Abstract
Type 2 diabetes mellitus (T2DM) is a metabolic disorder with intricate pathogenic mechanisms. Despite the availability of various oral medications for controlling the condition, reports of poor glycemic control in type 2 diabetes persist, possibly involving unknown pathogenic mechanisms. In recent years, the gut microbiota have emerged as a highly promising target for T2DM treatment, with the metabolites produced by gut microbiota serving as crucial intermediaries connecting gut microbiota and strongly related to T2DM. Increasingly, traditional Chinese medicine is being considered to target the gut microbiota for T2DM treatment, and many of them are edible. In studies conducted on animal models, edible traditional Chinese medicine have been shown to primarily alter three significant gut microbiotal metabolites: short-chain fatty acids, bile acids, and branched-chain amino acids. These metabolites play crucial roles in alleviating T2DM by improving glucose metabolism and reducing inflammation. This review primarily summarizes twelve edible traditional Chinese medicines that improve T2DM by modulating the aforementioned three gut microbiotal metabolites, along with potential underlying molecular mechanisms, and also incorporation of edible traditional Chinese medicines into the diets of T2DM patients and combined use with probiotics for treating T2DM are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the latest estimates from the International Diabetes Federation (IDF), China ranks first in the world in terms of the number of people with diabetes, with 140.9 million cases in 2021, surpassing all other countries or regions. Furthermore, it is predicted that China will continue to hold this position as the country with the most diabetes patients in the world until 2045 with 174.4 million cases [1]. Among the various types of diabetes mellitus, type 2 diabetes (T2DM) stands out as a chronic metabolic disease caused by insulin resistance. These specific condition accounts for approximately 90% of all DM cases [2]. Research has shown that T2DM significantly increases the risk of conditions such as coronary heart disease, myocardial disorders, hypertension, and hyperlipidemia [3]. Furthermore, as the condition progresses, it can lead to chronic damage in various organ tissues, presenting a significant threat to the human body. This not only affects the physical well-being of patients but also places considerable economic and psychological strains on them [4]. Even with the presence of oral medications in clinical practice for treating T2DM, there are still numerous reports of suboptimal blood sugar control among these patients. This highlights the need for more diversified treatment strategies, methods, and auxiliary therapeutic measures for T2DM, especially for those who don't respond effectively to traditional therapies [5, 6].
The gut microbiota consists of a community of microorganisms that inhabit the human intestinal tract. This includes a vast array of hundreds of distinct bacterial species, with their combined population reaching up to 1014 organisms [7]. As the largest microbial system within the human body, extensive research has substantiated the association between gut microbiota and various human diseases, including neurodegenerative diseases, cardiovascular diseases, as well as mental health diseases [8].
In 2016, Zhernakova et al. definitively established the connection between gut microbiota and T2DM through metagenomic analysis [9]. In 2019, Sanna et al. demonstrated a causal relationship between the gut microbiota and T2DM through a mendelian randomization study [10]. In 2020, Reitmeier et al. found that disruptions in the rhythmicity of the gut microbiota are even considered to be predictive of the onset of T2DM. This provides compelling evidence for the potential of the gut microbiota to serve as a clinically relevant biomarker for predicting the development of T2DM [11]. The same year, a random clinical trial conducted by Perraudeau et al. involved supplementing T2DM patients with probiotics formulation of producing butyrate and consuming sugars. The results revealed that, without any adverse effects, this intervention moderately improved the blood glucose levels of the patients with T2DM [12]. More and more researches are highlighting the gut microbiota as a promising and potential therapeutic target for the management of T2DM [13]. The gut microbiotal metabolites, which mainly include short chain fatty acids (SCFAs), secondary bile acids (SBAs), branched chain amino acid (BCAAs) and others, serving as intermediaries connecting the gut microbiota with the host, play vital roles between the relationship between gut microbiota and T2DM [14].
In recent years, an increasing amount of research has demonstrated that oral medications, such as metformin, have led to changes in the gut microbiota [15, 16]. This alters the profile of gut microbiotal metabolites, reduces inflammation, promotes improvements in blood glucose levels and others [17]. These mechanisms may potentially become new therapeutic targets for the treatment of T2DM [14]. However, A randomized clinical trial suggests that, compared to metformin, traditional Chinese medicines demonstrate a greater advantage in treating T2DM through targeting the gut microbiota [16]. In addition to taking medications, diet also holds a significant role in the treatment of T2DM [18], since in traditional Chinese medicines, there is a category of substances that can be used both as medicines and as food ingredients. This category is referred to as edible traditional Chinese medicines [19,20,21], the most obvious advantage of this medication is that it can be incorporated into the patient's diet. Researches showed that edible traditional Chinese medicines can also exert a considerable therapeutic effect on T2DM by altering the gut microbiotal metabolites associated with the T2DM [22,23,24] (Fig. 1).
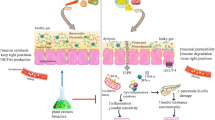
Delineates the holistic amelioration mechanism of type 2 diabetes mellitus (T2DM) through the reverse modulation of gut microbiota by edible traditional Chinese medicine (TCM). When TCM designated for consumption is integrated into the diet of T2DM patients, it regulates the intestinal microbial community, leading to an increased abundance of probiotic bacteria and a decreased abundance of pathogenic bacteria. Accompanying this microbiota modulation, there is an observed upsurge in the concentration of short-chain fatty acids, a decline in branched-chain amino acids, and an enhanced metabolism of bile acids. The fluctuations in these three metabolites correlate with a range of biological responses, including suppressed appetite, heightened satiety, reduced insulin resistance, increased glucose uptake in muscle cells, and alleviated inflammatory reactions. Consequently, this cascade of mechanistic changes collectively contributes to the improvement of T2DM. Drawn using the software Adobe Illustrator 2023
In this review, we delve into the impact of edible traditional Chinese medicines on gut microbiota and its metabolites in T2DM animal models, summarize the potential mechanisms by which these metabolite alterations might alleviate T2DM and discuss strategies to harness these edible medicines alongside other treatments for comprehensive T2DM management.
Gut microbiota and T2DM
Overall view of gut microbiota, obesity and T2DM
The gut microbiota changes in many diseases [8]. However, researches on the changes in the gut microbiota composition of T2DM are not exactly the same. Especially, some studies had found an increased abundance of bacteria in the Firmicutes phylum in individuals with T2DM, or observed a decreased abundance in the Bacteroidetes phylum [25,26,27,28,29], and other studies have reported the opposite conditions [30, 31], but the changes in the Firmicutes/Bacteroidetes ratio may be considered to be a potential biomarker for predicting T2DM [32]. Even before the emergence of T2DM, alterations in the gut microbiota may have already taken place, particularly among obese individuals [33]. Obesity is widely recognized as one of the primary risk factors for T2DM. Yet, it's not the obesity per se that triggers T2DM. Instead, it's the insulin resistance and inflammation induced by obesity that pave the way for T2DM. Intriguingly, the gut microbiota appears to have a role in this intricate process. Studies have indicated that the Firmicutes/Bacteroidetes ratio might undergo changes in obesity, mirroring similar shifts observed in T2DM [34]. Typically, obesity manifests before T2DM, emerging when the caloric intake surpasses the body's metabolic capabilities. The gut microbiota is actively involved in the metabolic activities of the intestines. As the body endeavors to mitigate this metabolic strain, the composition of the gut microbiota undergoes changes [35]. These collective alterations lead to shifts in their metabolic byproducts, encompassing variations in SCFAs, BCAAs, and disturbances in bile acid metabolism. Such changes in gut microbiota-derived metabolites heighten inflammation, influencing the body's glucose metabolism [34]. This can intensify insulin resistance, potentially culminating in the development of T2DM (Fig. S1).
Gut microbiotal metabolites changes in T2DM
As intermediaries between the gut microbiota and the host, the changes in the metabolites of the gut microbiota in T2DM are relatively consistent [36]. Since the improvement of T2DM through edible traditional Chinese medicine primarily involves three types of gut microbiotal metabolites—short-chain fatty acids, secondary bile acids, and branched-chain amino acids—this review will mainly focus on these three categories of gut microbiotal metabolites [22,23,24, 37,38,39,40,41,42,43,44,45].
