Abstract
Purpose of Review
This paper aims to provide an overview of sarcopenia, an enigmatic skeletal muscle disease where age, disuse, injury, and chronic disease can all contribute to the accelerated loss of mass and strength beyond normal variation, and that can negatively affect a person’s physical function and quality of life.
Recent Findings
A rapid and pervasive “graying” of societies worldwide is expected to continue in the coming decades. Due to this projected increase in the number of older adults, sarcopenia and its associated costs will be a significant public health concern. New international guidelines address the need for clinic-based approaches to identify vulnerable patients through quick and simple screening, while lifestyle-based interventions including resistance exercise training, general physical activity, and adequate nutrition remain the mainstays of treatment. The development of new, viable treatment options, including nutrition products and pharmacotherapy, are progressing with results expected in the near future.
Summary
The refinement of diagnostic criteria, recent designation as an internationally recognized medical condition, and the introduction of evidence-based treatment, is advancing sarcopenia as a treatable disease for a rapidly growing population of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
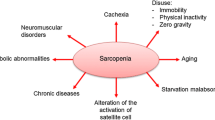
Originally introduced to describe the loss of skeletal muscle mass with advanced age, sarcopenia has evolved to include the accompanying loss of muscle function (e.g., strength) and consequent limitation in physical capability [1,2,3,4]. While the loss of muscle mass and strength is common with aging, sarcopenia is a distinct condition [5]. To differentiate sarcopenia from normal aging, several consensus criteria have been developed in the past decade (see Table 1). The current definitions contain thresholds in three key areas: (1) skeletal muscle mass (normalized by height or body mass index (BMI)), (2) muscle strength, and (3) functional performance [2,3,4]. On review of the criteria detailed in Table 1, it is evident that the key criterion has been skeletal muscle mass. However, the most recently updated definition prioritizes muscle weakness as the primary clinical symptom and revises diagnostic thresholds to promote better clinical awareness of sarcopenia [7••]. As such, sarcopenia is currently considered a systemic skeletal muscle disease defined as an age- and condition-related loss of muscle strength and mass with associated low physical function, most often seen in older adults (> 65 years old) [7••]. In addition to the traditional application to older adults, the label of sarcopenia has been used increasingly to describe the loss of skeletal muscle mass, strength, and function associated with chronic diseases [8,9,10], injuries [11], cancer [12], and other conditions [13, 14]. Primary sarcopenia is used to describe the state of sufficient muscle weakness or low functional performance and loss of muscle mass associated primarily with aging without another identifiable cause. In contrast, secondary sarcopenia describes the characteristics seen as a consequence of another condition (e.g., prolonged hospitalization, COPD) independent of age [7••]. This nomenclature remains somewhat controversial, however, as these same conditions may also be categorized according to a proposed muscle wasting classification schema [15].
Diagnosis and Methods of Assessment
Sarcopenia is diagnosed using the domains of (1) muscle mass (via body composition measurement), (2) muscle strength, and (3) lower extremity physical performance (summarized in Table 1). Current recommendations support the use of dual energy X-ray absorptiometry (DXA) and bioelectrical impedance to quantify total body and appendicular lean body mass as proxies for skeletal muscle mass in the clinic setting [2, 4], although the most recent treatment guidelines specify DXA only [16••]. Appendicular lean body mass (aLBM) is normalized by height to form appendicular skeletal muscle index (ASMI) [ASMI = aLBM/height (m)2] or by body mass index (BMI) [ = aLBM/BMI]. Measures that are more accurate, but costly or less accessible, are endorsed for research purposes (e.g., computed tomography, magnetic resonance imaging, and ultrasound). Muscle strength is commonly assessed as unilateral or bilateral grip strength using a calibrated dynamometer and standardized assessment position; notably, low grip strength can identify adults at risk for mobility disability [17, 18•]. The timed five-repetition chair stand test, (time in seconds to stand five times quickly without using the arms for assistance), provides a more functional strength test of