Abstract
Purpose
Carcinoembryonic antigen (CEA) has limited value as a standalone predictor of the survival in patients with colorectal cancer (CRC). D-dimer (DD) is a predictor of the survival in patients with metastatic CRC. We aimed to predict the prognosis in patients undergoing curative resection for the treatment of CRC by integrating the evaluation of preoperative CEA and DD concentrations with the pathological classification for stage grouping (pStage).
Methods
The study enrolled 304 patients between 2007 and 2012. The Combination of DD and CEA Score (CDCS) awarded 1 point each for a CEA concentration of > 5.0 ng/ml and DD concentration of > 1.0 μg/ml. Patients were classified according to the total points: CDCS 2, increased DD and CEA concentrations; CDCS 1, increased concentration of either DD or CEA; CDCS 0, normal concentrations.
Results
The overall survival (OS) and relapse-free survival (RFS) were significantly lower in patients with CDCS 2 than in those with CDCS 1 or 0. The pStage and CDCS were not independent prognostic predictors of the OS but were predictors of the RFS. The C-index value of the combination of the pStage and CDCS was better than that of either alone for the OS and RFS.
Conclusion
The combination of the pStage and CDCS accurately predicts relapse in patients with CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is a frequently observed malignancy. Recently, there have been advancements in surgical techniques, perioperative management, and chemotherapy (e.g., molecular-targeted drugs) for patients with CRC; however, some CRC patients experience disease recurrence even after curative resection.
Colorectal cancer is the fourth-most common cause of death from cancer worldwide [1]. Thus, predicting the postoperative prognosis in CRC patients is critical for determining proper treatment strategies and facilitating personalized therapy. At present, the tumor–node–metastasis (TNM) staging system of the Union for International Cancer Control (UICC) is the best prognostic system for CRC patients [2]. However, there is accumulating evidence that a blood analysis can contribute to the prediction of the prognosis in CRC patients [3, 4]. Serum tumor marker levels can be easily measured and are useful for making a disease diagnosis, predicting the prognosis, and detecting recurrence following surgery in CRC patients [4].
The College of American Pathologists Consensus Conference 1999 suggested that the preoperative carcinoembryonic antigen (CEA) concentration can be a category I prognostic marker for CRC [5]. However, its utility cannot be fully exhibited using a single cut-off value (e.g., 5 ng/ml). Using a different cut-off value for each TNM stage based on the CEA concentration in the corresponding stages, the preoperative CEA concentration can be used as a prognostic factor in combination with the corresponding TNM stage [6].
D-dimer (DD), a degradation product of cross-linked fibrin, is a sensitive marker of fibrinolysis [7]. A previous study showed that DD is a strong predictor of the survival in patients with metastatic CRC [8].
The present study investigated the prognostic value of the combination of preoperative DD and CEA concentrations in terms of the Combination of DD and CEA Score (CDCS) and examined the CDCS integrated with pStage as a predictive model.
Materials and methods
Patients
We performed a retrospective analysis of data from 1014 consecutive CRC patients who underwent surgery at the Hiroshima City Hiroshima Citizens Hospital (Hiroshima, Japan) from January 2007 to December 2012.
Patients with histologically confirmed stage I–III colorectal adenocarcinoma who had undergone curative resection from the cecum to the rectosigmoid were included. Patients whose laboratory examination data (e.g., preoperative serum DD and CEA concentrations) were not available were excluded from the study. Furthermore, patients with multiple synchronous primary CRCs and those with a location of the upper or lower rectum and proctos were also excluded.
The clinicopathological findings were assessed according to the 8th edition of the Japanese Classification of Colorectal Carcinoma [9]. Preoperative serum CEA and DD concentrations were measured within 1 month before surgery.
Ethics statement
The ethics committee at the Hiroshima City Hiroshima Citizens Hospital approved the study in accordance with the Declaration of Helsinki 1996 (approval number: 2020-22).
