Abstract
Aims
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disorders worldwide. Some hypoglycemic drugs can improve NAFLD. However, it is unclear which of these types of hypoglycemic drugs are more effective for NAFLD. Therefore, we conducted a network meta-analysis to determine the effect of thiazolidinediones (TZDs), sodium-glucose cotransporter 2 (SGLT2) inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists on NAFLD patients.
Methods
A literature search of PubMed, EMBASE, the Cochrane Library, and Medline was conducted, and the literature from database inception up to April 30, 2021 was obtained. Liver function tests, lipid profiles, body mass index (BMI) and glycemic parameters were obtained from randomized controlled trials. Weighted mean differences (WMDs), relative risks and 95% confidence intervals (CIs) were calculated for continuous outcomes, and the I2 statistic was used to evaluate the heterogeneity of the studies.
Results
In total, 22 trials, including 1361 patients, were selected. In direct meta-analysis, GLP-1 receptor agonists were superior to TZDs in decreasing alanine aminotransferase (WMD, −0.40, 95% CI: −0.60 to −0.20), γ-glutamyl transferase (WMD, −5.00, 95% CI: −6.47 to −3.53), BMI (WMD, −4.10, 95%CI: −6.55 to −1.65) and triglycerides (WMD, − 0.50, 95% CI: −0.68 to −0.32). Based on Bayesian network meta-analysis, the effect of SGLT-2 inhibitors on weight loss was superior to that of TZDs (WMD, −1.80, 95%CI: −3.30 to −0.41).
Conclusions
GLP-1 receptor agonists and SGLT-2 inhibitors improved liver enzymes, BMI, blood lipid, blood glucose and insulin resistance in NAFLD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a pathological state of excess hepatic fat accumulation in the absence of significant alcohol consumption or other known factors that induce chronic liver damage [1]. NAFLD comprises a broad spectrum of diseases, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), liver cirrhosis and hepatocellular carcinoma [2]. NAFLD is a leading cause of chronic liver disorders, affecting approximately 25% of the global population [3, 4]. The prevalence of NAFLD is increasing.
The management of NAFLD mainly focuses on interventions pertaining to diet and lifestyle, including changes in energy intake, focus on weight loss, increased aerobic exercise and restriction of alcohol consumption [5]. Several drugs exhibited a definite histological effect on NAFLD in clinical trials and animal experiments. Insulin sensitizers, such as thiazolidinediones (TZDs), can improve the histological indications such as steatosis, inflammation, hepatocellular ballooning, and fibrosis in patients with NASH [6]. However, there are side effects such as weight gain and an increase in subcutaneous adipose tissue; these greatly reduce the efficacy of TZD treatment [7].
Novel oral hypoglycemic agents, such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, have gained attention for NAFLD treatment. SGLT-2 inhibitors increase urinary glucose excretion by inhibiting glucose reabsorption in the renal proximal tubule, thereby lowering blood glucose levels [8]. In addition, they significantly reduce the weight of mice while improving liver steatosis, inflammation, and fibrosis [9]. A prospective study showed that SGLT-2 inhibitors reduce liver fat and lower serum alanine aminotransferase (ALT) levels in patients with NAFLD and type 2 diabetes mellitus (T2DM) [10]. In addition, they improve liver function irrespective of the changes in the body weight in T2DM patients [11].
GLP-1, an incretin hormone, is secreted by the L cells in the distal ileum and proximal colon [12]. The role of GLP-1 receptor agonists in the treatment of T2DM is widely recognized in terms of promoting insulin secretion by pancreatic β cells and inhibiting glucagon secretion [13, 14]. A meta-analysis showed that GLP-1 receptor agonists reduce body weight in overweight or obese patients with or without T2DM [15]. GLP-1 receptor agonists and structured lifestyle interventions have similar effects pertaining to reducing body weight, liver fat fractions, and serum transaminase levels in obese adults with NAFLD [16]. A retrospective study showed that the use of GLP-1 agonists after 12 weeks of treatment decreases the amount of total body fat mass and liver stiffness in patients with NAFLD and T2DM [17].