the body area of interest (i.e., lower extremities). Both tests can be administered quickly, inexpensively, and reliably in the clinic and are predictive of poor health outcomes and quality of life [19, 20]. Physical performance measures assess multi-joint movements, commonly required in daily life [21•]. Of the several tests recommended, the most common is usual gait speed over 4 m [2,3,4, 7••]. Often part of a comprehensive geriatric assessment, usual gait speed has been referred to as the “5th vital sign” and is simple, inexpensive, and quick to administer [22, 23]. Some facilities mark out a course and assess a patient while ambulating from the waiting area to the exam room or as part of the visit history intake. Epidemiological and intervention-based data show a strong association between low gait speed (< 0.8 m/s) and future incidence of adverse health outcomes and loss of independence [24, 25]. In addition to usual gait speed, the Short Physical Performance Battery (SPPB) score (composite of static balance, 4-m gait speed, and timed 5 chair stands), the Timed Up and Go test or longer walking tests (e.g., 400-m walk, 6-min walk) can be used to assess physical performance and document changes in patient function over time [7••, 26]. The latest summary of recommended endpoints for both clinical and research use, and the cut-points to diagnose sarcopenia can be found in the recent definition update from the European Working Group on Sarcopenia in Older Persons [7••]. The prevalence of sarcopenia is dependent upon the definition used, sex (e.g., women > men), race (e.g., Asian > Caucasian > Black), global location, and place of residence (e.g., nursing home > community) [27]. The lack of a universally accepted definition of sarcopenia makes determining the prevalence and the associated personal and societal costs difficult. However, an estimate using the most commonly used definition suggests that sarcopenia can be found in up to 29% of community-dwelling older adults > 65 years old [27], while a conservative definition estimated a prevalence as low as 1–3% [3].
Public Health Concern and Patient Characteristics and Risk
Sarcopenia is a growing global public health problem caused by the rapid aging of societies, the increasing number of older adults, and the associated personal, societal, and economic costs of adverse health outcomes and dependence. The fastest growing segment of many societies is those aged 65 years and older [28]. Due largely to the increase in older adults as a percentage of the overall population plus a stable life expectancy of approximately 80 years in industrialized countries, the World Health Organization estimates the number of older men and women will be more than triple by the year 2050 [28]. The healthcare burden of sarcopenia is due to the elevated risks for a variety of adverse health outcomes [29].
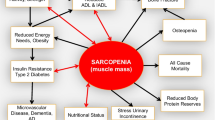
Muscle weakness, primarily of the lower extremities, alone or in conjunction with low lean body mass, is associated with a decline in physical function, mobility impairment, and increased physical disability [13, 28, 29]. Further, these states are associated with a reduced quality of life. Independently, the decline in muscle mass and function are risk factors for osteoporosis, which has been shown to frequently occur concurrent with sarcopenia [30]. The increased prevalence of both osteoporosis and sarcopenia has prompted the proposed use of the label “osteosarcopenia” to describe patients with both conditions [30]. In addition, low muscle mass and function are associated with a higher risk for adverse outcomes in patients with comorbidities including type 2 diabetes and obesity [31, 32], and with the exacerbation of functional decline associated with cancer, stroke, heart failure, and hip fracture [9, 11, 33, 34]. The risk for falls and fractures can be more than four times greater in someone with sarcopenia as compared to a healthy age-matched peer [35]. Health care costs associated with sarcopenia include increased hospitalizations, hospital lengths of stay, and readmission [36•, 37]. Furthermore, sarcopenia and its attendant skeletal muscle and mobility dysfunction are predictive of increased mortality in both middle aged and older adults [38]. These personal and illness-related expenses translate to measurable societal and economic costs of disability, while the loss of independence increases the demand on community resources for in-home and institutional care. Even the most industrialized nations lack the infrastructure and professional and skilled work force required to provide care to the rapidly increasing number of older people with sarcopenia and other related disabling conditions.