Methods
CDCS
CDCS incorporates the CEA and DD concentrations. The cut-off values were set at a CEA concentration > 5.0 ng/ml and DD concentration > 1.0 μg/ml. These were used as per our hospital’s standard cut-off values for DD (≤ 1.0 μg/ml) and CEA (≤ 5.0 ng/ml) concentrations. All patients’ DD and CEA concentrations were evaluated, and one point was allocated for each of these two, depending on the value. The points were summed, and the patients were then divided into three groups according to their CDCS (0, 1, or 2).
The CEA concentration was measured via electrochemiluminescence immunoassays using ECLusys® CEA II (Roche Diagnostics, Tokyo, Japan). The DD concentration was measured via a luminescence immunoassay using Reaswort® D-dimer Neo (Sysmex, Kobe, Japan).
Analyzed parameters
We analyzed the overall survival (OS) and relapse-free survival (RFS) after colectomy in patients categorized according to their CDCS. We investigated prognostic factors according to the OS and RFS rates using the following variables: age (< 70 vs. ≥ 70 years old), gender (male vs. female), DD concentration (> 1.0 vs. ≤ 1.0 μg/ml), CEA concentration (> 5.0 vs. ≤ 5.0 ng/ml), tumor location, invasion depth, lymph node involvement (pN), and surgical approach (laparoscopic vs. open surgery). Adjuvant chemotherapy included capecitabine, S-1, UFT/LV, FOLFOX, or XELOX and was administered for 6 months.
Statistical analyses
All data are expressed as medians with minimum and maximum values in parentheses. The Kruskal–Wallis test and χ2 test were used to compare groups and proportions between groups, respectively. Survival curves were estimated using the Kaplan–Meier method, and analyses were performed using the log-rank test. Univariate Cox proportional hazards models of all potential baseline predictors were built to compute hazard ratios and 95% confidence intervals.
Continuous variables were nonparametrically analyzed using the Kruskal–Wallis test, and categorical variables were analyzed using the χ2 test or Fisher’s exact test as appropriate. Variables with a p value of < 0.10 in the univariate analysis were considered candidates for the multivariate analysis using the Cox proportional hazards model. The cumulative OS and RFS were calculated using the Kaplan–Meier method, and differences between curves were evaluated using the log-rank test. A p value of < 0.05 was considered significant. C-indices were calculated using the area under the receiver-operating characteristic curve, which was obtained from a logistic regression analysis.
All statistical data were generated using the JMP 13 (SAS Institute, Cary, NC, USA) and Prism 6 (GraphPad Software, La Jolla, CA, USA) software programs.
Results
Patient characteristics
A total of 1,014 consecutive patients were evaluated for enrollment. Of them, 346 patients did not meet the inclusion criteria due to a lack of preoperative CEA and DD concentration measurements; furthermore, 346 patients were subsequently excluded for the reasons shown in Fig. 1. Thus, a total of 304 patients were enrolled. The demographic and clinical data of study patients are shown in Table 1. The median patient age was 70 (range 36–93) years old.
The median follow-up periods for the RFS and OS were 54.1 (range 0.7–99.1) months and 56.0 (range 0.7–99.1) months, respectively. During follow-up, 43 patients (14.1%) developed recurrence, and 27 patients (8.9%) died. Adjuvant chemotherapy was administered to 58 patients (19.1%).
Preoperative lower limb venous echo was performed in 88 patients with preoperative risk factors, such as severe obesity, varicose veins, lower limb paralysis, high DD concentration, and a history of estrogen treatment. Four patients were diagnosed with deep vein thrombosis, for which treatment was performed before surgery (Table 2).
A total of 114 patients (37.5%) exhibited increased DD concentrations (> 1.0 μg/ml), and 101 patients (33.2%) exhibited increased CEA concentrations (> 5.0 ng/ml). Of the 114 patients with increased DD concentrations, 52 (45.6%) also exhibited increased CEA concentrations.