Accumulating data indicate the potential therapeutic effects of the three antidiabetics on NAFLD; however, few studies have directly compared the efficacy of these agents for NAFLD. Several direct pairwise meta-analyses provide a comparison of each drug pair against a placebo or other agents [6, 18, 19]. We performed a systematic review and network meta-analysis (NMA) to compare the effectiveness of the three antidiabetic agents (TZDs, SGLT-2 inhibitors and GLP-1 receptor agonists) on BMI, liver enzymes, blood lipids and glycemic parameters in NAFLD patients.
Methods
This NMA was conducted following The Preferred Reporting Items for Systematic Reviews and Metaanalyses (PRISMA) extension statement for the reporting of systematic reviews incorporating NMAs of Health Care Interventions [20]. The protocol for this systematic review with NMA is registered on PROSPERO (CRD42020202514).
Selection criteria
Studies included in this NMA were randomized controlled trials (RCTs) that met the following inclusion criteria: (1) patients: adults with NAFLD (including NASH) proven through biopsy or imaging examination; (2) intervention: TZDs, GLP-1 receptor agonists or SGLT-2 inhibitors; (3) comparator: placebo or other agents that can be compared to the three interventions; (4) outcomes: primary outcomes were improvements in ALT, aspartate aminotransferase (AST) and γ-glutamyl transferase (GGT) levels; secondary outcomes included body mass index (BMI), blood lipids [total cholesterol, high density lipoprotein (HDL)-cholesterol, low density lipoprotein (LDL)-cholesterol and triglycerides (TG)], glycemic parameters [glycosylated hemoglobin (HbA1c), glucose and homeostasis model assessment (HOMA-IR)]. We excluded (1) observational and non-randomized controlled trial studies, (2) interventions other than pharmacological therapy, including diet and lifestyle changes, and (3) patients with comorbidities that could affect the outcomes.
Search strategy
We searched PubMed, EMBASE, the Cochrane library and Medline databases from database inception until April 2021. Databases were searched using a combination of MeSH terms and entry terms. We limited the language included in this study to English.
Data abstraction and quality assessment
Characteristics of the included literature were extracted onto a standardized form in Microsoft Excel 2016 and EndNote X9 as follows: (1) study characteristics, primary author, year of publication, study design, geographical location and study duration; (2) treatment characteristics, sample sizes of each group and schedule of intervention; (3) outcome assessment, change in liver aminotransferases (ALT, AST and GGT) levels, plasma levels of TG, total cholesterol (TC), LDL-C (low density lipoprotein cholesterol), HDL-C (high density lipoprotein cholesterol), FBG (fasting blood glucose), HOMA-IR and Hb1Ac from baseline in each treatment group. The risk of bias in each study was assessed with the Cochrane Risk of Bias assessment tool. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria for NMA to appraise the quality of evidence [21].
Statistical methods
When the data were reported as measures before and after intervention, we calculated the mean change and standard deviations for change. When only the median, range and size of a sample were provided, we estimated them using the method proposed by Stela Pudar Hozo [22]. When the mean and standard deviation of the calculated change in value were provided, we directly used these data.
Direct meta-analysis was performed using a random-effects model to estimate the weighted mean difference (WMD) and 95% confidence intervals (CIs). We used STATA software (Stata Statistical Software Release 16; Stata Corp, USA) to perform direct comparisons and draw the forest plots. Statistical heterogeneity was assessed using the I2 statistic, with values > 50% indicating significant heterogeneity.
Owing to the limited number of trials based on direct comparison, we used indirect comparisons to explore the difference in efficacy between the two treatments. To perform indirect comparisons, we performed a random-effects Bayesian network meta-analysis using R version 4.0.2 (R Core Team, Vienna, Austria) with the GeMTC package for statistical analyses. We modeled the comparative efficacy of any two interventions as a function of each intervention relative to the reference intervention (i.e., placebo and metformin in this study). The consistency of the network was evaluated by comparing direct estimates to indirect estimates. The node splitting method was used to estimate the effect of the indirect comparison. We assessed the probability that each intervention as the most efficacious in improving outcomes, the second best, the third best, and so on, to rank the intervention hierarchically in the NMA.
