Abstract
Despite improving the survival after repair of esophageal atresia (EA), the morbidity of EA repair remains high. Specifically, tracheomalacia (TM) is one of the most frequent complications of EA repair. Continuous positive airway pressure is generally applied for the treatment of TM. However, surgical intervention is required against an apparent life-threatening event or inability to perform extubation for a long period. According to our review, most cases of TM showed symptom improvement after aortopexy. The ratio of the trachea’s lateral and anterior–posterior diameter at the brachiocephalic artery crossing the trachea, which reflects the compression of the trachea by the brachiocephalic artery, is a good indicator of aortopexy. Our finding suggests that most TM cases associated with EA may not be caused by tracheal fragility alone, but may involve blood vessel compression. Posterior tracheopexy (PT) is also an effective treatment for TM. Recently, open or thoracoscopic PT was able to be performed simultaneously with EA repair. In many cases, aortopexy or PT is a safe and effective surgical treatment for TM with EA. Other surgical procedures, such as external stenting, should be considered for patients with diffuse-type TM for whom aortopexy and PT appear relatively ineffective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophageal atresia (EA) is one of the most common congenital disorders of the alimental tract, affecting an average of 1 in 4000 births [1]. Most EA cases are repaired unless patients have lethal chromosomal abnormalities, which reportedly occur in 6–10% of EA cases [1, 2], and the survival rate is improving, reaching approximately 90% [3, 4]. However, morbidities following EA repair, including anastomotic leakage, anastomotic stenosis, gastroesophageal reflux, and tracheomalacia (TM), remain frequent [5].
TM in particular is one of the most frequent complications of EA [5]. TM is a condition wherein the tracheal wall loses its stiffness, and its lumen is abnormally constricted because of the increased intrathoracic pressure during expiration or coughing. In previous studies, the incidence rate of TM in patients with EA has varied but was still relatively high, ranging from 15 [6] to 90% [7, 8].
TM treatment requires considerable effort owing to the fact that weaning from respiratory support or cyanotic spells is challenging [9]. One conservative treatment approach for TM is the administration of high positive end-expiratory pressure until growth improvement is observed [10]. Previously, positive pressure ventilation was often achieved in tracheostomy [11]. However, the administration of tracheostomy has become less popular over the years, owing to the need for long-term management with potentially fatal complications. Conversely, continuous positive airway pressure (CPAP) has become popular. Several surgical treatment options are also available, depending on the onset and severity of TM.
We herein review the treatment of TM with EA along with our surgical experience and intervention for TM with EA. The release of these descriptions of our surgical experience has been approved by the Ethics Committee of Nagoya University Hospital (Ref Nos. 2020-0589 and 2019-0421).
The diagnosis
TM is easily suspected in patients with EA because it frequently occurs in these patients. TM should be suspected in cases of suggestive symptoms or clinical histories, such as noisy respiration, tracheal rhonchi, harsh barking cough, or apneic spells because of expiratory obstruction [10]. The diagnosis of TM is relatively simple, but determining its severity is difficult. Examinations for the diagnosis of TM should therefore be started quickly.
Fiberoptic bronchoscopy (FB)
At present, the gold standard for diagnosing TM is FB. The degree of TM can be assessed. In clinical practice, anatomical changes are arbitrarily described as mild (50–75% reduction), moderate (75–90% reduction), or severe (> 90% reduction) on subjective visual inspection. However, this is a purely descriptive classification and does not reflect clinical severity, as the degree of lumen occlusion is not associated with disease morbidity [12]. In patients with TM, FB typically reveals widening of the posterior membrane with collapse anteriorly or invagination during exhalation. In more severe cases, the airway may be entirely occluded. Furthermore, patients with TM caused by external compression often show flattened anterior tracheal cartilage (Fig. 1) [9].