It is suggested that SCFAs might be reduced in individuals with T2DM [46, 47]. These SCFAs, namely acetate, butyrate, and propionate, are primarily generated by the gut microbiota through the fermentation of indigestible dietary components [48]. They confer several advantages to the human body, encompassing the preservation of intestinal barrier integrity, mitigation of inflammation, prevention of cancer, and control of blood glucose levels [49], and the main gut microbiota responsible for synthesizing SCFAs include Akkermansia, Lactobacillus, Clostridium, Roseburia, and Eubacterium [50].
An increase in the levels of BCAAs in patients with T2DM has been confirmed [51, 52]. BCAAs, which encompass leucine, isoleucine, and valine, are essential amino acids that the human body cannot produce on its own. This necessitates their acquisition through dietary sources [53]. These amino acids play pivotal roles in influencing host metabolism, immunity, and various other physiological functions. The gut microbiota, such as Lactobacillus, Bacteroides, and Prevotella, are instrumental in the absorption of BCAA precursors and synthesis of BCAAs [54].
The relationship between bile acids and T2DM is multifaceted. Notably, within the context of T2DM, bile acid concentrations deviate from those observed in normoglycemic individuals [55], e.g., patients with T2DM may have elevated bile acid levels [56]. Primary bile acids originate from cholesterol and undergo conjugation with taurine or glycine in the liver, resulting in the formation of conjugated bile acids. Once formed, they are secreted into the small intestine. Here, the gut microbiota exerts its metabolic influence, leading to the deconjugation of these bile acids and their subsequent transformation into secondary bile acids. A portion of these secondary bile acids undergo reabsorption in the small intestine and are recycled back to the liver, while the remainder is excreted from the body in conjunction with other metabolites [57]. The production of secondary bile acids requires the involvement of gut microbiota such as Lactobacillus, Bifidobacterium, Clostridium, Enterococcus, Eubacterium, and Bacteroides. In T2DM, it has been observed that the abundance of these bacteria changes, potentially lead to an undesirable change in the generation of secondary bile acids [13].
Edible traditional Chinese medicines change microbiota metabolites in T2DM
During the Qin and Han dynasties, the Yellow Emperor's Inner Canon mentioned: Excessive adiposity engenders internal heat, while the consumption of sweet substances induces a sensation of abdominal fullness. Consequently, this surplus energy ascends and transforms into an affliction characterized by unquenchable thirst, which corresponds to the modern medical condition known as diabetes mellitus. In the Eastern Han period, a renowned physician named Zhang Zhongjing dedicated a discourse to diabetes in his work Synopsis of the Golden Chamber proposing the use of formulations such as the Ginseng and White Tiger Decoction for treatment. In the Tang Dynasty, Sun Simiao further advocated dietary therapy for diabetes. In contemporary clinical practice, dietary therapy remains relevant for treating T2DM [58].
Within the realm of traditional Chinese medicines, there exists a distinct category of them that can serve not only as traditional remedies but also as ingredients for food. These medicines are known as edible traditional Chinese medicines. This group of traditional Chinese medicines has gradually gained attention in the treatment of T2DM and has been found to correlate with improvements in the metabolic products of gut microbiotal metabolites.
By conducting searches on PubMed and incorporating the list of edible traditional Chinese medicines released by the National Health Commission of the People's Republic of China on November 10, 2021 (Table S1), a total of twelve types of edible traditional Chinese medicines were identified as possibly improving T2DM by changing gut microbiota and clear the alterations in the three mentioned microbial metabolites (Table 1).
Ganoderma lucidum
Ganoderma lucidum, known as "Ling Zhi" in Chinese, is regarded in traditional Chinese medicines as a tonic that strengthens the body and extends life. Research conducted on rats with T2DM has revealed that the intake of Ganoderma lucidum can lower serum glucose levels by inhibiting the expression of the PEPCK gene in the liver, demonstrating its potential as a treatment for T2DM [59]. Furthermore, Ganoderma lucidum contains a compound named FYGL (Fudan-Yueyang-G. lucidum), which is believed to lower fasting blood glucose levels and enhance insulin levels. This effect may be achieved by inhibiting the expression and activity of PTP1B in skeletal muscles [60]. Additionally, FYGL may improve insulin secretion by suppressing the activation of NF-κB, JNK, and p38 MAPK signaling pathways, as well as by reducing the intracellular accumulation of reactive oxygen species and nitric oxide [61].
Current research findings indicate that the key components affecting the gut microbiotal metabolites in T2DM are Ganoderma lucidum polysaccharides (GLP) [22]. In Chen et al.’s study, GLP was administered to T2DM rats, resulting in a significant reduction in the fasting blood glucose and insulin levels of these rats. Further analysis revealed that GLP decreased the abundance of gut microbiota, such as Dorea and Ruminococcus, increased the abundance of Blautia, Dehalobacterium, Parabacteroides, and Bacteroides. Butyric acid and valeric acid, two kinds of SCFAs also increased. They are believed to enhance the anti-inflammatory cytokines IL-10 express and suppress pro-inflammatory cytokines [22].
Astragalus membranaceus
Astragalus membranaceus, known as "Huangqi" in Chinese, belongs to the plant family Leguminosae [62]. In traditional Chinese medicines, it is believed to possess functions such as enhancing immune function, improving digestion, and alleviating fatigue and weakness. Astragalus membranaceus has been shown to have effects in lowering blood glucose levels and enhancing pancreatic sensitivity [63,64,65,66,67]. Astragalus membranaceus (AMP) polysaccharides are the main active components of Astragalus membranaceus. When utilized to intervene with diabetic mice, AMP was found to lead to a significant increase in the abundance of Akkermansia and Faecalibaculum. Further analysis revealed a notable rise in the levels of SCFAs, particularly butyric acid and acetic acid. It is believed that SCFAs, through the activation of GPCR 41/43 in L cells, stimulate the release of GLP-1, thereby enhancing insulin sensitivity. Simultaneously, they elevate the levels of Occludin and ZO-1, thus improving gut integrity and reducing inflammation [23].
Lycium barbarum L.
The Chinese name for L. barbarum L. (LBL) is "Gou Qi", and one of the most common uses for L. barbarum L. is to brew it as a tea, which is believed to have the effect of nourishing the liver and kidneys [68]. Polysaccharides from L. barbarum (LBPs) are believed to possess anti-diabetic properties [69]. When intervening in T2DM mice with LBPs, alterations in the gut microbiota abundance were observed. Specifically, there was an increase in the abundance of Bacteroidetes and Actinobacteria, while Firmicutes and Lachnospiraceae declined. Furthermore, there was a notable rise in the levels of butyrate among the SCFAs. Butyrate plays a pivotal role in promoting epithelial cell proliferation, augmenting the expression of the mucin 2 gene, and facilitating the assembly of tight junctions. This aids in preserving the integrity of the gut, subsequently reducing inflammation and the progression of insulin resistance [24].
Panax ginseng
Panax ginseng, the Chinese herb known as "Ren Shen", with its root being the main medicinal part, is believed to possess functions such as enhancing immunity, alleviating fatigue, and boosting mental well-being [70]. Studies have indicated that Ginseng can alleviate insulin resistance in mice and hinder the progression of diabetes, which might be related to the gut microbiota and its metabolites [71, 72]. A polysaccharide known as GPS-1, derived from Ginseng, was found to play a significant role in improving T2DM. GPS-1 led to alterations in the composition of gut microbiota in T2DM rats. Notably the abundance of Akkermansia, Lactobacillus, Bacteroides increased, while Proteobacteria, Shigella and Firmicutes/Bacteroidetes ratio decreased. Further analysis of gut microbiotal metabolites showed a notable increase in the levels of SCFAs including acetate, propionate, butyrate, and valerate in T2DM rats treated with GPS-1. This suggests that GPS-1 potentially raised the SCFAs levels in these rats. SCFAs, upon binding to GPR41/GPR43 receptors, trigger signaling responses that activate gut hormones secretion, such as GLP-1 and PYY. This, in turn, contributes to the improvement of glucose levels and insulin resistance [37].
