Abstract
Background
Pelvic exenteration is potentially curative for locally advanced and recurrent pelvic cancers. Evolving technology has facilitated the use of minimally invasive surgical (MIS) techniques in selected cases. We aimed to compare outcomes between open and MIS pelvic exenteration.
Methods
A review of comparative studies was performed. Firstly, we evaluated the differences in surgical techniques with respect to operative time, blood loss, and margin status. Secondly, we assessed differences in 30-day morbidity and mortality rates, and length of hospital stay.
Results
Four studies that directly compared open and MIS exenteration were included. Analysis was performed on 170 patients; 78.1% (n = 133) had open pelvic exenteration, while 21.8% (n = 37) had a MIS exenteration. The median age for open exenteration was 57.7 years versus 63 years for MIS exenteration. Even though the operative time for MIS exenteration was 83 min longer (p < 0.001), it was associated with a median of 1,750mls less blood loss. The morbidity rate for MIS exenterative group was 56.7% (n = 21/37) versus 88.5% (n = 85/96) in the open exenteration group, with pooled analysis observing a 1.17 relative risk increase in 30-day morbidity (p = 0.172) in the open exenteration group. In addition, the MIS cohort had a 6-day shorter length of hospital stay (p = 0.04).
Conclusion
MIS exenteration can be performed in highly selective cases, where there is favourable patient anatomy and tumour characteristics. When feasible, it is associated with reduced intra-operative blood loss, shorter length of hospital stay, and reduced morbidity.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Pelvic exenteration surgery is a radical procedure that offers curative potential for both locally advanced and recurrent pelvic cancers [1]. Pelvic exenteration (PE) was first described by Alexander Brunschwig in 1948 as “the most radical surgical attack for advanced pelvic cancer.” [2]. The first series describing PE for colorectal cancer was reported by Butcher and Spjut in 1959 [3]. In the last few decades, more extensive exenterative resections have been performed for management of advanced colorectal, gynaecological, urological, and sarcoma neoplasm [4,5,6]. Though these resections pose a significant challenge for the operating surgeons, improved surgical techniques, technology, and reconstructive options have facilitated more radical resections [6, 7]. Despite improved surgical options, patients still have considerable post-operative morbidity and negative impact to quality of life [8,9,10]. However, non-surgical management options result in poor prognosis with only 3% survival at 5 years [11]. Pelvic exenteration in appropriately selected patients offers hope of long-term survival [12].
The development of minimally invasive surgery (MIS) has evolved substantially in recent years, especially regarding pelvic surgery [13,14,15]. There have been sporadic low-volume reports highlighting the potential promise for MIS exenterative surgery; however, many reports have been heterogeneous in disease sub-types. In addition, some advocate performing a laparoscopy initially to assess suitability of MIS exenteration, and if favourable anatomy is present, then proceeding with MIS exenteration could be considered [14]. A trial to examine the role of MIS exenteration is not feasible. This systematic review therefore aims to examine the current evidence regarding the use of MIS techniques in pelvic exenterative surgery for locally advanced and recurrent pelvic cancers and to assess surgical and survival outcomes in comparison to open exenterative surgery.
Methods
A systematic review was performed in accordance to the guidelines and recommendations from the preferred reporting items for systematic reviews and meta-analyses checklist (PRISMA) [16]. Institutional review board approval was not required.
Search strategy
An electronic search for relevant publications was performed using the following resources: PubMed, Embase, Google Scholar and the Cochrane collaboration database from 2000 to September 2017. The database was interrogated using the following MESH search terms: “pelvic exenteration” OR “pelvic” AND “exenteration” OR “pelvic exenteration” AND “rectal” OR " AND “laparoscopy” OR “laparoscopy.”