Baseline characteristics of patients categorized according to the CDCS
Patients were divided into the following three groups according to their CDCS: (1) CDCS 2, increased DD (> 1.0 μg/ml) and CEA (> 5.0 ng/ml) concentrations; (2) CDCS 1, increased concentration of either DD or CEA; (3) CDCS 0, normal DD and CEA concentrations.
Significant differences were observed in the age, invasion depth, pN, pStage, and surgical approach among the three groups (Tables 2, 3).
Recurrence pattern in patients classified according to the CDCS
Recurrence occurred in 13 patients (9.2%) with CDCS 0, 12 (10.8%) with CDCS 1, and 18 (34.6%) with CDCS 2. No differences were observed in the primary recurrence site or route among the groups (Table 3).
The OS and RFS after curative resection
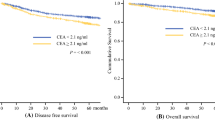
The Kaplan–Meier analysis showed that patients with CDCS 2 had the shortest OS among the 3 groups (5-year OS for patients with a CDCS of 0 = 94%, CDCS of 1 = 89%, and CDCS of 2 = 79%; log-rank p = 0.03). Similarly, the RFS in patients with CDCS 2 was shortest among the three groups (log-rank p = 0.0001).
OS and RFS curves for each group are shown in Fig. 2. The 5-year OS and RFS rates were significantly different among the three groups.
Risk factors for poor outcomes in patients undergoing colonic curative resection
A univariate analysis showed that the age, DD concentration, pT, pN, and CDCS were significantly associated with the OS (Tables 4, 5). The multivariate analysis showed that the age and pN were significant independent predictors of the OS (Tables 4, 5).
The univariate analysis revealed significant differences in outcomes among the three groups in terms of the age, CEA and DD concentrations, surgical approach, pT, pN, pStage, and CDCS for relapse after curative resection (Tables 6, 7). The multivariate analysis identified the following significant independent risk factors for relapse after curative colonic resection for CRC: age (≥ 70 years old), pStage, and CDCS (Tables 6, 7).
The C-index values of the prognostic score in this study are summarized in Table 8. The C-index value of the combination of pStage and CDCS was better than that of pStage or CDCS alone for the OS and RFS. Thus, the combination of pStage and CDCS was a better prognostic predictor than pStage or CDCS alone, especially in terms of the RFS, in CRC patients.
Discussion
The results of our retrospective study indicate that the CDCS, a combined evaluation of preoperative CEA and DD concentrations, is a useful prognostic predictor in CRC patients. This novel prognostic indicator may help clinicians decide on the best treatment strategy after curative resection in CRC patients.
Recent advances in chemotherapy have improved outcomes for patients with unresectable advanced CRC or recurrent CRC [10]; furthermore, the early detection of recurrence improves the survival after curative colectomy [11, 12]. Accordingly, it is essential to identify factors that can help correctly predict the prognosis of CRC patients. The CDCS can identify patients at a high risk of recurrence who require intensive follow-up and adjuvant chemotherapy even after curative surgery.
At present, the TNM staging system from UICC is the best survival predictor for CRC patients [2]; however, the prognosis differs among patients classified under the same TNM stage [13]. Therefore, prognostic factors supplementary to the TNM staging system should be identified.
Many predictive models have been reported to help improve the classification of CRC [14,15,16]. Several studies have shown that the preoperative CEA concentration is an independent prognostic factor for the OS [17] and RFS [18, 19] in CRC patients. However, a single CEA cut-off concentration of 5 ng/ml is not an appropriate prognostic marker for all CRC patients. In most studies, a fixed cut-off value, not a reference value, is used to predict outcomes across all stages of CRC [6]. The distribution of the CEA concentration is different at each TNM stage, which is why discrete cut-off values are necessary at different stages of the disease [6].