Results
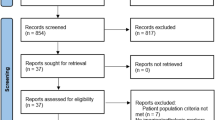
A schematic diagram of the study selection process is shown in Fig. 1. In total, 5301 unique studies were identified using our search strategy. By reading the full text, 22 RCT studies were reserved for this NMA [7, 16, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] (Fig. 2).
Characteristics and quality of included studies
Table 1 summarizes the characteristics of the RCTs included in the NMA. Overall, these 22 trials included 1351 participants with NAFLD. A summary of the risk of bias of the included RCTs is shown in Supplementary Fig. 1. All interventions were implemented as intended and completed outcome reporting; overall, the studies were at a low-to-moderate risk of bias.
Improvement in ALT
Direct Meta-analysis: Compared to TZDs, GLP-1 receptor agonists (WMD, −0.40, 95%CI: −0.60 to −0.20) were associated with an improvement in ALT levels in patients with NAFLD (Fig. 3). SGLT-2 inhibitors (WMD, −6.00, 95%CI: −9.13 to −2.86) were superior to the placebo in lowering ALT levels. Compared to metformin, SGLT-2 inhibitors (WMD, −3.60, 95%CI: −6.71 to −0.49) improved ALT levels in patients with NAFLD. However, metformin and placebo were associated with increased ALT levels. There was no difference between TZDs and SGLT-2 inhibitors in improving ALT levels in patients with NAFLD.
Network Meta-analysis: Compared to placebo, SGLT-2 inhibitors and GLP-1 receptor agonists were associated with significant improvements in ALT (Supplementary Fig. 4A). TZDs and GLP-1 receptor agonists had the highest probability of being ranked first and second for improving ALT, respectively, whereas SGLT-2 inhibitors had the highest probability of being ranked third (Supplementary Fig. 5A).
Improvement in AST levels
Direct Meta-analysis: TZDs were associated with a decrease in AST levels (WMD, −11.25, 95%CI: −17.16 to −5.35) when compared to that with a placebo (Fig. 4). In the only head-to-head trial, metformin was superior to GLP-1 receptor agonists in improving AST levels (WMD, 4.30, 95%CI: 3.03–5.57). No significant differences were found among the other interventions.
Network Meta-analysis. Consistent with the results of direct comparisons, TZDs were superior to placebo in decreasing AST levels (Supplementary Fig. 4B). Overall, TZDs were ranked the highest for improvements in AST, whereas GLP-1 receptor agonists and SGLT-2 inhibitors had the highest probabilities of being ranked third and fourth, respectively (Supplementary Fig. 5B).
Improvement in GGT
Direct Meta-analysis: GLP-1 receptor agonists were superior to TZDs in decreasing GGT (WMD, −5.00, 95%CI: −6.47 to −3.53) (Fig. 5). However, there was no significant difference between TZDs and SGLT-2 inhibitors.
Network Meta-analysis: Owing to the wide range of confidence intervals, no intervention was clearly superior to others in the comparative effectiveness NMA of active interventions (Supplementary Fig. 4C). However, the ranking of efficacy showed that GLP-1 receptor agonists and TZDs had the highest probability of being ranked first and second for improving GGT, respectively, whereas SGLT-2 inhibitors had the highest probability of being ranked third (Supplementary Fig. 5C).
Effect on Weight Loss.
Direct Meta-analysis: GLP-1 receptor agonists were significantly superior to TZDs (WMD, −4.10, 95%CI: −6.55 to −1.65), placebo (WMD,−1.50, 95%CI: −2.49 to −0.51), and metformin (WMD, − 0.60, 95%CI: −0.94 to − 0.26) for weight loss (Fig. 6). In addition, compared to that with the placebo and metformin, SGLT-2 inhibitors significantly reduced the weight of NAFLD patients.