The search protocol was registered on PROSPERO [17]. All titles were initially reviewed and appropriate abstracts were screened. Each of the relevant publication references was also screened for other applicable publications. Two reviewers (NS, GM) independently assessed all identified abstracts and titles of studies meeting the predetermined selection criteria to confirm eligibility. Each reviewer extracted the following data variables: title and study details (first author, journal, year, country), study population characteristics (number in study, gender and age). In addition, surgical approach, operative times, blood loss, margin status, length of hospital stay, surgical and survival outcomes were recorded. All data were recorded independently by both reviewers in separate databases and compared at the end of the reviewing process to limit selection bias. The database was also reviewed by a third person (MK) and discrepancies or duplicates were clarified. The last date of search was 12th September 2017.
Inclusion criteria
To be included in the analysis, the studies had to meet the following criteria: (a) report on patients with locally advanced primary or recurrent pelvic malignancies; (b) the pelvic cancer must be amenable to exenterative resection; (c) report on minimally invasive exenterative surgery techniques and compare directly with open exenteration; (d) report on surgical or survival outcomes; and (e) have a clear research methodology.
Exclusion criteria
Studies were excluded from the analysis if: (a) they did not specifically report on locally advanced primary or recurrent pelvic malignancy; (b) they did not report on outcomes following minimally invasive exenterative techniques versus open pelvic exenteration; (c) the methodology was not clearly reported; (d) if only a conference abstract or (e) if the data were overlapping.
Outcomes of interest
The following parameters were used in the meta-analysis to compare the surgical approaches (open versus MIS exenterative resection) in the management of locally advanced primary or recurrent pelvic cancer:
Primary
Comparison of surgical techniques with respect to operative time, blood loss, and margin status was performed.
Secondary
Differences in associated 30-day morbidity, mortality and length of hospital stay were assessed.
Statistical analysis
Statistical analysis was performed using Stata Data Analysis and Statistical Software (Ver. 12 StataCorp LLC USA). Binary outcome data were reported as odd ratios (OR) and 95% confidence interval (95% CI), and were estimated using the Mantel–Haenszel method. For continuous data, standardized mean differences (SMD) and 95% CI were estimated using random effects models. SMD was calculated as [18]
In our analysis, the new treatment was MIS exenteration, compared to open exenteration as the standard treatment. An SMD equal zero denotes equivalent effects between MIS and open exenteration. For continuous data such as operative length, blood loss, and length of stay, SMD less than zero indicates that MIS is better than open, and vice versa.
Comparative parameters were recorded either as mean and standard deviation (SD) or median and range. For continuous data, the mean and SD were estimated from the median and range using formula described by Hozo et al. [19]. Heterogeneity was assessed by I-squared statistics, with > 50% being considered as considerable heterogeneity. Statistical significance was attributed to p value < 0.05.
Results
Eligible studies
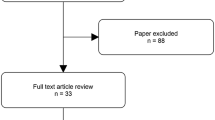
1866 articles were initially identified using the search strategy. After adjusting for duplicates and non-relevant articles, 39 publications were examined in detail. On full text screening, four publications met the predefined inclusion/exclusion criteria (Fig. 1). All studies were retrospective, and were published between 2011 and 2016. On review of the extracted data, there was 100% agreement between the two reviewers. Study details, participation numbers, and characteristics are outlined in Table 1.
Demographics
Analysis was performed on 170 patients; 78.1% (n = 133) had open pelvic exenteration, while 21.8% (n = 37) had a MIS exenteration. Table 1 outlines the histopathological indication for the pelvic exenteration. The studies spanned from 2006 to 2016. The median age for open exenteration was 57.7 years versus 63.0 years for MIS exenteration. The median body mass indices (BMI) for open and MIS exenteration were similar (22.9 vs. 25.6 respectively) (Table 2).
Surgical differences
All four studies reported median operative times (Table 3). MIS exenteration had an 83 min longer median operative time (range 35–130 min, p < 0.001). Pooled analysis showed that the standardized mean difference (SMD) in operative times between MIS and Open exenteration was 0.66 (95% CI 0.27–1.05; p = 0.001; I2 = 74%) in favour of open exenteration (Fig. 2).