Treatment Guidelines
Recently, the first formal treatment guidelines for sarcopenia were published; these include recommendations for screening and interventions for men and women aged 65 years and older (summarized in Table 2) [16••]. The evolving definition of sarcopenia has limited the number of high quality, randomized controlled trials in this specific patient population. However, these initial guidelines were drafted using published data in men and women with sarcopenia where available, and using data from older adults with similar skeletal muscle and mobility characteristics when sufficient sarcopenic patient data were lacking. Screening for sarcopenia is recommended annually or following a major health event (e.g., a fall requiring medical care or bedrest for more than 3 days) for adults 65 years of age or older. Screening can be performed either by questionnaire (e.g., SARC-F) or by the assessment of usual gait speed. The SARC-F is a five-item screening tool developed to identify people at risk for sarcopenia and associated adverse health consequences [39]. The five questions assess self-reported difficulties in the domains of strength, walking, rising from a chair, climbing stairs, and falls; the total score ranges from 0 to 10 with a score > 4 predictive of sarcopenia. The SARC-F is simple, inexpensive, quick to administer, and designed to be used in the clinic. For patients who screen “positive” (i.e., total score > 4), it is prudent to make certain positive responses are not due to comorbid medical conditions (e.g., difficulty rising from a chair or climbing stairs due to significant hip or knee osteoarthritis). As described earlier, usual gait speed over 4 m is a simple performance assessment that quantifies a person’s normal walking speed [19, 24]. Gait speed assessment only requires a stopwatch and a measured course, can be easily administered in the clinic setting (e.g., hallway), and can be completed by most ambulatory older adults. Individuals who meet the screening criterion for the SARC-F (≥ 4 points) or gait speed over 4 m (< 0.8 m/s or time > 5 s) should be referred for evaluation of lean body mass to confirm the diagnosis of sarcopenia [16••]. If confirmed, the International Classification of Diseases (ICD-10) code for sarcopenia (M62.84) may be utilized.
The first-line therapy recommendation for sarcopenia includes the non-pharmacological approaches of exercise and diet [16••]. Among the various forms of exercise, resistance training has shown to be the most effective for maintaining and increasing muscle mass and strength and improving functional performance in older adults [40,41,42,43]. Resistance exercise can be performed using a variety of methods including machines, dumbbells/free weights, resistance bands, or body weight at various speeds and using single or multi-joint movements. To date, no single exercise regimen has been identified as being the most efficacious for improving muscle mass, strength, and function, although several have been successful at improving one or more of these characteristics [41, 42••, 43,44,45,46].
A review of studies with healthy older adults suggests that a resistance exercise program to improve strength should be performed twice weekly, contain 8–10 basic exercises including the major muscle groups of the upper and lower extremities, should be performed at approximately 75% of a person’s one-repetition maximum (1-RM; i.e., maximal effort) with 10–12 repetitions per set of each exercise, and with each repetition requiring approximately 6 s of muscle tension [42••]. Typically, the longer a person performs the intervention (i.e., more weeks) the greater the benefits though increases tend to be less pronounced after 12–24 weeks. To increase mass, a program of similar duration, would generally require three sessions per week, 2–3 sets per exercise with 7–9 repetitions per set performed at approximately 60–80% of the 1-RM [42••]. A second review in older adults identified as “physically frail,” showed that 1–6 exercise sessions per week, performing 1–3 sets and 6–15 repetitions per set of resistance exercise at an intensity of 30–70% of 1-RM led to increases in muscle strength and power as well as functional performance [47].
While resistance training may be preferred for addressing muscle weakness and lower functional performance, increasing the overall level of physical activity is independently beneficial for overall health [40]. Regular physical activity of approximately 150 min per week at moderate or greater intensity (e.g., 5 or greater intensity on a 0–10 scale) has numerous health benefits, including up to a 50% reduction in relative risk of developing limitations in physical function and a 31% reduction in mortality [40, 48, 49]. For patients unfamiliar with resistance exercise training or physical activity, a number of options are available for education and instruction including the following: referral to a physical therapist, identifying a certified fitness trainer (e.g., American College of Sports Medicine), and/or utilize the resources from the National Institute on Aging (https://go4life.nia.nih.gov), or patient advocacy organizations like the Arthritis Foundation (https://www.arthritis.org/living-with-arthritis/exercise/).
