Abstract
Purpose
Tracheomalacia (TM) is a frequent complication after esophageal atresia (EA) repair. This study aimed to review patients who underwent aortopexy for TM after EA repair and to compare their imaging features.
Methods
The patients who underwent thoracoscopic EA repair and contrast-enhanced computed tomography (CECT) at our hospital between 2013 and 2020 were retrospectively reviewed. The ratio of the lateral and anterior–posterior diameter of the trachea (LAR) where the brachiocephalic artery (BCA) crosses the trachea was defined. The LAR of the patients who underwent CECT for asymptomatic pulmonary disease was set as a normal reference. The Z-score of each LAR was calculated and compared between the patients that did or did not undergo aortopexy.
Results
A total of 51 patients represented the controls, 5 patients underwent aortopexy, and 12 patients were discharged without surgery. The mean LARs in the patients who underwent aortopexy, did not undergo aortopexy, and controls were 3.54, 1.54, and 1.15, respectively. The mean Z-score of the aortopexy group was 21.2. After successful aortopexy, each patient’s LAR decreased to < 1.5.
Conclusion
Aortopexy was preferred if the trachea was compressed by the BCA. The LAR is a useful indicator for predicting the therapeutic effect of aortopexy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophageal atresia (EA) is one of the most common congenital disorders in the alimental tract and affects approximately 1 in 4000 births [1]. Almost all EA cases are successfully treated except for patients with lethal chromosomal abnormalities, which have been reported to occur in 6–10% of EA cases [1, 2]. The survival rate of EA patients is improving and is already over 90% [3]. Nevertheless, the morbidity of EA repair remains high, owing to anastomotic leakage, anastomotic stenosis, gastroesophageal reflux, and tracheomalacia (TM) [4].
In particular, TM is reportedly one of the most frequent complications in EA patients [4]. There are several possible reasons for the association between TM and EA, including the fact that both viscera share a common embryological foregut origin. Therefore, innate tracheal anomalies are often observed in EA patients. For example, the ratio between the anterior cartilaginous ring and posterior membranous trachea is reduced from 4–5:1 to 2–3:1 in EA cases, which predisposes the airway to collapse more easily [5, 6].
Furthermore, treatment is difficult because children with TM can experience difficulty weaning from respiratory support as well as recurrent bacterial bronchitis or cyanotic spells [7]. One conservative method for treating TM is waiting for improvement during growth by applying high positive pressure at the end of expiration [5]. Tracheostomy is often created as a means to achieve positive pressure ventilation [8]. Furthermore, aortopexy is a form of surgical treatment that is often performed [4] and has been the mainstay of surgical therapy for pediatric intrathoracic TM [5]. Aortopexy was already long ago reported as a surgical solution for trachea compressed by the brachiocephalic artery (BCA) [9]. However, the criteria for performing aortopexy are still not clear.
TM may occur in some patients after EA repair due to compression of the trachea by the BCA; these cases may need to undergo aortopexy, for the management of TM. We hypothesized that some patients with TM after EA had a peculiar tracheal morphology, which resulted in high aortopexy effectiveness. This study aimed to compare the imaging features of the trachea of the patients who underwent aortopexy for TM after EA repair in our hospital with those of normal people.
Methods
We reviewed the records of patients who underwent the repair for EA at our hospital between 2013 and 2020. The patients who underwent tracheoesophageal fistula (EA type E) closure and repair via a cervical incision were excluded. The patients who underwent chest contrast-enhanced computed tomography (CECT) after repair were enrolled. Patients that were less than 6 months old and underwent chest CECT for asymptomatic pulmonary disease during the same period were selected as controls (control group). We intended to perform CECTs on patients without providing respiratory support, such as non-invasive positive pressure ventilation (NIPPV).
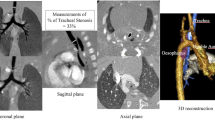
According to the CECT findings, a numerical reference that could be easily evaluated was defined as follows: the lateral and anterior–posterior diameters of the trachea on the slice where the BCA crosses the trachea were measured, and their ratio was calculated (lateral/anterior–posterior ratio; LAR) (Fig. 1). If there were multiple images in different phases, the image that appeared closer at exhalation was selected.