Fibrobroncoscpy of the patient with TM due to the anterior compression by the brachiocephalic artery. Black triangles indicate the tracheal cartilage deformed due to the anterior compression by the brachiocephalic artery. This deformation can be observed at both expiration and inspiration. The white arrow indicates an excessive dynamic movement of the posterior tracheal membrane at expiration. a Image at expiration. The trachea is constricted. b Image at inspiration. The constriction of the trachea is reduced
Computed tomography (CT)
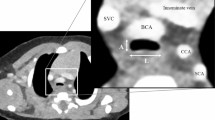
In the recent years, multiphasic scanning by multirow-detector helical CT, by which we can obtain multiple images that appear at exhalation and inhalation in infants, has been recognized as a useful modality for diagnosing TM in children [13]. By obtaining the precise information about the caliber change and spread of lesions in large airways at both end-inspiration and end-expiration, CT can be used to make a diagnosis (Fig. 2). Furthermore, CT can show the relationship between the trachea and the position where the brachiocephalic artery (BCA) branches from the aorta. In nearly half of children, the BCA branches off the left side of the trachea and traverses it [14]. In children with respiratory problems, the shape of the trachea is often flattened where the BCA crosses the trachea because of compression by the BCA [14]. The diagnostic accuracy of TM by CT is sufficient [15], and a good concordance was found between FB and CT findings concerning the presence of TM [16].
Sagittal views of computed tomography in a patient with TM. The two images were taken at different time points of the contrast phase. They were taken unintentionally at expiration and inspiration. BCA indicates brachiocephalic artery. a Image at expiration. The trachea is constricted. b Image at inspiration. The constriction of the trachea above the BCA is reduced. However, the pressure from the BCA remained
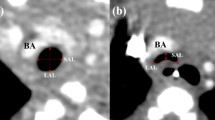
In our hospital, we apply the numerical reference from CT that can easily evaluate the severity of TM. The numerical reference was defined according to the contrast-enhanced CT findings. The lateral and the anterior–posterior diameters of the trachea on the slice where the BCA crosses the trachea were measured, and their ratio was calculated (lateral/anterior–posterior ratio [LAR]) (Fig. 3). A large LAR indicates that the trachea is compressed and compressed by the BCA. Through this approach, the mean LAR calculated from the results of 51 control cases (patients without TM) was 1.2, whereas that of patients with TM requiring aortopexy was 3.9. Patients with TM thus had much larger LAR values than controls [17].
Definition of LAR. The lateral diameter (L) and the anterior–posterior diameter (A) of the trachea at the slice where the brachiocephalic artery crosses the trachea in the computed tomography. LAR was defined as the ratio of the lateral diameter and the anterior–posterior diameter. BCA brachiocephalic artery, ICA internal carotid artery, SCA subclavian artery, INV innominate vein
Other approaches
Other diagnostic examinations for TM include pulmonary function testing and fluoroscopy. However, pulmonary function testing can be performed only on older children and is unsuitable for evaluating TM in infants. Fluoroscopy is reportedly a highly specific, but poorly sensitive test for evaluating TM (Fig. 4) [18]. Although fluoroscopy lacks reasonable sensitivity [19], it may be worth performing, as it can show TM easily and clearly. However, imaging examinations, including CT and fluoroscopy, only reveal narrow airways anatomically. Therefore, determining the indication for surgery based only on the imaging examinations is challenging. The surgical indications should also be decided based on the symptoms, such as the presence of an apparent life-threatening event (ALTE) or recurrent pneumonia.
Treatment
CPAP
Children with mild TM can be treated nonsurgically. The abnormal soft trachea tends to become more rigid with growth. Mild TM is reported to be relieved by around 2 years old. CPAP effectively prevents the posterior membranous trachea from protruding toward the tracheal lumen during exhalation. It provides support by maintaining breathing until the improvement of TM symptoms [10]. A recent report revealed that a high-flow nasal cannula was able to maintain CPAP with noninvasive respiratory management for patients with TM after EA repair [20].
Tracheostomy
Tracheostomy is usually created for respiratory support with CPAP for a long period. However, it is not a very safe procedure. A study reported that 15–19% of children experience tracheostomy-related complications. Adverse events following tracheostomy placement in children range from mild to life threatening [21]. Therefore, tracheostomy has not been performed as a primary treatment in the recent years, and various types of noninvasive positive pressure ventilation (e.g., masked CPAP) have become the preferred procedure.
Surgical interventions
For severe TM, surgical intervention is recommended to improve or alleviate symptoms and sequelae of TM, such as an apparent life-threatening events or recurrent pneumonia.
Aortopexy
Aortopexy has been the mainstay of surgical therapy for pediatric intrathoracic TM [10]. Aortopexy was long ago reported as a surgical solution for tracheal compression by a great artery [22]. Aortopexy has become a generally accepted treatment mode, although thoracotomy’s invasion is considerable. The thoracoscopic approach was introduced in the current era of minimally invasive surgery [23].