However, intra-operative blood loss was significantly less in the MIS exenterative cohort, (median 550 ml (200–5225) versus 2300 ml (200–9619), respectively). Pooled analysis of all four studies showed the standardized mean difference (SMD) between MIS and Open exenteration was − 1.32 (95% CI − 2.02 to − 0.61; p = 0.000; I2 = 63%) in favour of MIS exenteration (Fig. 3).
Only two studies (Uehara and Martinez) compared the difference in achieving clear margins (R0 resection) between MIS and open exenteration techniques. Pooled analysis showed an Odds Ratio = 1, with no statistical difference between either exenterative methods (p = 0.963) (Fig. 4). There was only one conversion from MIS exenteration to open exenteration reported.
Post-operative outcomes
The overall morbidity rate for MIS exenterative group was 56.7% (n = 21/37) versus 88.5% (n = 85/96) for the open exenterative group. Even though not statistically significant, pooled analysis of three studies showed a 1.17 relative risk increase in 30-day morbidity (p = 0.172) in the open exenteration group (Fig. 5).
There were no 30-day mortality recorded across the four studies; however, there was a significant difference in the median length of hospital stay, with MIS exenterative cohort staying 22 days in hospital versus 28 days for the open exenterative cohort (p = 0.04). Pooled analysis of all four studies showed that the SMD between MIS and Open exenteration was − 0.39 (95% CI − 0.77 to − 0.01; p = 0.047; I2 = 69%) in favour of MIS exenteration (Fig. 6).
Discussion
Pelvic exenteration remains an important surgical procedure for advanced pelvic malignancies [12]. Locally advanced or recurrent pelvic malignancies are technically demanding, due to involvement of several organs within the tight confines of a narrow pelvis [5]. Since first being described in the 1940s, there have been numerous modifications to exenterative surgical techniques that have facilitated more radical excisions with satisfactory short- and long-term outcomes [24, 25].
Improved surgical techniques combined with better peri-operative care have resulted in a substantial reduction in peri-operative mortality, but morbidity remains variable (27–86%) [26, 27]. There has also been increased focus in employing minimal invasive surgical techniques for advanced pelvic cancers. This analysis showed that MIS exenteration is utilized in a very selective case-by-case basis, with relatively low-volume compared to open exenterative surgery. When feasible it was associated with less intra-operative blood loss and shorter length of hospital stay, but did not impact on margin/resection status, despite association with longer procedural times. The emergence of laparoscopic surgery has been widely adapted for major resections involving the colon, rectum, prostate, bladder, and gynaecological organs [28,29,30]. Even in advanced abdominal neoplasms, initial laparoscopy can be performed to assess tumour anatomy and MIS resectability [28]. Laparoscopic surgery is noted to have less intra-operative blood loss, quicker recovery, and reduced length of hospital stay [31]. However, the role of laparoscopic surgery for multi-visceral resection has been controversial, with sporadic reports published. Our review shows that open exenterative surgery is not always required, and MIS exenteration is feasible when there is suitable anatomy, MIS experience, and favourable tumour characteristics. However, with large tumours and unfavourable anatomy (side-wall involvement, need for sacrectomy), MIS exenteration is not suitable or safe, especially when extraction of the tumour is not feasible through small incisions or need for extensive reconstruction options.
Pomel et al. in 2003 were first to describe the feasibility of laparoscopic pelvic exenteration for recurrent cervical cancer [32]. Since then, some centres have enthusiastically advocated laparoscopic exenteration for other advanced pelvic malignancies [33]. However, the majority of cases are highly selective. Proponents of laparoscopic (assisted) exenteration have cited that the excellent optics and magnified views aid meticulous dissection, resulting in comparable quality of dissection, but with less blood loss and morbidity [34]. This is similar to findings in this review. To date, gynaecologists have been keen adopters of laparoscopic (assisted) pelvic exenteration as it is associated with smaller wounds, quicker recovery, and reduced length of hospital stay [23]. All of these factors would likely impact positively on health expenditure costs.