The definition of LAR. The lateral (L) and anterior–posterior diameter (A) of the trachea in the computed tomography slice where the brachiocephalic artery crosses the trachea. The ratio of the lateral diameter and the anterior–posterior diameter was defined as LAR. BCA brachiocephalic artery, CCA common carotid artery, LAR lateral and anterior–posterior diameter of the trachea, SCA subclavian artery, SVC supra vena cava
The mean and standard deviation (SD) were calculated according to the LAR of the control group. The Z-scores of the LARs were calculated for each chest CECT. The Z-score was calculated using the formula ([each LAR] – [mean LAR of control group])/[SD of LAR of control group], i.e., a LAR Z score of 2.0 of one case implied that the LAR of the case was 2.0 SD away from the mean LAR of normal controls. The Z-scores were compared between the EA patients who underwent aortopexy and the EA patients who were discharged without requiring surgical treatment. The factors associated with the tracheostomy were so variable that the patients who underwent a tracheostomy were only displayed the LAR of some patients.
This study was performed under the approval of the Ethics Committee of our institution (Ref No. 2019–0421). The authors have no conflicts of interest to declare.
Results
In total, 42 patients underwent EA repair during the review period and all of them were repaired thoracoscopically, of which 25 patients underwent chest CECT. All patients except for those with multiple congenital malformations were able to safely undergo the CECT without respiratory support.
Among these 25 patients, 12 patients were discharged from the hospital without surgical intervention. The type of EA was C in 11 patients and D in 1. Four patients required continuous positive airway pressure at discharge, whereas eight patients were discharged without such respiratory support. Five patients underwent aortopexy after EA repair under diagnosis of TM. All of them had EA of type C.
The remaining eight patients underwent tracheostomy for the management of various conditions; three patients had multiple congenital malformations, one had recurrent nerve palsy, three had TM with obvious bronchomalacia, and one had TM alone. The patients with multiple congenital malformations and recurrent nerve palsy were excluded from the review because they had difficulty in breathing regardless of their tracheal condition.
As for the control group, 51 patients underwent chest CECT for asymptomatic pulmonary disease in the neonatal period during the review period.
The CECT of each patient was reviewed. The BCA branched from the aortic arch on the left side of the trachea in every patient in both the control and EA group. The mean LAR of the control group was 1.15 ± 0.13 (Fig. 2). According to this result, the LAR and Z-score of each patient in the control group was not more than 1.5 and 2.5, respectively.
The mean LAR and Z-score of the 12 patients who were discharged without surgical intervention were 1.53 and 2.90, respectively (Table 1). Except one case whose LAR was 2.71, their LAR values were not > 2. Conversely, the mean LAR and Z-scores of the five patients who underwent CECT just before the aortopexy were 3.93 and 21.2, respectively. Their LAR values were not < 2.8 (Table 1). Their trachea appeared to be compressed by the BCA even in the three-dimensional CT images (Fig. 3).
Three-dimensional computed tomography reconstruction of the same case as in Fig. 1. a The anterior–posterior view. The brachiocephalic artery branched from the left side of the trachea (white arrowhead) and passed through the anterior of the trachea. b The left anterior oblique view. Due to the compression by the brachiocephalic artery, the trachea was flattened (white arrow). However, the trachea in the other areas is thick enough
The LARs of the patients who underwent tracheostomy were between 1.76 and 2.78 in the three patients who had TM with obvious bronchomalacia, and 1.54 in the patient who had TM alone.
Five patients underwent aortopexy at 81 days (in median) after EA repair (range 45–869 days). Post-operative CECT was performed in all cases. Four patients exhibited improved LARs (to < 1.5) and alleviation of their respiratory disorder. However, one patient without an improved LAR was unable to achieve improvements in their respiratory disorder after aortopexy. This patient underwent redo-aortopexy. The subsequent CECT showed an improved LAR of < 1.5 and simultaneous improvement of the respiratory disorder. The LARs and Z-scores of those who underwent aortopexy are shown in Fig. 4. Their respiratory disorders were improved to the point that they could be discharged from the hospital. Two patients were discharged without respiratory support and three were discharged with NIPPV. The support was able to be withdrawn within 3–6 months after aortopexy.