In PubMed, we searched articles published after 2012 using the term “aortopexy”, except for articles on general topics or case reports, and selected only those related to EA. Accordingly, we found 1 large review [24] and 10 case series [23, 25,26,27,28,29,30,31,32,33].
The review article by Torre et al. analyzed 40 articles published from 1980 to 2011 including a total of 758 patients with TM, with 581 undergoing aortopexy. TM was associated with EA in 328 patients. Regarding the 10 case series reports, the three articles [26, 28, 32] had overlapping objects; therefore, only 1 [26] was adopted for review. In total, 215 aortopexies were performed, among which 130 (60%) were performed for EA.
Consequently, 64–100% of the patients achieved satisfactory improvement of symptoms. The incidence of re-do aortopexy ranged from 0 to 31%, and the mortality ranged from 0 to 17%. However, this appeared to include deaths from preexisting complications (Table 1). Postoperative complications in each article were reviewed, including pneumothorax or pleural effusion [24, 27, 29], respiratory tract infection or lung atelectasis [24, 25], phrenic nerve palsy [24, 29, 30], bleeding [24, 30], vocal fold palsy [25, 33], and wound infection [25].
When compared with other populations, aortopexy for patients with TM associated with EA achieved better results than in patients with associated severe cardiac anomalies [24].
In our hospital, aortopexy was introduced in 2016 for treating TM associated with EA. In brief, regarding our surgical technique, the left thymus lobe was excised to enable pericardial and aortic arch visualization after median sternotomy or a left thoracoscopic approach. In some cases, the innominate vein was cut. The aortic wall at the origin of the BCA was sutured with 4-0 or 5-0 nonabsorbable sutures (Prolene Polypropylene Suture, Ethicon Inc., Somerville, NJ, USA) reinforced with pledgets. More than two sutures were placed on the BCA. These strings were passed inside-out intercostally and tied down to pull the aorta and BCA away from the trachea. This procedure was performed under observation with FB to ensure that the trachea was relieved of compression by the BCA.
Five patients underwent aortopexy after chest CT and EA repair to assess TM. The indication for aortopexy was ALTE for two patients and dependent on respiratory support, such as masked CPAP, for the remaining three patients. The LARs of all cases were > 2.5 (range, 2.88–5.57). Although respiratory symptoms were not improved after the first aortopexy in one patient and required re-do-aortopexy, all patients achieved relief of their respiratory symptoms and exhibited improved in their LAR to < 1.5 (range 1.19–1.49) (Fig. 5) [17].
Transition in LAR before and after surgery for each patient who required aortopexy. The LAR of the four patients decreased to < 1.5 after the first aortopexy. The LAR of the remaining patient in whom respiratory support was continued did not decrease after the first aortopexy. The patient underwent re-do-aortopexy, and LAR decreased to < 1.5 after the second surgery
Posterior tracheopexy (PT)
PT is the suture fixation of the membranous posterior tracheal wall to the anterior longitudinal spinal ligament [34]. In the trachea of patients with EA, the ratio between the anterior cartilaginous ring and the posterior membranous trachea is reduced, and a floppy pars membranacea causes TM [10]. Therefore, some researchers argue that PT is a good indication for patients with TM associated with EA, as PT can directly address posterior membranous tracheal intrusion [35].
A literature search in PubMed after 2012 using the terms “posterior tracheopexy,” excluding articles of general topic or case reports and including only articles related to EA, revealed four case series reports (Table 2) [36,37,38,39].
One article reported 118 patients with TM associated with EA. Among them, 18 underwent PT at the time of the EA repair, whereas 100 underwent PT after initial EA repair. No obvious early postoperative complications or postoperative deaths were reported. Following PT, the incidence of symptoms was significantly reduced, and bronchoscopy showed significant improvement. All 11 patients who had tracheostomy before PT were able to be weaned off mechanical ventilation. Fifteen patients required further interventions [39]. Another article reported the outcomes of eight patients with severe TM who underwent PT, of which six patients had EA. Two patients had chylothorax postoperatively. All patients reported improvement of TM symptoms, although one required re-do PT for recurrence [37].