A recent study showed that the DD concentration was increased in CRC patients who developed distant metastasis after curative resection [20]. Another report found that the DD concentration was a predictor of the OS in patients with metastatic CRC [8]. The ability of the combination of DD and CEA concentrations to detect subgroups of patients with a poor survival may be due to coexisting micro-metastatic systemic disease not preoperatively diagnosed via cancer staging. DD concentrations may also be a nonspecific marker of poor general health. Indeed, this possibility has been supported by several studies that have shown DD concentrations to be associated with a poor survival among patients with various nonmalignant diseases, including pneumonia [21], pancreatitis [22], and cardiovascular disease [23].
Interestingly, we also revealed the ability of the CDCS to detect a subgroup of patients with a favorable 5-year mortality of 6%. This result may also be explained by the high accuracy of the CDCS for ruling out the presence of systemic micro-metastases.
Previous studies have shown that disorders of the coagulation system, such as thrombocytosis [24, 25], hyperfibrinogenemia [26, 27], and increased DD concentrations [28], are associated with a worse prognosis in CRC patients, independent of TNM staging. Although the detailed mechanism underlying the involvement of various pro-coagulant factors in carcinogenesis remains unknown, increasing evidence suggests that two key coagulation factors—platelets [24, 25] and tissue factor (TF) [29,30,31]—play an important role in the malignancy-associated hypercoagulable state. TF, also known as clotting factor III, is the primary initiator of the extrinsic coagulation cascade [32]. TFs are expressed in 70% of tumor cells and 53% of tumor vascular endothelial cells in CRC patients [29]. A previous report showed that the TF expression was associated with the prognosis and could be considered a prognostic predictor in CRC patients [29]. Because DD is located downstream of TF in the extrinsic coagulation cascade [32], patients with a high TF expression might exhibit increased DD concentrations.
When examining the C-index, the combination pStage and CDCS enables a more accurate prediction of relapse in CRC patients than pStage or CDCS alone. Patients with a poor prognosis can thus be selected from those of the same stage using CDCS.
Several limitations associated with the present study warrant mention. First, this study adopted a retrospective, single-center design. Second, the sample size was small. Thus, further studies are needed to prospectively confirm the prognostic usefulness of the combination of CEA and DD concentrations in CRC patients.
In conclusion, combined CEA and DD concentrations were shown to be useful for predicting the prognosis of CRC patients. Integration of the pStage and CDCS provides important information for deciding on the best treatment strategy for CRC patients in a routine clinical setting.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell; 2009. p. 100–9.
Yamashita K, Watanabe M. Clinical significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009;100:195–9.
Stiksma J, Grootendorst DC, van der Linden PWG. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13:239–44.
Compton C. American joint committee on cancer prognostic factors consensus conference. Cancer. 1999;88:1739–57.
Jeon BG, Shin R, Chung JK, Jung IM, Heo SC. Individualized cutoff value of the preoperative carcinoembryonic antigen level is necessary for optimal use as a prognostic marker. Ann Coloproctol. 2013;29:106–14.
Hong T, Shen D, Chen X, Wu X, Hua D. Preoperative plasma fibrinogen, but not D-dimer might represent a prognostic factor in non-metastatic colorectal cancer: a prospective cohort study. Cancer biomarkers. 2017;19:103–11.
Blackwell K, Hurwitz H, Lieberman G, Novotny W, Snyder S, Dewhirst M, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101:77–82.
Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal carcnioma. Tokyo: Kanehara; 2013.
Jawed I, Wilkerson J, Prasad V, Duffy AG, Fojo T. Colorectal cancer survival gains and novel treatment regimens: a systematic review and analysis. JAMA Oncol. 2015;1:787–95.
Fora A, Patta A, Attwood K, Wilding G, Fakih M. Intensive radiographic and biomarker surveillance in stage II and III colorectal cancer. Oncology. 2012;82:41–7.
Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, Lopez-Calvino B, Seoane-Pillado T, Pertega-Diaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644–56.