Dendrobium officinale
Dendrobium officinale is an orchidaceous plant widely used in traditional Chinese medicines to nourish the lungs and stomach, among other applications [73]. The potential of D. officinale in improving T2DM has been confirmed, likely through the modulation of gut microbiota or microbiota metabolites [74, 75]. Dendrobium officinale polysaccharide (DOP) has the ability to reshape gut microbiota and repair intestinal barrier damage, possibly achieved by promoting the growth of beneficial bacteria and suppressing harmful ones like Helicobacter pylori [75]. A study revealed that DOP primarily consists of glucose and mannose, with mannose being the major component. Following the administration of DOP to prediabetic mice, significant alterations were observed in the gut microbiota composition. Abundances of Roseburia, Lactobacillus and Bifidobacterium increased, while the Firmicutes/Bacteroides (F/B) ratio and Colidextribacter decreased. The overall content of SCFAs, particularly acetic acid, increased in gut microbiotal metabolites. SCFAs are closely linked to the free fatty acid receptors FFAR2 and FFAR3. After DOP treatment, the expression of FFAR2 and FFAR3 significantly increased, possibly stimulated by the elevated SCFAs. FFAR2 and FFAR3 are intricately connected with gut hormones GLP-1 and PYY and they improved insulin resistance, enhanced glucose metabolism, and appetite control, SCFAs/FFAR2/FFAR3 pathway also connected with NF-κB, the pathway can inhibit NF-κB and improve inflammation [38].
Mulberry
Mulberry are common plants, also called Morus alba and their leaves are often used for silk production by silkworms. Additionally, these leaves are frequently infused to make tea, which is believed to help regulate blood glucose levels and promote metabolism. Research has demonstrated that mulberry leaf can serve as a complementary dietary component for individuals with T2DM [76, 77].
One bioactive compound found in mulberry leaves called 1-deoxynojirimycin (DNJ) has been shown to reduce levels of plasma lipopolysaccharides, IL-6, and TNF-α in T2DM rats. This reduction alleviates inflammation in the liver and colon tissues. Additionally, DNJ inhibits the expression of suppressor of SOCS3 and the activity of the TLR4/NF-κB signaling pathway. DNJ also enhances the expression of adiponectin and improves the phosphorylation ratio of IRS1 to Tyr896/IRS1. These effects suggest that DNJ may have a role in ameliorating T2DM and that these effects could be related to the modulation of gut microbiota and its metabolites [78].
Zheng et al. examined the relationship between the water extract of mulberry leaves (MLE) and gut microbiotal metabolites in T2DM rat models. Following MLE intervention, significant changes were observed in the abundance of gut microbiota such as Proteobacteria, Firmicutes, and Cyanobacteria. Furthermore, the levels of gut microbiotal metabolites BCAAs were notably reduced. This reduction could be attributed to the impact of MLE on the changes of enzymes such as BCKDH E1α, BCKDH E1β, DBT, and PPM1K. These enzyme alterations facilitate the degradation of BCAAs [45]. Elevated BCAAs are associated with insulin resistance, mitochondrial dysfunction, accumulation of metabolic toxins, and inflammation—several metabolic abnormalities that interfere with insulin signaling, cellular energy metabolism, and inflammatory responses, thus promoting the development of T2DM [51, 52]. The water extract of mulberry leaves reduces the levels of BCAAs, which may potentially aid in the improvement of T2DM.
Coix seed
Coix seed (CS), a type of grain commonly added to porridge, is believed to have hypolipidemic and anti-inflammatory properties, offering potential relief for diabetes [79]. Studies in mouse models have indicated that including CS in the diet partially counteracts the adverse metabolic effects of a high-fat diet. This effect might be associated with gut bacteria such as Akkermansia, Muciniphila and Lactobacillus agilis, suggesting that CS could function as a prebiotic to benefit individuals with metabolic disorders like obesity [80].
Intervention with Coix seed polysaccharides (CSP) led to increased levels of serum insulin and high-density lipoprotein cholesterol in mice. Conversely, total cholesterol, triglycerides, and low-density lipoprotein cholesterol levels decreased. These changes could be attributed to shifts in the gut microbiota composition, particularly an increase in Bacteroides, Lactobacillus, and Akkermansia, along with elevated SCFAs. The rise in SCFAs was linked to the activation of the IGF1/PI3K/AKT signaling pathway, associated with improved blood lipid and glucose levels [39, 81]. Essentially, the activation of the IGF1/PI3K/AKT pathway contributed to lower fasting blood glucose and total cholesterol levels, thereby ameliorating T2DM [39].
Gastrodia elata Blume
Gastrodia elata Blume (GEB), also known as "Tian Ma" in Chinese, belongs to the orchidaceae family. It is believed that GEB has effects in treating symptoms such as migraines, rheumatic pain, and limb numbness [82]. Research has shown that GEB also holds potential in the treatment of diabetes [83,84,85]. Gastrodin, the primary chemical constituent of GEB, has been identified as playing a key role. In type 2 diabetes, Gastrodin can activate the PI3K/AKT signaling pathway to promote the GATA1, enhancing its transcriptional activity. This, in turn, increases the expression of USP4, reducing the ubiquitination and degradation of insulin receptors, ultimately improving insulin resistance [83]. A study by Wang et al. discovered that Gastrodia elata Blume extract (GEBE) can increase the abundance of Faecalibaculum and Lactobacillus, leading to elevated concentrations of deoxycholic acid. The study also highlighted that deoxycholic acid can bind to FXR and TGR5 receptors, elevating the levels of GLP-1. Additionally, bile acids facilitate the expression of the GLUT-4 transporter in adipocytes and inhibit the TLR4-NF-κB signaling pathway, thereby mitigating inflammation [44].
Cornus officinalis
Cornus officinalis is a versatile plant valued for both its medicinal and nutritional properties, believed to nourish the liver and kidneys. Its extracts play a pivotal role in anti-inflammatory, antioxidant, and immunoregulatory activities [86]. Furthermore, Cornus officinalis may have a significant impact on the improvement of T2DM [87]. Radix Rehmanniae is exclusively used as a traditional Chinese medicine, when Radix Rehmanniae was combined with Cornus officinalis to treat KK-Ay mice, there was a notable increase in the abundance of Clostridiaceae in the gut microbiota, while the abundance of Catabacter, Marvinbryantia, and Helicobacter significantly decreased. Concurrently, the concentration of butyric acid saw a marked rise. This could be linked to the acid/GLP-1/GLP-1 Receptor Pathway. By activating this pathway, the glucose metabolism in mice improved, and the butyrate also led to a downregulation in the inflammatory markers IL-6 and TNF-α. However, the main active components of Cornus officinalis and Radix Rehmanniae were not mentioned [40].
Vigna radiata L.
Vigna radiata L., also known as the Mung bean, is believed to have properties that can improve glucose levels, combat hyperlipidemia, and hypertension, and even offer potential cancer prevention benefits [88]. It is frequently added to rice porridge as a part of daily diets [89]. The anti-diabetic effects of mung beans are increasingly being validated. In research conducted by Sating et al., they extracted active compounds from mung beans using boiling water and then applied this extract to insulin-resistant HepG2 cells, the results demonstrated a significant improvement in insulin sensitivity [90]. Another study by Charoensiddhi et al. found that mung bean extract intervention reduced inflammatory responses in insulin-resistant HepG2 cells. Furthermore, this mung bean extract also altered the composition of the gut microbiota, leading to an increase in the concentration of SCFAs in the feces of healthy individuals [91]. Hou et al. treated prediabetic mice with mung bean coat extract. This treatment resulted in significant changes in the mice's gut microbiota. Specifically, there was an increase in the abundance of Firmicutes, Roseburia, Bifidobacterium, and Romboutsia, while abundance of Lactobacillus, Rikenella, Odoribacter, and Proteobacteria decreased. Further analysis of SCFAs revealed elevated levels of acetic acid, propionic acid, and butyric acid in the feces, associated with an improvement in the mice's insulin resistance [41].
Laminaria japonica
Laminaria japonica, commonly referred to as "kelps" when used as a food ingredient, takes on the name "Kun Bu" in traditional Chinese medicines [92]. The soluble dietary fiber found in Laminaria japonica may offer therapeutic benefits for T2DM and has been shown to alter the gut microbiota composition in T2DM mice [93]. Additionally, a polysaccharide known as sulfated fucogalactan, present in Laminaria japonica, might enhance mitochondrial function and treat β-cell failure by activating the SIRT1-PGC1-α Signaling Pathway [94]. Another polysaccharide, Fucoidan, found in Laminaria japonica, was identified as the primary active ingredient against diabetes in a study by Zhang et al. In their research, both high and low doses of Laminaria japonica fucoidan (LJF) were administered to diabetic mice. The findings revealed that LJF increased the abundance of gut microbiota such as Lactobacillus and Allobaculum, while decreasing the levels of Bacteroides and Klebsiella. Furthermore, mice treated with LJF exhibited a significant rise in the levels of SCFAs, including acetic acid, propionic acid, iso-butyric acid, iso-valeric acid, and valeric acid. Notably, this increase was directly proportional to the dosage of LJF administered [42].