Tytgat et al. introduced a new approach for TM with EA, i.e., PT was performed during thoracoscopic repair of EA when TM was diagnosed on FB before EA repair. Four patients underwent additional PT during thoracoscopic EA repair, with an average additional time of 6 min. No complications occurred. No additional procedures were required in any cases [38]. Another article reported 14 patients who underwent PT for thoracoscopic EA repair. Accordingly, there were no significant differences in the respiratory outcomes of patients with PT compared with those in whom PT was deemed preoperatively by bronchoscopy to be unnecessary. No patients who underwent PT required further interventions [36].
In our hospital, intraoperative PT during thoracoscopic EA repair was introduced in 2020 and performed in all patients with EA complicated by TM as defined by bronchoscopy performed preoperatively or intraoperatively. In brief, regarding our surgical technique, PT was performed during primary EA repair. The tracheal suture height was determined by FB to identify the location where the posterior tracheal wall collapsed after esophagus anastomosis. Several nonabsorbable sutures (5-0 Prolene Polypropylene Suture; Ethicon Inc.) were placed from the membranous posterior tracheal wall to the anterior longitudinal spinal ligament to pull the trachea.
Eight patients underwent PT during thoracoscopic EA repair. The median operative time required for PT was 10 (range, 8–15) min. One patient developed chylothorax postoperatively, which was resolved conservatively. The postoperative LAR and incidence of dependence on respiratory support were sufficiently low in patients who underwent PT during primary thoracoscopic repair for EA with TM [40].
External tracheobronchial stabilization
External splinting may offer airway support in selected patients with severe TM. External splints have been used to stabilize the malacic or deformed airway. A ring-reinforced expanded polytetrafluoroethylene was used for the prosthesis and achieved good results [41]. Recently, a resorbable plate has also been used to provide temporary airway support in cases with full resorption predicted to occur within several years, hopefully providing enough time for the cartilage to reform in a more favorable shape and to allow for growth of the airway in pediatric patients [42]. However, the implantation of external prosthetic splints has raised concerns regarding its long-term effects and complications, including infection and erosion into nearby structures. Therefore, this procedure should be performed only when other methods have not been sufficiently effective.
Discussion
Improvements in neonatal care have improved the survival rate of infants with low-risk EA. The focus has thus shifted gradually from the survival after repair to managing early and late morbidities. TM is recognized as a particularly frequent complication of EA.
Patients with EA show innate tracheal anomalies. For example, in approximately 80% of patients with EA, the esophageal muscle and squamous epithelium were identified in the membranous part of the trachea; this may have been the result of the trachea and esophagus sharing a common embryological foregut origin [43]. In addition, the ratio between the anterior cartilaginous ring and the posterior membranous trachea is reduced from 4–5:1 to 2–3:1 in patients with EA [10, 19]. Disturbance of the normal tracheal development by tracheal compression caused by dilated proximal esophagus pouch in utero [44] or normal intratracheal pressure loss in utero through a TEF [45] leads to this phenomenon. The occurrence of the resulting flaccid trachea can be attributable to the abovementioned factors.
TM is classified as follows: (i) airway compression by BCA or aberrant subclavian artery, (ii) double aortic arch or vascular ring causing circumferential airway compression, (iii) diffuse or focal cartilage weakness with dynamic large airways collapse (as observed in certain genetic conditions and tracheal inflammation), and (iv) excessive posterior tracheal membrane dynamic movement during forced exhalation [46]. TM associated with EA concurrently shows histological or structural anomalies; thus, this form of TM is considered to be based on congenital factors, as the third or the fourth category [10, 43]. However, most TM cases in patients with EA are actually the segmental type. This type of TM is also associated with other thoracic lesions, such as vascular compression, mainly by the BCA or aberrant artery (i.e., belonging to the first category), and aortopexy is effective in many such cases [10].
In most children, the BCA branches off from the aortic arch at the left side of the trachea [14]. However, the only phenomenon that BCA crosses the trachea anteriorly does not cause clinically significant dyspnea in most children [47]. In contrast, TM due to compression by the BCA is frequently observed in patients with EA. TM with EA appears to be caused by the combination of congenital fragility, a marked width of the posterior membranous trachea, and BCA compression. Given that aortopexy improves TM symptoms in most cases, compression by the BCA may be one of the most significant causes of TM with EA.