The ratio of the lateral and anterior–posterior diameter of the trachea (LAR) and Z-score before and after surgery for each patient requiring aortopexy. a The LARs of four patients decreased to < 1.5 after the first aortopexy. The LAR of one patient without withdrawal of respiratory support was not decreased after the first aortopexy. This patient underwent redo aortopexy, and their LAR decreased to < 1.5 after the second surgery. b The Z-scores of four patients who were discharged without surgical intervention decreased to < 3.6. The Z-score of the patient without withdrawal of respiratory support was not decreased by the first aortopexy. This patient underwent redo aortopexy, and their Z-score decreased to < 3.6 after the second surgery
Discussion
TM occurs frequently in EA patients and is thus often suspected. The incidence rate of TM reported in previous studies is relatively high, ranging from 15 [10] to 79% [11]. TM should be suspected in the presence of the following symptoms or clinical history: noisy respiration, tracheal rhonchi, harsh barking cough, or apneic spells due to expiratory obstruction [5]. In suspected cases of TM, diagnostic examinations should be conducted quickly and include pulmonary function testing, fluoroscopy, CT, and fiberoptic bronchoscopy (FB). The gold standards for diagnosing TM are CT and FB.
Children with minor degrees of TM can be treated non-surgically. The abnormally soft trachea tends to become more rigid with growth. TM is reported to be relieved by about 2 years of age in mild cases [7]. An effective approach consists of securing the lumen with continuous positive airway pressure and to maintain breathing until symptoms improve [5].
For long-term respiratory support with continuous positive airway pressure, tracheostomy is usually performed. However, tracheostomy is not a completely safe procedure. Reportedly, 15–19% of children experience a tracheostomy-related complication. Adverse events following tracheostomy placement in children range from mild to life-threatening [12].
Therefore, for severe TM, surgical intervention without tracheostomy is recommended to improve or alleviate symptoms and sequelae of TM. The surgical indication should also be decided based on the symptoms, such as dying spell or recurrent pneumonia. In our hospital, aortopexy had been performed as the prior intervention than tracheostomy in recent years. Our indication of that was the occurrence of apparent life-threatening event (ALTE) despite proper NIPPV.
Aortopexy has been the mainstay of surgical therapy for pediatric intrathoracic TM [5]. According to the review on aortopexy by Torre et al., > 80% of patients show satisfactory symptom improvement. Redo aortopexy was reported in < 1% of cases, and mortality was approximately 6%. The morbidities were: pneumothorax or pleural effusion (3%), lung atelectasis (2.5%), pericardial effusion (2%), phrenic nerve palsy (1.3%), and bleeding (1%) [13]. Aortopexy is considered a relatively safe surgery and is greatly effective for TM patients with EA.
Some cases of TM after EA repair are caused by compression of the trachea by the BCA. We hypothesized that these cases would have a large LAR and that the patients with a large LAR would show good aortopexy effectiveness. We believe that the anterior-posterior diameter of the trachea alone was inappropriate to gauge the index of compression by the BCA because the diameter of the trachea varies due to the differences in the body weight of patients when CT was examined. Therefore, we adopted LAR as an index of the degree of tracheal compression and the effectiveness of aortopexy.
We reviewed the CECT scans of controls and found that their BCA branched from the aortic arch left of the trachea and crossed the anterior of the trachea in every case. However, none of the controls suffered from respiratory troubles. These LARs were all < 1.5. We believe that this was because each of the cases in the control group had a sufficiently rigid trachea that did not deform due to compression by the BCA.
According to our results, the LARs in patients requiring surgical intervention for respiratory disorder were much greater than those in controls. Their mean LAR and Z-score was 3.93 and 21.2, respectively. This Z-score meant the outlier that 21.2 SD larger than the mean of LAR of normal control. Conversely, the LAR values of patients who underwent EA repair and were discharged without surgical intervention were not > 2, except for one case.
Among the four cases who underwent tracheostomy for tracheomalacia, three cases had obvious bronchomalacia. LAR is considered as an indicator of compression of the trachea by BCA. Therefore, LAR does not reflect their severity of TM when the malacia exists at other position of air way. We believed that aortopexy alone could not improve their symptom if bronchomalacia existed with the patients of TM, regardless of the value of LAR. Therefore, tracheostomy was undergone. On the other hands, the residual one patient could withdraw the tracheostomy after about 1 year of respiratory support. Considering from a retrospective perspective, the patient may have been able to successfully withdraw respiratory support only with NIPPV. However, it was considered the limit of the research method.