Siraitia grosvenorii
Siraitia grosvenorii, also known as monk fruit, is a fruit that serves both medicinal and dietary purposes and often used as a sweetener in various foods [95]. Siraitia grosvenorii boasts a wide range of medicinal benefits, believed to reduce inflammation and enhance immunity [96]. Mogrosides, a natural compound found in Siraitia grosvenorii, are thought to have potential therapeutic effects on T2DM [97]. Further research suggests that the anti-diabetic effects of mogrosides might be related to the AMPK pathway [98]. However, mogrosides might also exert their therapeutic effects on T2DM through the modulation of gut microbiota. In a study by Zhang et al., T2DM rats were treated with both high and low doses of Siraitia grosvenorii glycosides (SGgly). The results showed a significant increase in the abundance of Elusimicrobium and Acetitomaculum, while the levels of Proteobacteria, Escherichia-Shigella, and Desulfovibrio significantly decreased. Further analysis of the gut microbial metabolites revealed an increase in SCFAs, primarily acetic acid, butyric acid, propionic acid, and also glycohyocholic acid while decrease in the level of 1β-hydroxycholic acid, 12α-hydroxylated bile acids, 12α-hydroxylated bile acids. The study suggests that such changes might lead to a rise in GLP-1 levels, subsequently improving insulin resistance in diabetic rats [43].
In general, the majority of edible traditional Chinese medicines lead to elevated levels of SCFAs [22,23,24, 37,38,39,40,41,42,43]. Additionally, intervention with Gastrodia elata Blume has been linked to an increase in deoxycholic acid levels [44], Siraitia grosvenorii administration led to glycohyocholic acid increasing and 12α-hydroxylated bile acids, deoxycholic acid and 1β-hydroxycholic acid decreased. Also, Mulberry leaf extract has been associated with a reduction in levels of BCAAs [45]. These changes may all contribute to the improvement of T2DM.
Molecular mechanisms and biological effects
Anti-inflammation
Inflammation has been proven to have a strong correlation with insulin resistance in T2DM [99]. Although the anti-inflammatory mechanisms of these gut microbiotal metabolites may be diverse, it is interesting to note that the NF-κB signaling pathway is involved both SBAs and SCFAs.
NF-κB serves as a pivotal transcription factor, playing a critical role in the orchestration of inflammatory responses [100]. Under normal conditions, NF-κB forms a complex with the inhibitory protein IκB within the cytoplasm, rendering NF-κB inert. Upon initiation of an inflammatory response, IKK phosphorylates IκB, triggering its subsequent degradation. This liberation of NF-κB enables its migration into the cell nucleus, where it binds to specific DNA sequences, thereby igniting the transcription of target genes—among them, pro-inflammatory factors like TNFα and IL-6 [101].
SCFAs exhibit the capacity to quell NF-κB activity, with a particular potency observed in butyric acid, leading to the attenuation of inflammatory reactions [102]. The underlying mechanism is postulated as follows: FFA2 and FFA3, both types of G protein-coupled receptors (GPCR), possess an affinity for binding to SCFAs [103, 104]. Upon SCFA binding to the them, especially FFA2 also called GPR43, they facilitate the recruitment of β-arrestins-2 [105]. This recruitment process holds the potential to bolster the stability of IκBα, thereby curbing its degradation and subsequently repressing NF-κB activity [106].
TGR5 is a GPCR that serves as a binding receptor for bile acids [107]. Research has shown that when secondary bile acids bind to TGR5, there is an elevation in cAMP levels, which in turn activates protein kinase A, leading to the inhibition of NF-κB activity [108]. Xiao et al. suggest that the activation of TGR5 may also recruit β-arrestin2, similar to the mechanism with SCFAs, resulting in sustained inhibition of NF-κB activity [109]. Additionally, the FXR is a nuclear receptor primarily located in the liver. It plays a pivotal role in bile acid metabolism and stands as another receptor capable of binding with bile acids. Under conditions of ischemic-reperfusion injury, the activation of FXR suppresses the activity of TLR4, subsequently inhibiting the signaling of the NF-κB pathway [110]. However, the activation of FXR may serve as a biological signal promoting the NF-κB pathway as well [111] (Fig. 2).
Delineates the mechanism by which short-chain fatty acids (SCFAs) and secondary bile acids modulate inflammatory responses via the inhibition of the NF-κB pathway. SCFAs and secondary bile acids exert their actions by interacting with their respective G-protein-coupled receptors (GPCRs)—FFA2 for SCFAs and TGR5 for secondary bile acids. This interaction recruits β-arrestins-2, which subsequently suppresses the degradation of IkB by IKK, ensuring sustained inhibition of NF-κB activity. Concurrently, secondary bile acids can also directly activate protein kinase A (PKA) via the TGR5 receptor, resulting in further inhibition of NF-κB. Additionally, the farnesoid X receptor (FXR), a nuclear receptor that binds with secondary bile acids, can impede NF-KB activity through the suppression of TLR4. With the inhibition of NF-κB activity, there is a consequent reduction in the expression of pro-inflammatory cytokines such as IL-6 and TNF-α, thereby alleviating inflammation. Created with BioRender.com. Adapted from “NF-κB Signaling Pathway”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates (accessed on 24th Aug 2023)
In addition, SCFAs can also stimulate Occludin and ZO-1 by binding to receptors FFA2 and FFA3, as well as enhance tight junctions between intestinal epithelial cells, which improves the intestinal barrier. thereby preventing the initiation of inflammatory responses [112].
Improving glucose metabolism
In enteroendocrine L cells, receptors FFAR2/FFAR3 are present, and the gut hormones GLP-1 and PYY are stored within vesicles of these cells [113]. The binding of SCFAs to FFAR2/FFAR3 promotes the fusion of vesicles containing GLP-1 and PYY with the cell membrane, leading to the release of GLP-1 from the cell [114]. Similarly, the binding of secondary bile acids to the TGR5 receptor can produce the same effect [115]. Once released by the enteroendocrine L cells into the circulatory system, GLP-1 and PYY primarily target five organs: the liver, pancreatic islets, hypothalamus, skeletal muscle, and intestine. GLP-1 activates its receptor on pancreatic β-cells, enhancing insulin secretion. Additionally, GLP-1 acts on its liver receptor, reducing hepatic glucose production, and on its skeletal muscle receptor, increasing glucose uptake. Both GLP-1 and Y2 receptors are present in the hypothalamus, where they receive signals from GLP-1 and PYY, respectively, leading to reduced appetite and food intake. In the intestine, these receptors, when activated by GLP-1 and PYY, can slow down intestinal motility, enhancing the feeling of satiety [116].
Furthermore, an increase in bile acids, especially secondary bile acids, can influence GLUT-4, a glucose transporter primarily expressed in adipocytes and myocytes. Typically, GLUT-4 resides intracellularly when glucose uptake is not required. The binding of bile acids to FXR may induce GLUT-4 translocation, enhancing glucose uptake [117].
BCAAs, besides potentially inducing insulin resistance through impaired lipid metabolism, might also interfere with insulin signaling via the mTOR pathway [118]. mTOR is an atypical serine/threonine kinase, a member of the phosphoinositide 3-kinase-related kinase protein family. Two distinct complexes, mTORC1 and mTORC2, exist within cells [119]. Studies have shown that mTORC1 activity is modulated by BCAAs, especially leucine [120]. When BCAAs accumulate in cells, mTORC1 can be activated, which induces serine phosphorylation of IRS-1, inhibiting its activity, impairing insulin signaling, and inducing insulin resistance [121]. Additionally, BCAAs expression might also suppress Glut4 expression, potentially reducing the number of GLUT-4 transporters and glucose uptake in myocytes and adipocytes [122] (Fig. 3).
