feasibility and safety of a two-team approach. Ann Surg Treat Res. 2021;101:102–10. https://doi.org/10.4174/astr.2021.101.2.102.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by TM, YF, and TN. All resected specimen was pathologically evaluated by HK. The first draft of the manuscript was written by TM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Consent to participate
Informed consent was obtained from all participants included in the study.
Consent to publication
Patients gave their signed informed consent regarding the publication of their data and photographs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukai, T., Nagasaki, T., Akiyoshi, T. et al. The impact of staple transection of the dorsal venous complex and urethra on intraoperative blood loss in cooperative laparoscopic and transperineal endoscopic pelvic exenteration. Surg Today 54, 23–30 (2024). https://doi.org/10.1007/s00595-023-02693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02693-x