The LAR accurately represents BCA compression of the trachea. In some cases, the soft tracheal cartilage combined with the BCA crossing the trachea was considered to cause tracheal compression and respiratory distress. Therefore, the efficacy of aortopexy for these cases is obvious considering that the mean LAR improved to < 1.5 when the respiratory disorder resolved after aortopexy.
In summary, the LARs of the normal neonates were < 1.5, those of the patients with EA who were discharged without surgical intervention were < 2.8, and those of the patients who underwent aortopexy were all > 2.8. After successful aortopexy, the LAR decreased to < 1.5.
Nevertheless, aortopexy is not always effective for TM with EA. Such cases seem to have TM throughout the entire trachea, and this type of TM did not improve by releasing the compression of the BCA. We believe that further studies will elucidate this distinction.
In conclusion, the compression of the trachea by the BCA seems to be one of the major causes of TM after EA repair in some cases. The LAR was considered to reflect the degree of compression of the trachea by the BCA. According to our results, aortopexy is preferred if the trachea is compressed by the BCA, and our indicator, LAR, is useful in predicting the therapeutic effect of aortopexy.
References
Nassar N, Leoncini E, Amar E, Arteaga-Vazquez J, Bakker MK, Bower C, Canfield MA et al (2012) Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol 94:893–899
Quiroz HJ, Turpin A, Willobee BA, Ferrantella A, Parreco J, Lasko D, Perez EA, Sola JE, Thorson CM (2020) Nationwide analysis of mortality and hospital readmissions in esophageal atresia. J Pediatr Surg 55:824–829
Masuya R, Kaji T, Mukai M, Nakame K, Kawano T, Machigashira S, Yamada W, Yamada K, Onishi S, Yano K, Moriguchi T, Sugita K, Kawano M, Noguchi H, Suzuhigashi M, Muto M, Ieiri S (2018) Predictive factors affecting the prognosis and late complications of 73 consecutive cases of esophageal atresia at 2 centers. Pediatr Surg Int 34:1027–1033
Rayyan M, Embrechts M, Van Veer H, Aerts R, Hoffman I, Proesmans M, Allegaert K, Naulaers G, Rommel N (2019) Neonatal factors predictive for respiratory and gastro-intestinal morbidity after esophageal atresia repair. Pediatr Neonatol 60:261–269
Fraga JC, Jennings RW, Kim PC (2016) Pediatric tracheomalacia. Semin Pediatr Surg 25:156–164
Snijders D, Barbato A (2015) An update on diagnosis of tracheomalacia in children. Eur J Pediatr Surg 25:333–335
Hysinger EB, Panitch HB (2016) Paediatric tracheomalacia. Paediatr Respir Rev 17:9–15
Overman AE, Liu M, Kurachek SC, Shreve MR, Maynard RC, Mammel MC, Moore BM (2013) Tracheostomy for infants requiring prolonged mechanical ventilation: 10 years’ experience. Pediatrics 131:e1491-1496
Gross RE, Neuhauser EB (1948) Compression of the trachea by an anomalous innominate artery; an operation for its relief. Am J Dis Child 75:570–574
Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR 3rd (1995) Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Arch Surg 130:502–508 (discussion 508-509)
DeBoer EM, Prager JD, Ruiz AG, Jensen EL, Deterding RR, Friedlander JA, Soden J (2016) Multidisciplinary care of children with repaired esophageal atresia and tracheoesophageal fistula. Pediatr Pulmonol 51:576–581
Watters KF (2017) Tracheostomy in Infants and Children. Respir Care 62:799–825
Torre M, Carlucci M, Speggiorin S, Elliott MJ (2012) Aortopexy for the treatment of tracheomalacia in children: review of the literature. Ital J Pediatr 38:62
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sumida, W., Tainaka, T., Shirota, C. et al. An imaging study on tracheomalacia in infants with esophageal atresia: the degree of tracheal compression by the brachiocephalic artery is a good indicator for therapeutic intervention. Pediatr Surg Int 37, 1719–1724 (2021). https://doi.org/10.1007/s00383-021-04985-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-021-04985-0