We propose considering the LAR to reflect the degree of compression of the trachea by the BCA. Previous reports have described the shape of the trachea as being flattened when the trachea was compressed by the BCA, although a specific value has not been described [14]. In our experience, the LARs of patients who underwent aortopexy were all > 2.5, with these values decreasing to < 1.5 after successful aortopexy [17]. This indicates that LAR is indeed useful for predicting the therapeutic effect of aortopexy.
Recently, PT during thoracoscopic EA repair has become the preferred procedure in TM, as it involves a short operation time and little morbidity. In our experience, the postoperative LAR and incidence of respiratory support dependence are sufficiently low among patients who underwent PT. Interestingly, we found that PT improves the LAR not only by the direct effect of PT on the tracheal membranes, which are not pushed forward, but also by changing the position of the trachea in relation to the esophagus and BCA by fixing the trachea [40].
However, not all patients who underwent PT were completely relieved of post-curative respiratory support. We encountered a patient with long-segment TM with bronchomalacia who did not show any improvements after PT. The patient required tracheostomy and external stenting of the trachea [40]. This suggests that PT alone is not sufficient, so other surgical treatments should be considered for patients with broad airway lesions. In such patients, malacia exists in the broad area of the trachea at sites other than where the BCA crosses the trachea, including the bronchus. LAR values alone therefore cannot predict the severity of TM. Aortopexy alone will not likely improve respiratory disorders in such patients, and external tracheal stenting will be required.
Our therapeutic strategy for EA patient in recent years has been as follows (Fig. 6): At the time of EA repair, FB is performed to confirm TM presence. If TM is observed, PT is simultaneously performed during EA repair. In this way, the subsequent incidence of dependence on respiratory support because of TM can be reduced. However, if the patient continues to show dependence on respiratory support after EA repair, CT is performed, and the LAR is measured. This is a good predictor of the effectiveness of aortopexy. External stenting should be considered for patients with diffuse TM, as aortopexy or PT appear to be less effective for extensive airway involvement than external stenting. This EA with TM strategy based on the literature review appears reasonable.
Cases in which occurrence of TM has complicated EA have reportedly increased recently, possibly due to the fact that treatment outcomes of EA have improved. Another reason for this is that TM as a postoperative complication is now being prioritized for treatment by clinicians.
We summarized the recent TM diagnoses and treatment and discussed our treatment strategy.
Data availability
All data generated or analyzed during this study are included in the published articles in the references.
References
Nassar N, Leoncini E, Amar E, Arteaga-Vazquez J, Bakker MK, Bower C, Canfield MA, Castilla EE, Cocchi G, Correa A, Csaky-Szunyogh M, Feldkamp ML, Khoshnood B, Landau D, Lelong N, Lopez-Camelo JS, Lowry RB, McDonnell R, Merlob P, Metneki J, Morgan M, Mutchinick OM, Palmer MN, Rissmann A, Siffel C, Sipek A, Szabova E, Tucker D, Mastroiacovo P. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2012;94:893–9. https://doi.org/10.1002/bdra.23067.
Quiroz HJ, Turpin A, Willobee BA, Ferrantella A, Parreco J, Lasko D, Perez EA, Sola JE, Thorson CM. Nationwide analysis of mortality and hospital readmissions in esophageal atresia. J Pediatr Surg. 2020. https://doi.org/10.1016/j.jpedsurg.2020.01.025.
Masuya R, Kaji T, Mukai M, Nakame K, Kawano T, Machigashira S, Yamada W, Yamada K, Onishi S, Yano K, Moriguchi T, Sugita K, Kawano M, Noguchi H, Suzuhigashi M, Muto M, Ieiri S. Predictive factors affecting the prognosis and late complications of 73 consecutive cases of esophageal atresia at 2 centers. Pediatr Surg Int. 2018;34:1027–33. https://doi.org/10.1007/s00383-018-4326-1.
Keefe G, Culbreath K, Edwards EM, Morrow KA, Soll RF, Modi BP, Horbar JD, Jaksic T. Current outcomes of infants with esophageal atresia and tracheoesophageal fistula: a multicenter analysis. J Pediatr Surg. 2022;57:970–4. https://doi.org/10.1016/j.jpedsurg.2022.01.060.