Network Meta-analysis: Compared to TZDs, GLP-1 receptor agonists (WMD, −2.20, 95%CI: −3.80 to −0.85) and SGLT-2 inhibitors (WMD, −1.80, 95%CI: −3.30 to −0.41) were associated with weight loss (Supplementary Fig. 4D). GLP-1 receptor agonists and SGLT-2 inhibitors had the highest probability of being ranked as first- and second-best interventions for weight loss, respectively, whereas TZDs had the highest probability of being ranked last (Supplementary Fig. 5D).
Improvement in blood lipid profiles
Direct Meta-analysis: Compared to TZDs, GLP-1 receptor agonists (WMD, −0.50, 95%CI: −0.68 to −0.32) were associated with decreased TG (Supplementary Fig. 6). Compared to metformin, GLP-1 agonists could be more beneficial in reducing TG. Moreover, SGLT-2 inhibitors were superior to TZDs in improving TC (WMD, −0.10, 95%CI: −0.15 to −0.05). Compared to that with placebo, SGLT-2 inhibitors, GLP-1 receptor agonists, and TZDs slightly increased HDL cholesterol levels in NAFLD patients. In the only head-to-head trial, GLP-1 receptor agonists reduced TC, when compared to that with metformin.
Network Meta-analysis: Compared to metformin and placebo, GLP-1 receptor agonists significantly reduced TG levels in NAFLD patients. GLP-1 receptor agonists were superior to metformin in decreasing LDL cholesterol. The effects of TZDs on HDL and LDL cholesterol levels, compared to that with the placebo, were consistent with the results of the direct comparison (Supplementary Fig. 7). Overall, GLP-1 receptor agonists were ranked highest in improving blood lipid levels, except TC levels. SGLT-2 inhibitors had the highest probability of being ranked first to improve TC levels (Supplementary Fig. 8).
Other outcomes
We compared the effects of several drugs on FBG, HOMA-IR and HbA1c (Supplementary Fig. 9 and 10). The effects of these hypoglycemic drugs on fasting blood sugar levels are well established. Our results corroborated this through direct comparisons or indirect comparisons. The effect of GLP-1 receptor agonists on FBG was better than that of metformin, a classic hypoglycemic drug. Compared to metformin, SGLT-2 inhibitors were more effective in reducing Hb1Ac. The ranking of efficacy showed that GLP-1 receptor agonists had the highest probability of being ranked first for improving FBG, HOMA-IR, and HbA1c (Supplementary Fig. 11).
Sensitivity analysis and network coherence
We performed a sensitivity analysis to identify sources of heterogeneity in this NMA (Supplementary Tables 1–4). The results of the sensitivity analysis were similar to that of the primary results. Our analysis revealed that the studies by Rana [31] were the main sources of heterogeneity in terms of the results pertaining to BMI; excluding these results effectively removed the variations. The NMA between TZDs and metformin showed no significant differences, but the difference between these two groups was not the main concern of our study. The network evaluating ALT showed some inconsistency in one (SGLT-2 inhibitors versus placebo, P = 0.049) comparison. There was one inconsistency in the comparison of the efficacy of HOMA-IR between the TZDs and placebo groups (P = 0.045). Overall, there were no significant differences between the direct and indirect estimates (Supplementary Table 5–15). Therefore, we believe that our results are robust.
Quality of evidence
The quality of the indirect evidence was generally low owing to the imprecision. For the ALT, AST and GGT outcomes, the effects of GLP-1 receptor agonists, TZDs and SGLT-2 inhibitors were supported by low-quality evidence, as compared to outcomes with the placebo (reduced owing to imprecision and inconsistency). For the same outcomes, the effect of GLP-1 receptor agonists, TZDs and SGLT-2 inhibitors, compared to that with metformin, was supported by low-quality evidence (reduced owing to imprecision caused by a low number of events). Table 2 and Supplementary Table 16 and 17 provide details of the GRADE quality of evidence for this NMA.