Vasilescu et al. in 2011 reported an entirely robotic pelvic exenteration for recurrent endometrial carcinoma [35]. However, to date, there has been no large multicentre evaluation of MIS exenterative surgery. In addition, the majority of MIS exenterative procedures have been performed in female patients, due to more favourable anatomy [21]. More evaluation is needed to assess if MIS exenteration truly reduces post-operative pain scores, improves quality of life and whether it positively impacts on time to commencing adjuvant therapies (if needed) [23]. Furthermore, there has been no large-scale assessment on the effect that MIS exenteration has on long-term survival.
Gadkari et al. have reported the largest series of laparoscopic exenteration. In their series of 74 cases on laparoscopic anterior pelvic exenteration between 2005 and 2015, they reported a median operative time of 180 min and mean blood loss of 160 ml [36]. They demonstrated that laparoscopic exenteration is not just feasible, but that it is comparable oncologically in terms of the quality of resection [36]. The same group in 2014 reported ten cases of robot-assisted anterior pelvic exenteration [37]. They observed similar operative times and post-operative length of hospital stay, but noted reduced average blood loss (110 ml) with no patient needing blood transfusion. Again, they showed that robot-assisted exenteration provided good oncological resections, with no positive margins [37].
Despite these findings, widespread adoption of MIS exenteration is unlikely at present, as the number of patients deemed suitable for MIS exenteration is low, surgeon experience is limited and there remains a steep learning curve [33]. In addition, there are no data on long-term survival (5-year follow-up), or a cost-effective analysis that supports its role. This article serves to raise increased consideration of MIS exenteration as a reasonable option in suitable patients. However, selection bias issues must be acknowledged as the evidence concerning MIS exenteration is retrospective from single centres with small sample size and short-term follow-up.
Pelvic exenteration covers a heterogeneous group of multi-visceral surgical procedures. This meta-analysis has shown that MIS exenterative surgery is feasible in highly selected cases, with favourable tumour anatomy. MIS exenterative surgery is associated with reduced intra-operative blood loss but similar pathological resection rates. It is not associated with increased morbidity rates; however, there remains a lack of data regarding long-term oncological safety. Ultimately, MIS exenteration may not be a goal in itself, but its utilization in exenterative surgery should be explored.
Conclusion
Minimally invasive pelvic exenteration is feasible, but on highly selected cases with favourable anatomy and therefore caution is warranted. However, when feasible, MIS exenteration can have reduced intra-operative blood loss and length of hospital stay, with no adverse impact on resectability.
References
Zoucas E, Frederiksen S, Lydrup ML, Mansson W, Gustafson P, Alberius P (2010) Pelvic exenteration for advanced and recurrent malignancy. World J Surg 34:2177–2184
Brunschwig A (1948) Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1(2):177–183
Butcher HR Jr, Spjut HJ (1959) An evaluation of pelvic exenteration for advanced carcinoma of the lower colon. Cancer 12(4):681–687
Hockel M, Dornhofer N (2006) Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol 7:837–847
Pawlik TM, Skibber JM, Rodriguez-Bigas MA (2005) Pelvic exenteration for advanced pelvic malignancies. Ann Surg Oncol 13(5):612–623
Brown KGM, Koh CE, Solomon MJ, Qasabian R, Robinson D, Dubenec S (2015) Outcomes after en bloc iliac vessel excision and reconstruction during pelvic exenteration. Dis Colon Rectum 58:850–856
Brown KGM, Solomon MJ, Koh CE (2017) Pelvic exenteration surgery: the evolution of radical surgical techniques for advanced and recurrent pelvic malignancy. Dis Colon Rectum 60:745–754
Rodriguwz-Bigas MA, Petrelli NJ (1996) Pelvic exenteration and its modifications. Am J Surg 171(2):293–298
Lopez MJ, Standiford SB, Skibba JL (1994) Total pelvic exenteration. A 50-year experience at the Ellis Fischel Cancer Center. Arch Surg 129(4):390–395 (discussion 5–6).