Rayyan M, Embrechts M, Van Veer H, Aerts R, Hoffman I, Proesmans M, Allegaert K, Naulaers G, Rommel N. Neonatal factors predictive for respiratory and gastro-intestinal morbidity after esophageal atresia repair. Pediatr Neonatol. 2019;60:261–9. https://doi.org/10.1016/j.pedneo.2018.07.003.
Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR 3rd. Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Archiv Surg (Chicago Ill: 1960). 1995;130:502–8. https://doi.org/10.1001/archsurg.1995.01430050052008. (discussion 508-509).
DeBoer EM, Prager JD, Ruiz AG, Jensen EL, Deterding RR, Friedlander JA, Soden J. Multidisciplinary care of children with repaired esophageal atresia and tracheoesophageal fistula. Pediatr Pulmonol. 2016;51:576–81. https://doi.org/10.1002/ppul.23330.
Fayoux P, Morisse M, Sfeir R, Michaud L, Daniel S. Laryngotracheal anomalies associated with esophageal atresia: importance of early diagnosis. Eur Archiv Otorhinolaryngol. 2018;275:477–81. https://doi.org/10.1007/s00405-017-4856-5.
Hysinger EB, Panitch HB. Paediatric tracheomalacia. Paediatr Respir Rev. 2016;17:9–15. https://doi.org/10.1016/j.prrv.2015.03.002.
Fraga JC, Jennings RW, Kim PC. Pediatric tracheomalacia. Semin Pediatr Surg. 2016;25:156–64. https://doi.org/10.1053/j.sempedsurg.2016.02.008.
Overman AE, Liu M, Kurachek SC, Shreve MR, Maynard RC, Mammel MC, Moore BM. Tracheostomy for infants requiring prolonged mechanical ventilation: 10 years’ experience. Pediatrics. 2013;131:e1491-1496. https://doi.org/10.1542/peds.2012-1943.
Wallis C, Alexopoulou E, Anton-Pacheco JL, Bhatt JM, Bush A, Chang AB, Charatsi AM, Coleman C, Depiazzi J, Douros K, Eber E, Everard M, Kantar A, Masters IB, Midulla F, Nenna R, Roebuck D, Snijders D, Priftis K. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J. 2019. https://doi.org/10.1183/13993003.00382-2019.
Lee EY, Boiselle PM. Tracheobronchomalacia in infants and children: multidetector CT evaluation. Radiology. 2009;252:7–22. https://doi.org/10.1148/radiol.2513081280.
Fawcett SL, Gomez AC, Hughes JA, Set P. Anatomical variation in the position of the brachiocephalic trunk (innominate artery) with respect to the trachea: a computed tomography-based study and literature review of innominate artery compression syndrome. Clin Anat (New York, NY). 2010;23:61–9. https://doi.org/10.1002/ca.20884.
Ngerncham M, Lee EY, Zurakowski D, Tracy DA, Jennings R. Tracheobronchomalacia in pediatric patients with esophageal atresia: comparison of diagnostic laryngoscopy/bronchoscopy and dynamic airway multidetector computed tomography. J Pediatr Surg. 2015;50:402–7. https://doi.org/10.1016/j.jpedsurg.2014.08.021.
Lee EY, Mason KP, Zurakowski D, Waltz DA, Ralph A, Riaz F, Boiselle PM. MDCT assessment of tracheomalacia in symptomatic infants with mediastinal aortic vascular anomalies: preliminary technical experience. Pediatr Radiol. 2008;38:82–8. https://doi.org/10.1007/s00247-007-0672-1.
Sumida W, Tainaka T, Shirota C, Yokota K, Makita S, Takimoto A, Yasui A, Okamoto M, Nakagawa Y, Hinoki A, Uchida H. An imaging study on tracheomalacia in infants with esophageal atresia: the degree of tracheal compression by the brachiocephalic artery is a good indicator for therapeutic intervention. Pediatr Surg Int. 2021;37:1719–24. https://doi.org/10.1007/s00383-021-04985-0.
Sanchez MO, Greer MC, Masters IB, Chang AB. A comparison of fluoroscopic airway screening with flexible bronchoscopy for diagnosing tracheomalacia. Pediatr Pulmonol. 2012;47:63–7. https://doi.org/10.1002/ppul.21517.
Snijders D, Barbato A. An update on diagnosis of tracheomalacia in children. Eur J pediatr Surg. 2015;25:333–5. https://doi.org/10.1055/s-0035-1559816.