Discussion
There is increasing interest in the potential value of hypoglycemic drugs for the treatment of NAFLD [43,44,45]. The classic hypoglycemic drugs, TZDs are proven to improve the histology of patients with NAFLD. Recently, two novel hypoglycemic drugs, SGLT-2 inhibitors and GLP-1 receptor agonists, were shown to ameliorate NAFLD [18, 46]. The main aim of this NMA was to evaluate the effect of these medications on NAFLD, specifically on liver enzymes and other metabolism-related indicators. To the best of our knowledge, this is the first meta-analysis to compare the efficacies of TZDs, GLP-1 receptor agonists and SGLT-2 inhibitors in patients with NAFLD.
The natural course of NAFLD is dynamic, and liver enzyme levels are closely related to NAFLD activity. ALT, a marker of hepatic inflammation, is regarded as an important indicator of disease improvement [47]. A recent study confirmed that the serum ALT level is a good indicator of histological changes and can be used as an effective indicator of treatment [48]. For patients with lower baseline AST levels, NASH is more likely to resolve spontaneously. Meanwhile, higher AST levels are associated with a greater risk of progression to advanced fibrosis [49]. Serum GGT levels are strongly associated with increased mortality in patients with hepatic steatosis [50]. In the direct comparison, compared to TZDs, GLP-1 receptor agonists significantly decreased serum concentrations of ALT, AST and GGT in NAFLD patients. In the subsequent network analysis, GLP-1 receptor agonists ranked first in the possibility of improving the effects of ALT and GGT. Therefore, GLP-1 receptor agonists have beneficial effects on liver injury in NAFLD. A previous meta-analysis showed that compared to TZDs, SGLT-2 inhibitors reduced ALT levels more significantly [19]. However, in this study, there was no statistically significant difference in the improvements in response to the two medications, with respect to ALT levels. This could be attributed to the differences in the background glucose-lowering therapies in some studies included in the previous meta-analysis.
BMI is positively related to the presence of NAFLD, and significant weight loss can improve NAFLD [51]. Several studies have confirmed that obesity is an independent risk factor for the occurrence and development of NAFLD [52, 53]. GLP-1 receptor agonists and SGLT-2 inhibitors significantly reduced the BMI, while TZDs induced weight gain. NMA indicated that GLP-1 receptor agonists had the best effect on improving BMI. Diet and exercise that results in sustained weight loss of 7–10% can improve liver fat content and fibrosis [54]. A previous meta-analysis reported that weight loss ≥ 7% can improve the disease activity of NAFLD [55]. Therefore, the reduction in BMI indicates that GLP-1 receptor agonists and SGLT-2 inhibitors are beneficial in reducing liver fat. This is of great significance for the application of these two drugs for treating NAFLD patients, who are overweight or obese. TZDs, in addition to weight gain, exhibit serious adverse effects such as bladder cancer, osteoporosis, female fractures, and even congestive heart failure [56]. This limits the application of TZDs in treating NAFLD. Based on the histological improvements in the NASH patients in response to TZDs, we believe that TZDs can be used for patients with NASH, especially those with T2DM, who have previously been treated with TZDs.
NAFLD is regarded as a liver manifestation of metabolic syndrome [57, 58]. Dyslipidemia and insulin resistance are the basis of NAFLD pathogenesis. Approximately 50% of patients with hyperlipidemia have fatty infiltration of the liver [59]. Therefore, we analyzed the related indicators of glucose and lipid metabolism. GLP-1 receptor agonists had the best effect on improving blood lipids, except TC; SGLT-2 inhibitors had the strongest effect on reducing TC levels. In addition, GLP-1 receptor agonists were better than TZDs in reducing FBG levels.
To facilitate comparison, we included metformin as an intermediate comparator to perform a NMA of a group other than the placebo group. Metformin, a first line hypoglycemic drug, has a positive therapeutic effect on NAFLD [60,61,62,63]. A clinical trial comparing the effects of metformin and vitamin E in NAFLD patients showed that the intrahepatic triacylglycerol content was significantly reduced in patients treated with metformin [60]. When metformin was combined with diet management, the degree of lipid content reduction in the liver was greater than that in patients undergoing diet management alone; the weight loss was similar between the two groups [61]. In addition, 25–30% of patients exhibited improved histological characteristics of steatosis following the treatment with metformin [62]. In this study, the two novel hypoglycemic drugs were better than metformin in improving ALT, BMI, FBG and HbA1c levels. At the same time, SGLT-2 inhibitors were more effective in improving insulin resistance. Considering the relationship between these indicators and NAFLD and comparing the results between the two drugs and metformin, we believe that GLP-1 receptor agonists and SGLT-2 inhibitors might have additional benefits for NAFLD patients with type 2 diabetes or obesity.