Yu HH, Leong CH, Ong GB (1976) Pelvic exenteration for advanced pelvic malignancies. Aust N Z J Surg 46(3):197–201
Dobrowsky W, Schmid AP (1985) Radiotherapy of presacral recurrence following radical surgery for rectal carcinoma. Dis Colon Rectum 28(12):917–919
Ferenschild FTJ, Vermaas M, Verhoef C, Ansink AC, Kirkels WJ, Eggermont AMM, deWilt JHW (2009) Total pelvic exenteration for primary and recurrent malignancies. World J Surg 33:1502–1508
Feigel A, Sylla P (2016) Role of minimally invasive surgery in the reoperative abdomen or pelvis. Clin Colon Rectal Surg 29(2):168–180
Keller DS, Flores-Gonzalez JR, Ibarra S, Haas EM (2016) Review of 500 single incision laparoscopic colorectal surgery cases—lessons learned. World J Gastroenterol 22(2):659–667
Medlin EE, Kushner DM, Barroilhet L (2015) Robotic surgery for early stage cervical cancer: evolution and current trends. J Surg Oncol 112(7):772–781
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open medicine: a peer-reviewed, independent. Open-Access J 3(3):e123–e130
Srinivasaiah N, Malietzis G, Jenkins I (2015) Use of laparoscopy in pelvic exenteration surgery. PROSPERO 2015 CRD42015023284. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42015023284
Faraone SV (2008) Interpreting estimates of treatment effects: implications for managed care. P&T 33(12):700–711
Hozo SP, Djulbegovic G, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Winters BR, Mann GN, Louie O, Wright JL (2015) Robotic total pelvic exenteration with laparoscopic rectus flap: Initial experience. Case Rep Surg 2015:835425
Yang K, Cai L, Yao L, Zhang Z, Zhang C, Wang X, Tang J, Li X, He Z, Zhou L (2015) Laparoscopic total pelvic exenteration for pelvic malignancies: the technique and short-time outcome of 11 cases. World J Surg Oncol 15:13:301
Uehara K, Nakamura H, Yoshino Y, Arimoto A, Kato T, Yokoyama Y, Ebata T, Nagino M (2016) Initial experience of laparoscopic pelvic exenteration and comparison with conventional open surgery. Surg Endosc 30(1):132–138
Martinez A, Filleron T, Vitse L et al (2011) Laparoscopic pelvic exenteration for gynaecological malignancy: is there any advantage? Gynecol Oncol 120(3):374–379
Austin KK, Solomon MJ (2009) Pelvic exenteration with en bloc iliac vessel resection for lateral pelvic wall involvement. Dis Colon Rectum 52(7):1223–1233
Heriot AG, Byrne CM, Lee P, Dobbs B, Tilney H, Solomon MJ et al (2008) Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum 51(3):284–291
Nielsen M, Rasmussen P, Lindegaard J et al (2012) A 10-year experience of total pelvic exenteration for primary advanced and locally recurrent rectal cancer based on prospective database. Colrectal Dis 14(9):1076–1083
Ike H, Shimada H, Yamaguchi S et al (2003) Outcomes of total pelvic exenteration for primary rectal cancer. Dis Colon Rectum 46:474–480
Bretagnol F, Dedieu A, Zappa M, Guedj N, Ferron M, Panis Y (2011) T4 colorectal cancer: is laparoscopic resection contraindicated? Colorectal Dis 13(2):138–143
Healy KA, Gomella LG (2013) Retropubic, laparoscopic, or robotic radical prostatectomy: is there any real difference? Semin Oncol 40(3):286–296