Masui D, Fukahori S, Hashizume N, Ishii S, Yagi M. High-flow nasal cannula therapy for severe tracheomalacia associated with esophageal atresia. Pediatr Int. 2019;61:1060–1. https://doi.org/10.1111/ped.13953.
Watters KF. Tracheostomy in infants and children. Respir Care. 2017;62:799–825. https://doi.org/10.4187/respcare.05366.
Gross RE. Neuhauser EB (1948) Compression of the trachea by an anomalous innominate artery; an operation for its relief. Am J Dis Child. 1911;75:570–4. https://doi.org/10.1001/archpedi.1948.02030020585007.
van der Zee DC, Straver M. Thoracoscopic aortopexy for tracheomalacia. World J Surg. 2015;39:158–64. https://doi.org/10.1007/s00268-014-2798-2.
Torre M, Carlucci M, Speggiorin S, Elliott MJ. Aortopexy for the treatment of tracheomalacia in children: review of the literature. Ital J Pediatr. 2012;38:62. https://doi.org/10.1186/1824-7288-38-62.
Williams SP, Losty PD, Dhannapuneni R, Lotto A, Guerrero R, Donne AJ. Aortopexy for the management of paediatric tracheomalacia—the Alder Hey experience. J Laryngol Otol. 2020. https://doi.org/10.1017/s0022215120000031.
Wong ZH, Hewitt R, Cross K, Butler C, Yeh YT, Ramaswamy M, Blackburn S, Giuliani S, Muthialu N, De Coppi P. Thoracoscopic aortopexy for symptomatic tracheobronchomalacia. J Pediatr Surg. 2020;55:229–33. https://doi.org/10.1016/j.jpedsurg.2019.10.034.
Haveliwala Z, Yardley I. Aortopexy for tracheomalacia via a suprasternal incision. J Pediatr Surg. 2019;54:247–50. https://doi.org/10.1016/j.jpedsurg.2018.10.073.
Rijnberg FM, Butler CR, Bieli C, Kumar S, Nouraei R, Asto J, McKavanagh E, de Coppi P, Muthialu N, Elliott MJ, Hewitt RJ. Aortopexy for the treatment of tracheobronchomalacia in 100 children: a 10-year single-centre experience. Eur J Cardio Thorac Surg. 2018;54:585–92. https://doi.org/10.1093/ejcts/ezy076.
Gruszka A, Sachweh JS, Schnoering H, Tenbrock K, Muehler EG, Laschat M, Vazquez-Jimenez JF. Aortopexy offers surgical options for a variety of pathological tracheal conditions in paediatric patients. Interact Cardiovasc Thorac Surg. 2017;25:589–94. https://doi.org/10.1093/icvts/ivx163.
Kay-Rivest E, Baird R, Laberge JM, Puligandla PS. Evaluation of aortopexy in the management of severe tracheomalacia after esophageal atresia repair. Dis Esophagus. 2015;28:234–9. https://doi.org/10.1111/dote.12179.
Jennings RW, Hamilton TE, Smithers CJ, Ngerncham M, Feins N, Foker JE. Surgical approaches to aortopexy for severe tracheomalacia. J Pediatr Surg. 2014;49:66–70. https://doi.org/10.1016/j.jpedsurg.2013.09.036. (discussion 70-61).
Arnaud AP, Rex D, Elliott MJ, Curry J, Kiely E, Pierro A, Cross K, De Coppi P. Early experience of thoracoscopic aortopexy for severe tracheomalacia in infants after esophageal atresia and tracheo-esophageal fistula repair. J Laparoendosc Adv Surg Tech A. 2014;24:508–12. https://doi.org/10.1089/lap.2013.0376.
Montgomery J, Sau C, Clement W, Danton M, Davis C, Haddock G, McLean A, Kubba H. Treatment of tracheomalacia with aortopexy in children in Glasgow. Eur J Pediatr Surg. 2014;24:389–93. https://doi.org/10.1055/s-0033-1351662.
Bairdain S, Zurakowski D, Baird CW, Jennings RW. Surgical treatment of tracheobronchomalacia: a novel approach. Paediatr Respir Rev. 2016;19:16–20. https://doi.org/10.1016/j.prrv.2016.04.002.