Currently, there are only a few studies on the combined use of these three drugs for NAFLD. Considering the serious adverse effects of TZDs, a drug with similar therapeutic effects but relatively lesser side effects is a more appropriate therapeutic choice. This was the goal of this NMA. GLP-1 receptor agonists and SGLT-2 inhibitors exhibit complementary effects (on gluconeogenesis, thermogenesis and energy expenditure) [64, 65]; therefore, the combination of these two drugs could have a favorable effect on weight and blood glucose control in NAFLD patients. A recent meta-analysis confirmed that the combination of GLP-1 receptor agonists and SGLT-2 inhibitors reduces body weight, HbA1c levels and systolic blood pressure in diabetic patients, when compared to that in patients treated with GLP-1 receptor agonists or SGLT-2 inhibitor alone [66]. In addition, unlike TZDs, the side effects of these two types of drugs are minor and rarely require the discontinuation of therapy [67, 68]. Therefore, the combination of GLP-1 receptor agonists with SGLT-2 inhibitors has broad application prospects for the treatment of NAFLD; however, this hypothesis needs to be verified through well-designed RCTs in the future.
We limited this analysis to well-designed RCTs and performed quality assessment to reduce possible bias; however, this meta-analysis still had several limitations. The low acceptance of invasive biopsy and the limited results of abdominal ultrasound in the clinical studies resulted in insufficient data on the histological changes and quantification of liver steatosis. Considering the relationship among NAFLD, obesity and metabolic syndrome, this study mainly evaluated whether these medicines could improve NAFLD from the perspective of biochemical indicators, and further research is needed to evaluate the effect of these drugs on liver histology. In addition, the number of studies included was relatively small, which might have caused inhomogeneity and inconsistency between the direct and indirect comparisons. Therefore, larger RCTs are needed to further validate these results.
Conclusion
This study suggests that GLP-1 receptor agonists and SGLT-2 inhibitors can improve liver enzymes, the BMI, blood lipid, blood glucose, and insulin resistance in NAFLD patients. Therefore, these two drugs could be effective for treating NAFLD. This article summarizes existing literature on NAFLD and provides promising insights into potential therapeutics for its treatment. Nevertheless, the investigation of these agents for the treatment of NAFLD is new and the number and quantity of published studies are limited. To further verify the specific effects of these drugs on NAFLD and the underlying mechanisms, larger RCTs with imaging and tissue data are necessary in the future.
References
Chalasani N, Younossi Z, Lavine JE et al (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American gastroenterological association, american association for the study of liver diseases, and american college of gastroenterology. Gastroenterology 142:1592–1609
Adams LA, Lymp JF, St Sauver J et al (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129:113–121
Eslam M, George J (2020) Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol 17:40–52
Ratziu V, Goodman Z, Sanyal A (2015) Current efforts and trends in the treatment of NASH. J Hepatol 62:S65-75
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140.
Mahady SE, Webster AC, Walker S, Sanyal A, George J (2011) The role of thiazolidinediones in non-alcoholic steatohepatitis–a systematic review and meta analysis. J Hepatol 55:1383–1390
Belfort R, Harrison SA, Brown K et al (2006) A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355:2297–2307
Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 66:255–270
Qiang S, Nakatsu Y, Seno Y et al (2015) Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr 7:104
Kuchay MS, Krishan S, Mishra SK et al (2018) Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 41:1801–1808
Komiya C, Tsuchiya K, Shiba K et al (2016) Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS ONE 11:e0151511
Amori RE, Lau J, Pittas AG (2007) Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298:194–206
Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet (London, England) 368:1696–1705
Meier JJ (2012) GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 8:728–742
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL (2012). Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical research ed.). 344:d7771.