Illustrates the integrated mechanisms by which short-chain fatty acids (SCFAs), secondary bile acids, and branched-chain amino acids (BCAAs) modulate blood glucose metabolism. SCFAs bind to G-protein-coupled receptors FFAR2/FFAR3, while secondary bile acids connect with the GPCR TGR5, promoting the fusion of vesicles in intestinal L-cells and subsequent release of GLP-1 and PYY. These peptides enhance satiety, reduce appetite, decrease hepatic glucose synthesis, increase muscle cell glucose uptake, and augment insulin secretion. BCAAs enter cells via membrane channels, activating mTORC1, which inhibits the IRS-1 and reduces GLUT-4 expression. As GLUT-4 facilitates glucose uptake into fat cells, its modulation affects blood glucose. Additionally, the secondary bile acid receptor FXR promotes GLUT-4 translocation, suggesting a role for secondary bile acids in glucose regulation, drawn using the software Adobe Illustrator 2023
Edible traditional Chinese medicines combined with other therapeutic methods in T2DM
Diet
Diet plays a fundamental role in the treatment and prevention of diabetes and obesity [58]. Currently, several dietary approaches have been proposed for the improvement of T2DM, including the Mediterranean diet, low-carbohydrate diet, high-fiber diet, high-protein diet, vegan diet, and ketogenic diet [123]. These diets share common characteristics such as being low in carbohydrates and fat, and high in fiber and protein. In essence, these dietary strategies are aimed at transforming unhealthy eating habits of individuals with T2DM.
The integration of edible traditional Chinese medicines into these dietary practices not only promotes healthier eating habits for individuals with type 2 diabetes but also holds potential therapeutic benefits. By incorporating such medicinal herbs into these dietary regimens, there is an opportunity to not only improve the overall healthiness of the dietary habits in managing type 2 diabetes but also explore potential therapeutic effects.
Probiotics and probiotics
Probiotics refer to beneficial microbial communities that confer health benefits to the host. Administered orally, probiotics can improve or maintain the equilibrium of the gut microbiota [124]. Common probiotic products primarily contain microbiota such as Lactobacillus and Bifidobacterium [124]. Consumption of these products is associated with an increased abundance of these microbial communities within the intestinal tract, thereby modulating metabolic profiles and enhancing the metabolic products of the gut microbiota to potentially alleviate or prevent diseases like T2DM [125]. Prebiotics, on the other hand, are specialized forms of dietary fiber, such as oligofructose [126]. Typically, prebiotics are consumed synergistically with probiotics. Prebiotics serve as substrates, fueling the growth and metabolic activities of probiotics. This symbiotic consumption is believed to foster a more conducive environment for the ingested probiotics to thrive and proliferate within the gut, thereby amplifying the beneficial effects of probiotic supplementation [124].
Some edible traditional Chinese medicines are considered highly promising prebiotic. Studies in animal models have shown that edible traditional Chinese medicines significantly increase the abundance of probiotic bacteria such as Lactobacillus, Bifidobacterium, and Proteobacteria [37,38,39, 42]. This suggests that components in edible traditional Chinese medicines provide nutritional support for the growth and metabolism of these probiotics. The synergistic use of edible traditional Chinese medicines and probiotics could potentially greatly enhance the therapeutic effectiveness of probiotics in treating patients with T2DM.
Discussion
Edible traditional Chinese medicines show promising potential in mitigating T2DM by focusing on the gut microbiota. This objective can be realized through the modification of gut microbiota composition, resulting in elevated levels of SCFAs, reduced BCAAs, and improved SBAs metabolism. These alterations collectively contribute to the reduction of inflammation and the improvement of glucose metabolism, thus offering a comprehensive approach to tackling T2DM.
An intriguing phenomenon arises when focusing on the impact of interventions using edible traditional Chinese medicines on secondary bile acids. In the context of Siraitia grosvenorii intervention, inconsistent results have been observed. Following the administration of Siraitia grosvenorii, an increase in glycohyocholic acid was detected in the fecal samples of T2DM rats, while 12α-hydroxylated bile acids and 1β-hydroxycholic acid decreased. Zhang et al.'s study noted a positive correlation between 1β-hydroxycholic acid and insulin resistance, and a negative correlation between 12α-hydroxylated bile acid and insulin action [43]. Additionally, research by Petersen et al. suggested a negative association between glycohyocholic acid and insulin resistance [127]. Consequently, the elevation of glycohyocholic acid levels and the reduction of 12α-hydroxylated bile acids and 1β-hydroxycholic acid would seemingly be beneficial for T2DM. However, intervention using Gastrodia elata Blume in mice resulted in an increase in deoxycholic acid levels, while intervention using Siraitia grosvenorii led to a decrease in deoxycholic acid levels. Although both studies improved diabetes symptoms in the mice, they exhibited contradictory changes in deoxycholic acid levels, and this discrepancy might stem from the utilization of disparate sample types for analysis of serum and fecal samples, respectively [43, 44]. The elevation of deoxycholic acid due to Gastrodia elata Blume intervention is consistent with the conclusion drawn from the use of metformin in T2DM patients, as well as the inherent increase of deoxycholic acid in T2DM [44, 56]. Therefore, the elevation of deoxycholic acid in serum could potentially confer benefits for T2DM. As for the elevated deoxycholic acid concentration in T2DM individuals without any intervention, it might be attributed to a protective mechanism orchestrated by the body. Comprehensive research encompassing all relevant molecular mechanisms associated with deoxycholic acid in T2DM may be necessary to elucidate this matter. In conclusion, the diversity of secondary bile acid types corresponds to a duality in their effects. Given that this review focuses on the potential benefits of secondary bile acid modulation in improving T2DM and subsequently omits the potential drawbacks associated with bile acids.
Edible traditional Chinese medicines might become integral components of the daily diet for T2DM patients and may combined with other therapeutic methods in the future. Many edible traditional Chinese medicines, like Pueraria montana and Silybum marianum, have shown potential in improving T2DM through modulation of gut microbiota, but the specific changes in microbial metabolites remain unclear [128, 129]. Clinical trials have already demonstrated the efficacy like Astragalus membranaceus, Ginseng, and Mulberry in T2DM patients. However, more clinical trials are required to ascertain the effects of other edible traditional Chinese medicines. Additionally, it is worth noting that the source of raw materials or production method of these edible traditional Chinese medicine may lead to significant variations in the composition of active ingredients. Furthermore, the method of administration, or co-consumption with other foods, might also influence their effects. Inter-individual digestive differences and the complexity of certain foods could further increase the uncertainty in medical applications. To ensure the efficacy and safety of edible traditional Chinese medicines, we must consider these variables and make appropriate adjustments when applying them. Especially when transitioning from food applications to medicinal applications, we need to be extra cautious, ensuring that each ingredient is discussed from both a therapeutic and safety perspective, during the clinical trials, these issues should all be taken into consideration.
Current research on the effects of edible traditional Chinese medicines on T2DM mainly utilizes techniques such as 16S rDNA high-throughput sequencing and gas chromatography to identify changes in gut microbiota and their metabolites. However, only a limited number of studies have successfully proposed and validated specific molecular mechanisms. Achieving a comprehensive understanding of how edible traditional Chinese medicines impact T2DM through gut microbial metabolites necessitates a holistic approach that combines multi-omics research with in-depth molecular mechanistic studies. Future research efforts should aim to address this gap in knowledge and shed further light on this intricate relationship.
Abbreviations
- IDF:
-
International Diabetes Federation
- T2DM:
-
Type 2 diabetes mellitus
- SCFAs:
-
Short chain fatty acids
- SBAs:
-
Secondary bile acids
- BCAAs:
-
Branched chain amino acid
- GLP:
-
Ganoderma lucidum Polysaccharides
- FYGL:
-
Fudan-Yueyang-G. lucidum
- AMP:
-
Astragalus membranaceus Polysaccharides
- LBL:
-
Lycium barbarum L.
- LBPs:
-
L. barbarum Polysaccharides
- DOP:
-
Dendrobium officinale Polysaccharide
- DNJ:
-
1-Deoxynojirimycin
- MLE:
-
Mulberry Leaves extract
- CS:
-
Coix seed
- CSP:
-
Coix seed polysaccharides
- GEB:
-
Gastrodia elata Blume
- GEBE:
-
Gastrodia elata Blume Extract
- LJF:
-
Laminaria japonica fucoidan
- GPCR:
-
G protein-coupled receptors
References
Magliano DJ, Boyko EJ, committee IDFDAtes. IDF Diabetes Atlas. Idf diabetes atlas. Brussels: International Diabetes Federation© International Diabetes Federation, 2021 (2021).
Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ (2022) Type 2 diabetes. Lancet 400(10365):1803–1820
Boutari C, DeMarsilis A, Mantzoros CS (2023) Obesity and diabetes. Diabetes Res Clin Pract 202:110773
Li H, Wang L, Huang J, Li B, Qiu T (2022) The relationship between the knowledge of diabetes mellitus and the mental, psychological and emotional status of T2DM patients based on a structural equation model. Sci Rep 12(1):20714
Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC et al (2020) Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS). Diabetologia 63(4):711–721
Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ (2013) Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 36(11):3411–3417
Athanasopoulou K, Adamopoulos PG, Scorilas A (2003) Unveiling the human gastrointestinal tract microbiome: the past, present, and future of metagenomics. Biomedicines 511(3).
Chen Y, Zhou J, Wang L (2021) Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol 11:625913
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T et al (2016) Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352(6285):565–569
Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U et al (2019) Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet 51(4):600–605
Reitmeier S, Kiessling S, Clavel T, List M, Almeida EL, Ghosh TS et al (2020) Arrhythmic gut microbiome signatures predict risk of Type 2 diabetes. Cell Host Microbe 28(2):258–72.e6
Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, et al. (2020) Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care 8(1).
Liu L, Zhang J, Cheng Y, Zhu M, Xiao Z, Ruan G et al (2022) Gut microbiota: a new target for T2DM prevention and treatment. Front Endocrinol (Lausanne) 13:958218
Croci S, D'Apolito LI, Gasperi V, Catani MV, Savini I (2021) Dietary Strategies for Management of Metabolic Syndrome: Role of Gut microbiota metabolites. Nutrients 13(5).
Vallianou NG, Stratigou T, Tsagarakis S (2019) Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens) 18(2):141–144
Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L et al (2018) Structural Alteration of gut microbiota during the amelioration of human Type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio 9(3).
Ye J, Wu Z, Zhao Y, Zhang S, Liu W, Su Y (2022) Role of gut microbiota in the pathogenesis and treatment of diabetes mullites: advanced research-based review. Front Microbiol 13:1029890
Magkos F, Hjorth MF, Astrup A (2020) Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 16(10):545–555
Xue H, Wang W, Bian J, Gao Y, Hao Z, Tan J (2022) Recent advances in medicinal and edible homologous polysaccharides: extraction, purification, structure, modification, and biological activities. Int J Biol Macromol 222(Pt A):1110–1126
Li X, He Y, Zeng P, Liu Y, Zhang M, Hao C et al (2019) Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J Cell Mol Med 23(1):4–20
Zhang L (2019) Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv 26(1):860–869
Chen M, Xiao D, Liu W, Song Y, Zou B, Li L et al (2020) Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int J Biol Macromol 155:890–902
Song Q, Cheng SW, Li D, Cheng H, Lai YS, Han Q et al (2022) Gut microbiota mediated hypoglycemic effect of Astragalus membranaceus polysaccharides in db/db mice. Front Pharmacol 13:1043527
Zhou W, Yang T, Xu W, Huang Y, Ran L, Yan Y et al (2022)The polysaccharides from the fruits of Lycium barbarum L. confer anti-diabetic effect by regulating gut microbiota and intestinal barrier. Carbohydr Polym 291:119626.
Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C et al (2013) Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 8(8):e71108
Salamon D, Sroka-Oleksiak A, Kapusta P, Szopa M, Mrozińska S, Ludwig-Słomczyńska AH et al (2018) Characteristics of gut microbiota in adult patients with type 1 and type 2 diabetes based on next-generation sequencing of the 16S rRNA gene fragment. Pol Arch Intern Med 128(6):336–343
Zhao L, Lou H, Peng Y, Chen S, Zhang Y, Li X (2019) Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine 66(3):526–537
Wang J, Li W, Wang C, Wang L, He T, Hu H et al (2020) Enterotype bacteroides is associated with a high risk in patients with diabetes: a pilot study. J Diabetes Res 2020:6047145
Hung WC, Hung WW, Tsai HJ, Chang CC, Chiu YW, Hwang SJ et al (2021) The association of targeted gut microbiota with body composition in Type 2 Diabetes mellitus. Int J Med Sci 18(2):511–519
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK et al (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5(2):e9085
Fassatoui M, Lopez-Siles M, Díaz-Rizzolo DA, Jmel H, Naouali C, Abdessalem G et al (2019) Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Biosci Rep 39(6).
Xia F, Wen LP, Ge BC, Li YX, Li FP, Zhou BJ (2021) Gut microbiota as a target for prevention and treatment of type 2 diabetes: Mechanisms and dietary natural products. World J Diabetes 12(8):1146–1163
Liu BN, Liu XT, Liang ZH, Wang JH (2021) Gut microbiota in obesity. World J Gastroenterol 27(25):3837–3850
Saad MJ, Santos A, Prada PO (2016) Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 31(4):283–293
Ichimura A, Kimura I (2015) Editorial: obesity and diabetes: energy regulation by free fatty acid receptors. Front Endocrinol (Lausanne) 6:178
Zhou Z, Sun B, Yu D, Zhu C (2022) Gut microbiota: an important player in Type 2 diabetes mellitus. Front Cell Infect Microbiol 12:834485
Ren T, Liu F, Wang D, Li B, Jiang P, Li J et al (2023) Rhamnogalacturonan-I enriched pectin from steamed ginseng ameliorates lipid metabolism in type 2 diabetic rats via gut microbiota and AMPK pathway. J Ethnopharmacol 301:115862
Liu H, Xing Y, Wang Y, Ren X, Zhang D, Dai J et al (2023) Dendrobium officinale polysaccharide prevents diabetes via the regulation of gut microbiota in prediabetic mice. Foods 12(12).
Xia T, Liu CS, Hu YN, Luo ZY, Chen FL, Yuan LX et al (2021) Coix seed polysaccharides alleviate type 2 diabetes mellitus via gut microbiota-derived short-chain fatty acids activation of IGF1/PI3K/AKT signaling. Food Res Int 150(Pt A):110717
Chen Y, Song S, Shu A, Liu L, Jiang J, Jiang M et al (2022) The Herb Pair Radix Rehmanniae and Cornus officinalis attenuated testicular damage in mice with diabetes mellitus through butyric acid/glucagon-like peptide-1/glucagon-like peptide-1 receptor pathway mediated by gut microbiota. Front Microbiol 13:831881
Hou D, Zhao Q, Chen B, Ren X, Yousaf L, Shen Q (2021) Dietary supplementation with mung bean coat alleviates the disorders in serum glucose and lipid profile and modulates gut microbiota in high-fat diet and streptozotocin-induced prediabetic mice. J Food Sci 86(9):4183–4196
Zhang C, Jia J, Zhang P, Zheng W, Guo X, Ai C et al (2022) Fucoidan from Laminaria japonica Ameliorates Type 2 Diabetes Mellitus in association with modulation of gut microbiota and metabolites in streptozocin-treated mice. Foods 12(1).
Zhang Y, Peng Y, Zhao L, Zhou G, Li X (2021) Regulating the gut microbiota and SCFAs in the faeces of T2DM rats should be one of antidiabetic mechanisms of mogrosides in the fruits of Siraitia grosvenorii. J Ethnopharmacol 274:114033
Wang D, Wang JX, Yan C, Liu Y, Liu H, Li D et al (2022) Gastrodia elata Blume extract improves high-fat diet-induced type 2 diabetes by regulating gut microbiota and bile acid profile. Front Microbiol 13:1091712
Zheng XX, Li DX, Li YT, Chen YL, Zhao YL, Ji S et al (2023) Mulberry leaf water extract alleviates type 2 diabetes in mice via modulating gut microbiota-host co-metabolism of branched-chain amino acid. Phytother Res 37(8):3195–3210
He QL, Wang HC, Ma YK, Yang RL, Dai ZF, Yang JN et al (2023) Changes in the microbiota and their roles in patients with Type 2 diabetes mellitus. Curr Microbiol 80(4):132
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X et al (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359(6380):1151–1156
Tang R, Li L (2021) Modulation of short-chain fatty acids as potential therapy method for type 2 diabetes mellitus. Can J Infect Dis Med Microbiol 2021:6632266
Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y et al (2021) The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res 165:105420
Morrison DJ, Preston T (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7(3):189–200
Sankanagoudar S, Shukla R, Shukla KK, Sharma P (2022) Positive association of branched-chain amino acids with triglyceride and glycated haemoglobin in Indian patients with type 2 diabetes mellitus. Diabetes Metab Syndr 16(4):102481
Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N et al (2011) Cohort profile: the study of health in Pomerania. Int J Epidemiol 40(2):294–307
Neinast M, Murashige D, Arany Z (2019) Branched chain amino acids. Annu Rev Physiol 81:139–164
Ni Y, Lohinai Z, Heshiki Y, Dome B, Moldvay J, Dulka E et al (2021) Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. Isme J 15(11):3207–3220
Gao R, Meng X, Xue Y, Mao M, Liu Y, Tian X et al (2022) Bile acids-gut microbiota crosstalk contributes to the improvement of type 2 diabetes mellitus. Front Pharmacol 13:1027212
Mantovani A, Dalbeni A, Peserico D, Cattazzo F, Bevilacqua M, Salvagno GL et al (2021) Plasma bile acid profile in patients with and without type 2 diabetes. Metabolites 11(7).