Shieh HF, Smithers CJ, Hamilton TE, Zurakowski D, Rhein LM, Manfredi MA, Baird CW, Jennings RW. Posterior tracheopexy for severe tracheomalacia. J Pediatr Surg. 2017;52:951–5. https://doi.org/10.1016/j.jpedsurg.2017.03.018.
van Tuyll van Serooskerken ES, Tytgat S, Verweij JW, Bittermann AJN, Coenraad S, Arets HGM, van der Zee DC, Lindeboom MYA. Primary posterior tracheopexy in esophageal atresia decreases respiratory tract infections. Front Pediatr. 2021;9:720618. https://doi.org/10.3389/fped.2021.720618.
Dewberry L, Wine T, Prager J, Masaracchia M, Janosy N, Polaner D, DeBoer E, Somme S. Thoracoscopic posterior tracheopexy is a feasible and effective treatment for tracheomalacia. J Laparoendosc Adv Surg Tech A. 2019;29:1228–31. https://doi.org/10.1089/lap.2019.0156.
Tytgat S, van Herwaarden-Lindeboom MYA, van Tuyll van Serooskerken ES, van der Zee DC. Thoracoscopic posterior tracheopexy during primary esophageal atresia repair: a new approach to prevent tracheomalacia complications. J Pediatr Surg. 2018;53:1420–3. https://doi.org/10.1016/j.jpedsurg.2018.04.024.
Shieh HF, Smithers CJ, Hamilton TE, Zurakowski D, Visner GA, Manfredi MA, Baird CW, Jennings RW. Posterior tracheopexy for severe tracheomalacia associated with Esophageal Atresia (EA): primary treatment at the time of initial EA repair versus secondary treatment. Front Surg. 2017;4:80. https://doi.org/10.3389/fsurg.2017.00080.
Yasui A, Hinoki A, Amano H, Shirota C, Tainaka T, Sumida W, Yokota K, Makita S, Okamoto M, Takimoto A, Nakagawa Y, Uchida H. Thoracoscopic posterior tracheopexy during primary esophageal atresia repair ameliorate tracheomalacia in neonates: a single-center retrospective comparative cohort study. BMC Surg. 2022. https://doi.org/10.1186/s12893-022-01738-1.
Takazawa S, Uchida H, Kawashima H, Tanaka Y, Masuko T, Deie K, Nagase Y, Iwanaka T. External stabilization for severe tracheobronchomalacia using separated ring-reinforced ePTFE grafts is effective and safe on a long-term basis. Pediatr Surg Int. 2013;29:1165–9. https://doi.org/10.1007/s00383-013-3383-8.
Kamran A, Jennings RW. Tracheomalacia and tracheobronchomalacia in pediatrics: an overview of evaluation, medical management, and surgical treatment. Front Pediatr. 2019;7:512. https://doi.org/10.3389/fped.2019.00512.
Emery JL, Haddadin AJ. Squamous epithelium in respiratory tract of children with tracheo-oesophageal fistula. Arch Dis Child. 1971;46:236–42. https://doi.org/10.1136/adc.46.247.236.
Davies MR, Cywes S. The flaccid trachea and tracheoesophageal congenital anomalies. J Pediatr Surg. 1978;13:363–7. https://doi.org/10.1016/s0022-3468(78)80455-2.
Wailoo MP, Emery JL. Normal growth and development of the trachea. Thorax. 1982;37:584–7. https://doi.org/10.1136/thx.37.8.584.
Choi S, Lawlor C, Rahbar R, Jennings R. Diagnosis, classification, and management of pediatric tracheobronchomalacia: a review. JAMA Otolaryngol Head Neck Surg. 2019;145:265–75. https://doi.org/10.1001/jamaoto.2018.3276.
Gardella C, Girosi D, Rossi GA, Silvestri M, Toma P, Bava G, Sacco O. Tracheal compression by aberrant innominate artery: clinical presentations in infants and children, indications for surgical correction by aortopexy, and short- and long-term outcome. J Pediatr Surg. 2010;45:564–73. https://doi.org/10.1016/j.jpedsurg.2009.04.028.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sumida, W., Yasui, A., Shirota, C. et al. Update on aortopexy and posterior tracheopexy for tracheomalacia in patients with esophageal atresia. Surg Today 54, 211–219 (2024). https://doi.org/10.1007/s00595-023-02652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02652-6