Khoo J, Hsiang J, Taneja R, Law NM, Ang TL (2017) Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: a pilot randomized trial. Diabetes Obes Metab 19:1814–1817
Seko Y, Sumida Y, Tanaka S et al (2017) Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res : Off J Jpn Soci Hepatol 47:1206–1211
Carbone LJ, Angus PW, Yeomans ND (2016) Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol 31:23–31
Xing B, Zhao Y, Dong B, Zhou Y, Lv W, Zhao W (2020) Effects of sodium-glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Investig 11:1238–1247
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–784
Brignardello-Petersen R, Bonner A, Alexander PE et al (2018) Corrigendum to “Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis” [J Clin Epidemiol 2018;93:36–44]. J Clin Epidemiol 98:162
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Ito D, Shimizu S, Inoue K et al (2017) Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label. Act-Control Trial Diabetes Care 40:1364–1372
Kinoshita T, Shimoda M, Nakashima K et al (2020) Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, open-label, three-arm, active control study. J Diabetes Investig 11:1612–1622
Zhang LY, Qu XN, Sun ZY, Zhang Y (2020) Effect of liraglutide therapy on serum fetuin A in patients with type 2 diabetes and non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol 44:674–680
Cusi K, Orsak B, Bril F et al (2016) Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 165:305–315
Aithal GP, Thomas JA, Kaye PV et al (2008) Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135:1176–1184
Ratziu V, Giral P, Jacqueminet S et al (2008) Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology 135:100–110
Armstrong MJ, Gaunt P, Aithal GP et al (2016) Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet (London, England) 387:679–690
Eriksson JW, Lundkvist P, Jansson PA et al (2018) Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 61:1923–1934
Rana H, Yadav SS, Reddy HD, Singhal S, Singh DK, Usman K (2016). Comparative Effect of Insulin Sensitizers and Statin on Metabolic Profile and Ultrasonographical Score in Non Alcoholic Fatty Liver Disease. J Clin Diagnostic Res: JCDR. 10:Oc19–23.
Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H (2012) Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon 12:e6099
Razavizade M, Jamali R, Arj A, Matini SM, Moraveji A, Taherkhani E (2013) The effect of pioglitazone and metformin on liver function tests, insulin resistance, and liver fat content in nonalcoholic fatty liver disease: a randomized double blinded clinical trial. Hepat Mon 13:e9270
Omer Z, Cetinkalp S, Akyildiz M et al (2010) Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 22:18–23
Fan H, Pan Q, Xu Y, Yang X (2013) Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol 57:702–708
Feng WH, Bi Y, Li P et al (2019) Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: a randomized trial. J Diabetes Investig 10:399–407
Tian F, Zheng Z, Zhang D, He S, Shen J (2018). Efficacy of liraglutide in treating type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease. Biosci Rep. 38.
Shibuya T, Fushimi N, Kawai M et al (2018) Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab 20:438–442
Yoneda M, Honda Y, Ogawa Y, et al. (2021). Comparing the effects of tofogliflozin and pioglitazone in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus (ToPiND study): a randomized prospective open-label controlled trial. BMJ open diabetes research & care. 9.
Guo W, Tian W, Lin L, Xu X (2020) Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty-six weeks: a randomized placebo-controlled trial. Diabetes Res Clin Pract 170:108487
Phrueksotsai S, Pinyopornpanish K, Euathrongchit J et al (2021) The effects of dapagliflozin on hepatic and visceral fat in type 2 diabetes patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol 36:2952–2959
Akyüz F, Demir K, Ozdil S et al (2007) The effects of rosiglitazone, metformin, and diet with exercise in nonalcoholic fatty liver disease. Dig Dis Sci 52:2359–2367
Armstrong MJ, Hazlehurst JM, Parker R et al (2014) Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM : Monthly J Assoc Physi 107:33–41
Portillo-Sanchez P, Bril F, Maximos M et al (2015) High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 100:2231–2238
Forlani G, Giorda C, Manti R et al (2016) The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016:2931985
Inoue M, Hayashi A, Taguchi T et al (2019) Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease. J Diabetes Investig 10:1004–1011
Farrell GC, Chitturi S, Lau GK, Sollano JD (2007) Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 22:775–777
Seko Y, Sumida Y, Tanaka S et al (2015) Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatol Res: Off J Jpn Soci Hepatol 45:E53-61
Kleiner DE, Brunt EM, Wilson LA et al (2019) Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2:e1912565
Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H (2009). Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology (Baltimore, Md.). 50:1403–1411.
Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. (2005). Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology (Baltimore, Md.). 42:44–52.
Hazlehurst JM, Tomlinson JW (2013) Non-alcoholic fatty liver disease in common endocrine disorders. Eur J Endocrinol 169:R27-37
Tarantino G, Finelli C (2013) Pathogenesis of hepatic steatosis: the link between hypercortisolism and non-alcoholic fatty liver disease. World J Gastroenterol 19:6735–6743
Brunner KT, Henneberg CJ, Wilechansky RM, Long MT (2019) Nonalcoholic fatty liver disease and obesity treatment. Curr Obes Rep 8:220–228
Musso G, Cassader M, Rosina F, Gambino R (2012) Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 55:885–904
Yau H, Rivera K, Lomonaco R, Cusi K (2013) The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diab Rep 13(3):329–341
Loomba R, Abraham M, Unalp A, et al. (2012). Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology (Baltimore, Md.) 56:943–951.
Grundy SM, Cleeman JI, Daniels SR et al (2005) Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement: executive summary. Crit Pathw Cardiol 4:198–203
Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G (2000) Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci 45:1929–1934
Bugianesi E, Gentilcore E, Manini R et al (2005) A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 100:1082–1090
Garinis GA, Fruci B, Mazza A et al (2005) Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes 2010(34):1255–1264
Nar A, Gedik O (2009) The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol 46:113–118
Weng S, Luo Y, Zhang Z, Su X, Peng D (2020) Effects of metformin on blood lipid profiles in nondiabetic adults: a meta-analysis of randomized controlled trials. Endocrine 67:305–317
Petit JM, Vergès B (2017). GLP-1 receptor agonists in NAFLD. Diabetes Metab, 43 Suppl 1:2S28–2S33.
Seufert J (2015) SGLT2 inhibitors - an insulin-independent therapeutic approach for treatment of type 2 diabetes: focus on canagliflozin. Diabetes Metab Syndr Obes 8:543–554
Mantsiou C, Karagiannis T, Kakotrichi P, Malandris K, Avgerinos I, Liakos A, Tsapas A, Bekiari E (2020) Glucagon-like peptide-1 receptor agonists and sodium–glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 22(10):1857–1868
Riggs K, Ali H, Taegtmeyer H, Gutierrez AD (2015) The use of SGLT-2 inhibitors in type 2 diabetes and heart failure. Metab Syndr Relat Disord 13:292–297
Chalasani N, Younossi Z, Lavine JE et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology (Baltimore, Md) 67:328–357
Shahebrahimi K, Zulnoorian S, Almasi A, Sharifi A, KeshvarzAA Farshchian N et al (2017) A comparison of the therapeutic effects of metformin, pioglitazone and vitamin E in patients with non-alcoholic fatty liver. J Babol Univ Med Sci 19:32–38
Funding
This work was supported by the National Natural Science Foundation (81974281), the Natural Science Foundation of Hunan Province (2020JJ2052), and the Chinese Cardiovascular Association-Access fund (2019-CCA-ACCESS-2020JJ2052).
Author information
Authors and Affiliations
Contributions
C.D. reviewed the literature and designed the review, C.D. and Y.T. conducted the search for the systematic review and interpreted the data, W.Z. and P.H. conducted the statistical analysis, J.R. and P.L. prepared the figures and tables, X.H. revised the manuscript, and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, C., Tang, Y., Zhu, W. et al. Sodium-glucose cotransporter protein-2 inhibitors and glucagon-like peptide-1 receptor agonists versus thiazolidinediones for non-alcoholic fatty liver disease: A network meta-analysis. Acta Diabetol 59, 519–533 (2022). https://doi.org/10.1007/s00592-021-01830-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01830-7