Bogani G, Cromi A, Serati M, Di Naro E, Casarin J, Pinelli C, Ghezzi F (2014) Laparoscopic and open abdominal staging for early-stage ovarian cancer: our experience, systematic review, and meta-analysis of comparative studies. Int J Gynecol Cancer 24(7):1241–1249
Ferron G, Pomel C, Martinez A et al (2012) Pelvic exenteration: current state and perspectives. Gynecol Obstet Fertil 40:43–47
Pomel C, Rouzier R, Pocard M, Thoury A et al (2003) Laparoscopic total pelvic exenteration for cervical cancer relapse. Gynecol Oncol 91:616–618
Puntambekar S, Kudchadar RJ, Gurjar AM, Sathe RM et al (2006) Laparoscopic pelvic exenteration for advanced pelvic cancers: a review of 16 cases. Gynecol Oncol 102:513–516
Mukai T, Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Ikeda A et al (2013) Laparoscopic total pelvic exenteration with en bloc lateral lymph node dissection after neoadjuvant chemoradiotherapy for advanced primary rectal cancer. Asian J Endosc Surg 6(4):314–317
Vasilescu C, Tudor S, Popa M, Aldea B, Gluck G (2011) Entirely robotic total pelvic exenteration. Surg Laparosc Endosc Percutaneous Tech 21(4):e200–e202
Gadkari Y, Puntambekar SP et al (2015) Our experience of laparoscopic anterior exenteration in locally advanced cervical carcinoma. J Min Invasive Gynecol 22:S1–S253
Puntambekar S, Lawande A, Desai R, Puntambekar S, Joshi GA, Joshi SN (2014) Initial experience of robotic anterior pelvic exenteration at a single institute. Int J Gynaecol Obstet 126(1):41–44
PelvEx Collaborative authors
Srinivasaiah N, Shekleton F, Kelly ME, Harji D, Malietzis G, Askari A, Aalbers AGJ, Alberda W, Antoniou A, Austin KK, Beets GL, Berg PL, Beynon J, Bosman SJ, Brunner M, Burger JWA, Campain N, Christensen HK, Coscia M, Colquhoun AJ, Coyne P, Daniels IR, Davies RJ, de Wilt JHW, Denost Q, Deutsch C, Dietz D, Duff M, Eglinton T, Fearnhead N, Frizelle FA, Garcia-Sabrido JL, George ML, Gentilini L, Griffiths B, Harris DA, Evans M, Heriot AG, Hohenberger W, Hoe CM, Holm T, Kanemitsu Y, Chan KKL, Kim H, Koh CE, Kok NF, Kontovounisios C, Law WL, Laurberg S, Lee P, Lydrup ML, Lynch AC, Martling A, Meijerink J, Mentha A, Merkel S, McDermott FD, McGrath JS, Nielsen MB, Nieuwenhuijzen GAP, Nilsson PJ, Abraham-Nordling M, O’Connell PR, Patsouras D, Poggioli G, Radwan RW, Rasheed S, Rasmussen PC, Rothbarth J, Rutten HJT, Sagar PM, Schizas AMP, Shida D, Smart NJ, Solomon MJ, Sorensen MM, Stocchi L, Tekkis PP, Tsukamoto S, Turner WH, Tuynman JB, van Ramshorst GH, van Zoggel D, Vasquez-Jimenez W, Verhoef C, Verstegen M, Wakeman C, Warrier S, Yip J, Winter DC, Jenkins JT.
Author information
Authors and Affiliations
Consortia
Ethics declarations
Conflict of interest
All PelvEx Collaborative authors declare no conflict of interest.
Additional information
PelvEx Collaborative—JT Jenkins, St Mark’s Hospital, London, email: mrianjenkins@icloud.com.
All PelvEx Collaborative members are co-authors and approved this submission. The list of authors is provided at the end of the article.
Rights and permissions
About this article
Cite this article
The PelvEx Collaborative. Minimally invasive surgery techniques in pelvic exenteration: a systematic and meta-analysis review. Surg Endosc 32, 4707–4715 (2018). https://doi.org/10.1007/s00464-018-6299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6299-5