Majait S, Nieuwdorp M, Kemper M, Soeters M (2023) The black box orchestra of gut bacteria and bile acids: who is the conductor? Int J Mol Sci 24(3).
Archundia Herrera MC, Subhan FB, Chan CB (2017) Dietary patterns and cardiovascular disease risk in people with type 2 diabetes. Curr Obes Rep 6(4):405–413
Seto SW, Lam TY, Tam HL, Au AL, Chan SW, Wu JH et al (2009) Novel hypoglycemic effects of Ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine 16(5):426–436
Teng BS, Wang CD, Zhang D, Wu JS, Pan D, Pan LF et al (2012) Hypoglycemic effect and mechanism of a proteoglycan from Ganoderma lucidum on streptozotocin-induced type 2 diabetic rats. Eur Rev Med Pharmacol Sci 16(2):166–175
Liang H, Pan Y, Teng Y, Yuan S, Wu X, Yang H et al (2020) A proteoglycan extract from Ganoderma lucidum protects pancreatic beta-cells against STZ-induced apoptosis. Biosci Biotechnol Biochem 84(12):2491–2498
Zhang Y, Li X, Ruan J, Wang T, Dong Y, Hao J et al (2016) Oleanane type saponins from the stems of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. Fitoterapia. 2016;109:99–105.
Mao XQ, Yu F, Wang N, Wu Y, Zou F, Wu K et al (2009) Hypoglycemic effect of polysaccharide enriched extract of Astragalus membranaceus in diet induced insulin resistant C57BL/6J mice and its potential mechanism. Phytomedicine 16(5):416–425
Zhu Y, Su Y, Zhang J, Zhang Y, Li Y, Han Y et al (2021) Astragaloside IV alleviates liver injury in type 2 diabetes due to promotion of AMPK/mTOR‑mediated autophagy. Mol Med Rep 23(6).
Chen X, Chen C, Fu X (2022) Hypoglycemic effect of the polysaccharides from Astragalus membranaceus on type 2 diabetic mice based on the “gut microbiota-mucosal barrier.” Food Funct 13(19):10121–10133
Chen X, Chen C, Fu X (2022) Hypoglycemic activity in vitro and vivo of a water-soluble polysaccharide from Astragalus membranaceus. Food Funct 13(21):11210–11222
Wang WK, Fan L, Ge F, Li Z, Zhu J, Yin K et al (2022) Effects of Danggui Buxue decoction on host gut microbiota and metabolism in GK rats with type 2 diabetes. Front Microbiol 13:1029409
Sun Y, Rukeya J, Tao W, Sun P, Ye X (2017) Bioactive compounds and antioxidant activity of wolfberry infusion. Sci Rep 7:40605
Retraction: Polysaccharide IV from Lycium barbarum L. improves lipid profiles of gestational diabetes mellitus of pregnancy by upregulating ABCA1 and downregulating sterol regulatory element-binding transcription 1 via miR-33. Front Endocrinol (Lausanne). 13:1130062 (2022).
Yang S, Li F, Lu S, Ren L, Bian S, Liu M et al (2022) Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J Ethnopharmacol 283:114739
Jiang L, Fu Q, Wang S, Chen Y, Li J, Xiao Y et al (2022) Effect of RG (Coptis root and ginseng) formula in patients with type 2 diabetes mellitus: a study protocol for a randomized controlled and double-blinding trial. Trials 23(1):305
Li J, Qiang FU, Shidong W, Jinxi Z, Yu C, Jiayue LI et al (2023) Effects of Shenlian formula on microbiota and inflammatory cytokines in adults with type 2 diabetes: a double-blind randomized clinical trial. J Tradit Chin Med 43(4):760–769
Wang Y, Tong Y, Adejobi OI, Wang Y, Liu A (2021) Research advances in multi-omics on the traditional Chinese herb Dendrobium officinale. Front Plant Sci 12:808228
Peng D, Tian W, An M, Chen Y, Zeng W, Zhu S et al (2023) Characterization of antidiabetic effects of Dendrobium officinale derivatives in a mouse model of type 2 diabetes mellitus. Food Chem 399:133974
Chen X, Chen C, Fu X (2023) Dendrobium officinale polysaccharide alleviates type 2 diabetes mellitus by restoring gut microbiota and repairing intestinal barrier via the LPS/TLR4/TRIF/NF-kB Axis. J Agric Food Chem 71(31):11929–11940
Long XS, Liao ST, Li EN, Pang DR, Li Q, Liu SC et al (2021) The hypoglycemic effect of freeze-dried fermented mulberry mixed with soybean on type 2 diabetes mellitus. Food Sci Nutr 9(7):3641–3654
Hu B, Yin T, Zhang J, Liu M, Yun H, Wang J et al (2023) Effect of “maccog” TCM tea on improving glucolipid metabolism and gut microbiota in patients with type 2 diabetes in community. Front Endocrinol (Lausanne) 14:1134877
Ren X, Xing Y, He L, Xiu Z, Yang L, Han A et al (2022) Effect of 1-Deoxynojirimycin on insulin resistance in prediabetic mice based on next-generation sequencing and intestinal microbiota study. J Ethnopharmacol 289:115029
Zhou Q, Yu R, Liu T, Li Y, Zhong J, Zhang T et al (2021) Coix seed diet ameliorates immune function disorders in experimental colitis mice. Nutrients 14(1).
Liu S, Li F, Zhang X (2019) Structural modulation of gut microbiota reveals Coix seed contributes to weight loss in mice. Appl Microbiol Biotechnol 103(13):5311–5321
Ferretti R, Moura EG, Dos Santos VC, Caldeira EJ, Conte M, Matsumura CY et al (2018) High-fat diet suppresses the positive effect of creatine supplementation on skeletal muscle function by reducing protein expression of IGF-PI3K-AKT-mTOR pathway. PLoS ONE 13(10):e0199728
Zhan HD, Zhou HY, Sui YP, Du XL, Wang WH, Dai L et al (2016) The rhizome of Gastrodia elata Blume—an ethnopharmacological review. J Ethnopharmacol 189:361–385
Bai Y, Mo K, Wang G, Chen W, Zhang W, Guo Y et al (2021) Intervention of Gastrodin in type 2 diabetes mellitus and its mechanism. Front Pharmacol 12:710722
Yang HJ, Kim MJ, Kwon DY, Kim DS, Lee YH, Kim JE et al (2016) Anti-diabetic activities of Gastrodia elata blume water extracts are mediated mainly by potentiating glucose-stimulated insulin secretion and increasing β-cell mass in non-obese type 2 diabetic animals. Nutrients 8(3):161
Ye T, Meng X, Zhai Y, Xie W, Wang R, Sun G et al (2018) Gastrodin ameliorates cognitive dysfunction in diabetes rat model via the suppression of endoplasmic reticulum stress and NLRP3 inflammasome activation. Front Pharmacol 9:1346
Gao X, Liu Y, An Z, Ni J (2021) Active components and pharmacological effects of Cornus officinalis: literature review. Front Pharmacol 12:633447
Peng ZC, He J, Pan XG, Zhang J, Wang YM, Ye XS et al (2021) Secoiridoid dimers and their biogenetic precursors from the fruits of Cornus officinalis with potential therapeutic effects on type 2 diabetes. Bioorg Chem 117:105399
Hou D, Yousaf L, Xue Y, Hu J, Wu J, Hu X et al (2019) Mung bean (Vigna radiata L.): bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients 11(6).