Puppa G, Sonzogni A, Colombari R, Pelosi G. TNM staging system of colorectal carcinoma. Arch Pathol Lab Med. 2010;134:837–52.
Fujino S, Myoshi N, Saso K, Sasaki M, Ishikawa S, Takahashi Y, et al. The inflammation-nutrition score supports the prognostic prediction of the TNM stage for colorectal cancer patients after curative resection. Surg Today. 2020;50:163–70.
McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–6.
Sasaki M, Miyoshi N, Fujino S, Ishikawa S, Saso K, Takahashi H, et al. Development of novel prognostic prediction models including the prognostic nutritional index for patients with colorectal cancer after curative resection. J Anus Rectum Colon. 2019;3:106–15.
Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010;101:396–400.
Takagawa R, Fujii S, Ohta M, Nagano Y, Kunisaki C, Yamagishi S, et al. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann Surg Oncol. 2008;15:3433–9.
Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol. 2009;16:3087–93.
Guo Y, Chen F, Cui W. Usefulness of plasma D-dimer level for monitoring development of distant organ metastasis in colorectal cancer patients after curative resection. Cancer Manag Res. 2018;10:4203–16.
Salluh JIF, Rabello L, Rosolem MM, Soares M, Bozza FA, Verdeal JCR, et al. The impact of coagulation parameters on the outcomes of patients with severe community-acquired pneumonia requiring intensive care unit admission. J Crit Care. 2011;26:496–501.
Maeda K, Hirota M, Ichihara A, Ohmuraya M, Hashimoto D, Sugita H, et al. Applicability of disseminated intravascular coagulation parameters in the assessment of the severity of acute pancreatitis. Pancreas. 2006;32:87–92.
Morange PE, Bickel C, Nicaud V, Schnabel R, Rupprecht HJ, Peetz D, et al. Haemostatic factors and the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene study. Arterioscler Thromb Vasc Biol. 2006;26:2793–9.
Kandemir EG, Mayadagli A, Karagoz B, Bilgi O, Turken O, Yaylaci M. Prognostic significance of thrombocytosis in node-negative colon cancer. J Int Med Res. 2005;33:228–35.
Sasaki K, Kawai K, Tsuno NH, Sunami H, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36:192–200.
Yamashita H, Kitayama J, Taguri M, Nagawa H. Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. Is on the survival of patients with primary colorectal cancer. World J Surg. 2009;33:1298–305.
Tang L, Liu K, Wang J, Wang C, Zhao P, Liu J. High preoperative plasma fibrinogen levels are associated with distant metastases and impaired prognosis after curative resection in patients with colorectal cancer. J Surg Oncol. 2010;102:428–32.
Stender MT, Larsen TB, Sorensen HT, Thorlacius-Ussing O. Preoperative plasma D-dimer predicts 1-year survival in colorectal cancer patients with absence of venous thromboembolism (VTE): a prospective clinical cohort study. J Thromb Haemost. 2012;10:2027–31.
Benqiang R, Yuanhong G, Jun H, Xiaoyan G, Xinhui F, Meijin H, et al. Mutations of p53 and K-ras correlate TF expression in human colorectal carcinomas: TF downregulation as a marker of poor prognosis. Int J Colorectal Dis. 2011;26:593–601.
Seto S, Onodera H, Kaido T, Yoshikawa A, Ishigami S, Arii S, et al. Tissue factor expression in human colorectal carcinoma. Cancer. 2000;88:295–301.
Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–41.
Kawai K, Watanabe T. Colorectal cancer and hypercoagulability. Surg Today. 2014;44:797–803.
Acknowledgements
The authors would like to thank Minoru Hattori for statistical advice and Enago (https://www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ojima, Y., Harano, M., Sumitani, D. et al. Prognostic value of preoperative carcinoembryonic antigen and D-dimer concentrations in patients undergoing curative resection for colorectal cancer. Surg Today 51, 1108–1117 (2021). https://doi.org/10.1007/s00595-020-02187-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02187-0