Mayachiew P, Charunuch C, Devahastin S (2015) Physicochemical and thermal properties of extruded instant functional rice porridge powder as affected by the addition of soybean or mung bean. J Food Sci 80(12):E2782–E2791
Saeting O, Chandarajoti K, Phongphisutthinan A, Hongsprabhas P, Sae-Tan S (2021) Water extract of mungbean (Vigna radiata L.) inhibits protein tyrosine phosphatase-1B in insulin-resistant HepG2 cells. Molecules 26(5).
Charoensiddhi S, Chanput WP, Sae-Tan S (2022) Gut microbiota modulation, anti-diabetic and anti-inflammatory properties of polyphenol extract from mung bean seed coat (Vigna radiata L.). Nutrients 14(11).
Li HY, Yi YL, Guo S, Zhang F, Yan H, Zhan ZL et al (2022) Isolation, structural characterization and bioactivities of polysaccharides from Laminaria japonica: a review. Food Chem 370:131010
Wang X, Zhang L, Qin L, Wang Y, Chen F, Qu C et al (2022) Physicochemical properties of the soluble dietary fiber from Laminaria japonica and its role in the regulation of Type 2 diabetes mice. Nutrients 14(2).
Wu N, Jin W, Zhao Y, Wang H, He S, Zhang W et al (2022) Sulfated Fucogalactan from Laminaria japonica Ameliorates β-cell failure by attenuating mitochondrial dysfunction via SIRT1-PGC1-α signaling pathway activation. Front Endocrinol (Lausanne) 13:881256
Gong X, Chen N, Ren K, Jia J, Wei K, Zhang L et al (2019) The fruits of Siraitia grosvenorii: a review of a Chinese food-medicine. Front Pharmacol 10:1400
Li C, Lin LM, Sui F, Wang ZM, Huo HR, Dai L et al (2014) Chemistry and pharmacology of Siraitia grosvenorii: a review. Chin J Nat Med 12(2):89–102
Zhou G, Zhang Y, Li Y, Wang M, Li X (2018) The metabolism of a natural product mogroside V, in healthy and type 2 diabetic rats. J Chromatogr B Analyt Technol Biomed Life Sci 1079:25–33
Liu H, Qi X, Yu K, Lu A, Lin K, Zhu J et al (2019) AMPK activation is involved in hypoglycemic and hypolipidemic activities of mogroside-rich extract from Siraitia grosvenorii (Swingle) fruits on high-fat diet/streptozotocin-induced diabetic mice. Food Funct 10(1):151–162
Baker RG, Hayden MS, Ghosh S (2011) NF-κB, inflammation, and metabolic disease. Cell Metab 13(1):11–22
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651
Malko P, Jia X, Wood I, Jiang LH (2023) Piezo1 channel-mediated Ca(2+) signaling inhibits lipopolysaccharide-induced activation of the NF-κB inflammatory signaling pathway and generation of TNF-α and IL-6 in microglial cells. Glia 71(4):848–865
Tedelind S, Westberg F, Kjerrulf M, Vidal A (2007) Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol 13(20):2826–2832
Ulven T (2012) Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 3:111
Bindels LB, Dewulf EM, Delzenne NM (2013) GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci 34(4):226–232
Lee SU, In HJ, Kwon MS, Park BO, Jo M, Kim MO et al (2013) β-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-κB. Biol Pharm Bull 36(11):1754–1759
Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B et al (2004) Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell 14(3):303–317
Sinha SR, Haileselassie Y, Nguyen LP, Tropini C, Wang M, Becker LS et al (2020) Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 27(4):659–70.e5
Hu J, Wang C, Huang X, Yi S, Pan S, Zhang Y et al (2021) Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep 36(12):109726
Xiao H, Sun X, Liu R, Chen Z, Lin Z, Yang Y et al (2020) Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol Res 151:104559
Liu H, Wang J, Ding Y, Shi X, Ren H (2022) Antibiotic pretreatment attenuates liver ischemia-reperfusion injury by Farnesoid X receptor activation. Cell Death Dis 13(5):484
Yu JH, Zheng JB, Qi J, Yang K, Wu YH, Wang K et al (2019) Bile acids promote gastric intestinal metaplasia by upregulating CDX2 and MUC2 expression via the FXR/NF-κB signalling pathway. Int J Oncol 54(3):879–892
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K (2019) The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16(8):461–478
Jorsal T, Rhee NA, Pedersen J, Wahlgren CD, Mortensen B, Jepsen SL et al (2018) Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia 61(2):284–294
Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ (2018) The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol 315(1):G53-g65
Zheng X, Chen T, Jiang R, Zhao A, Wu Q, Kuang J et al (2021) Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab 33(4):791-803.e7
Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756
Maneschi E, Vignozzi L, Morelli A, Mello T, Filippi S, Cellai I et al (2013) FXR activation normalizes insulin sensitivity in visceral preadipocytes of a rabbit model of MetS. J Endocrinol 218(2):215–231
Dimou A, Tsimihodimos V, Bairaktari E (2022) The critical role of the branched chain amino acids (BCAAs) catabolism-regulating enzymes, branched-chain aminotransferase (BCAT) and branched-chain α-keto acid dehydrogenase (BCKD), in human pathophysiology. Int J Mol Sci 23(7).
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168(6):960–976
Chen J, Ou Y, Luo R, Wang J, Wang D, Guan J et al (2021) SAR1B senses leucine levels to regulate mTORC1 signalling. Nature 596(7871):281–284
Marafie SK, Al-Shawaf EM, Abubaker J, Arefanian H (2019) Palmitic acid-induced lipotoxicity promotes a novel interplay between Akt-mTOR, IRS-1, and FFAR1 signaling in pancreatic β-cells. Biol Res 52(1):44
Rivera ME, Rivera CN, Vaughan RA (2022) Excess branched-chain amino acids alter myotube metabolism and substrate preference which is worsened by concurrent insulin resistance. Endocrine 76(1):18–28
Jing T, Zhang S, Bai M, Chen Z, Gao S, Li S et al (2021) Effect of dietary approaches on glycemic control in patients with Type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients 15(14).
Liu Y, Wang J, Wu C (2021) Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front Nutr 8:634897
Salgaço MK, Oliveira LGS, Costa GN, Bianchi F, Sivieri K (2019) Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl Microbiol Biotechnol 103(23–24):9229–9238
Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5(4):1417–1435
Petersen A, Julienne H, Hyötyläinen T, Sen P, Fan Y, Pedersen HK et al (2021) Conjugated C-6 hydroxylated bile acids in serum relate to human metabolic health and gut Clostridia species. Sci Rep 11(1):13252
Wang Z, Du H, Peng W, Yang S, Feng Y, Ouyang H et al (2022) Efficacy and mechanism of Pueraria lobata and Pueraria thomsonii polysaccharides in the treatment of type 2 diabetes. Nutrients 14(19).
Xu F, Yang J, Negishi H, Sun Y, Li D, Zhang X et al (2018) Silibinin decreases hepatic glucose production through the activation of gut-brain-liver axis in diabetic rats. Food Funct 9(9):4926–4935
Acknowledgements
None.
Funding
This work was supported by the Jiangxi Provincial Natural Science Foundation Key Project (20224ACB206010); Key Project of Jiangxi Province Science and Technology Key R&D Plan (20201BBG71006); and Jiangxi Provincial Science and Technology Innovation Base Plan (2020BCG74001 and 20221ZDG02011).
Author information
Authors and Affiliations
Contributions
SC, YJ and YH contributed equally to this work. SC, JZ, WC and JX conceived the idea and designed the study. SC and YH drafted the original manuscript. YD, JW and SH reviewed and edited the manuscript. YZ formatted the manuscript according to journal guidelines. JX and WC obtained the funding. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights disclosure
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Gut Microbiome and Metabolic Disorders, managed by Massimo Federici.
Supplementary Information
Below is the link to the electronic supplementary material.
592_2023_2217_MOESM2_ESM.jpg
Figure S1 Illustrates the relationship between obesity and insulin resistance. Gut microbiota act as mediators. Obesity may change the metabolites of gut microbiota, which in turn leads to insulin resistance in the body, and ultimately lead to T2DM. Drawn using the software Adobe Illustrator 2023.
Supplementary file2 (JPG 53KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Jiao, Y., Han, Y. et al. Edible traditional Chinese medicines improve type 2 diabetes by modulating gut microbiotal metabolites. Acta Diabetol 61, 393–411 (2024). https://doi.org/10.1007/s00592-023-02217-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02217-6