Optimizing a patient’s diet is the other key component of first-line therapy. A reduction in the level of dietary protein intake, independent of exercise, can increase the risk of a loss of muscle mass and strength, and expedite the onset of mobility disability [50,51,52]. Adequate daily protein and calorie intakes are essential to provide the necessary nutrients and energy to promote positive nitrogen balance and to maintain muscle mass and function [51, 53,54,55]. Protein intake of ≥ 1.0–1.2 g/kg/day can reduce the likelihood of becoming dependent in several daily tasks (e.g., climbing stairs, walking 800 m, lifting heavy objects) and the onset of mobility disability by 41–86% [51, 52]. To achieve this level of nutrition intake, patient education and counseling, typically from a registered dietitian, for an overall healthy diet and possible use of an oral nutritional supplement are recommended. Regardless of the lack of sufficient robust evidence to suggest the direct benefits of dietary protein intake alone to increase muscle mass, strength, and physical function in people with sarcopenia, it is highly unlikely these salutary effects would be achieved with inadequate nutrition [51, 54]. Notably, a recent randomized placebo controlled trial examining 0.8 vs. 1.3 g/kg/day of protein in conjunction with testosterone supplementation in older functionally impaired men found no additional improvements in muscle mass or strength with the higher protein intake [55]. Although data in patients with sarcopenia are scarce, it is recommended that nutritional and exercise interventions be combined to optimize their beneficial health effects [16••]. Proposed second-line therapy for sarcopenia focuses on quality geriatric care in managing each patient’s overall health and comorbid medical conditions as well as addressing polypharmacy.
The recent sarcopenia treatment guidelines made no recommendations for stand-alone Vitamin D supplementation or the use of anabolic hormones, due to the paucity of sufficient evidence in older adults with sarcopenia and the often-inconclusive data available with these interventions in healthy or non-sarcopenic populations [55, 56]. Pharmacotherapy was also not recommended. The area of drug development in muscle wasting conditions, including sarcopenia, is active with several approaches being studied [6, 57•]. While some success has been achieved in stimulating muscle hypertrophy, data suggesting a consistent translation of this increase in muscle mass to significant improvements in physical function are lacking [58,59,60].
Conclusion
Sarcopenia is currently defined as a systemic disease of skeletal muscle characterized by weakness and atrophy that can compromise intrinsic functional capacity in older adults and leads to an increased risk of numerous adverse health outcomes including a loss of independence. Decreases in skeletal muscle mass, strength, and function (e.g., walking speed) that comprise the main criteria for sarcopenia are often accepted as “normal aging” rather than a symptom of a disease that can be ameliorated by exercise and nutritional interventions. The rapid aging of global societies and the corresponding increase in the number of older adults worldwide make sarcopenia a public health concern that requires the identification and treatment of individuals at risk. If left unchecked, this massive “wave” of older people will overwhelm the health care systems of many countries. New international treatment guidelines recommend that at risk patients identified through clinical screening participate in resistance exercise programs and consume a healthy diet with sufficient protein and calories. Prescribing and supporting a healthier lifestyle with sufficient resistance exercise, general physical activity, and quality nutritional intake, will result in a variety of health benefits for older patients. In addition, clinicians are encouraged to provide quality geriatric care to address comorbid medical issues including polypharmacy. The development of new, viable nutritional, and pharmaceutical treatment options for sarcopenia is progressing with results of recent trials expected in the near future. The recent refinement of diagnostic criteria and evidence-based treatment recommendations are advancing the management of sarcopenia as a treatable disease for a rapidly expanding population of patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rosenberg I. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutrition. 1989;50:1231–3.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. European working group on sarcopenia in older people. Sarcopenia: European consensus on definition and diagnosis report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–23.
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–58.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. JAMDA. 2014;15:95–101.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64.
Rooks D, Roubenoff R. Development of pharmacotherapies for the treatment of sarcopenia. J Frailty Aging 2019;8(3):120–130. https://doi.org/10.14283/jfa.2019.11.
•• Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. https://doi.org/10.1093/ageing/afy169. This is a formal update of the commonly used consensus definition from 2010. The update reprioritizes muscle weakness as the primary clinical symptom and recommends lean mass be used for confirmation of sarcopenia; it also introduces modified parameter cutoffs.
Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14:85–99.
Kinugasa Y, Yamamoto K. The challenge of frailty and sarcopenia in heart failure with preserved ejection fraction. Heart. 2017;103:184–9.
Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26:219–28.
Landi F, Calvani R, Ortolani E, Salini S, Martone AM, Santoro L, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28:1569–76.
Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer Cachexia: beyond weight loss. J Oncol Pract. 2016;12:1163–71.
Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1076–81.
Meek AC, Madill J. Sarcopenia in liver transplantation: a review. Clin Nutr ESPEN. 2017;22:76–80.
Anker SD, Coats AJS, Morley JE, Rosano G, Bernabei R, von Haehling S, et al. Muscle wasting disease: a proposal for a new disease classification. J Cachexia Sarcopenia Muscle. 2014;5:1–3.
•• Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. Nutr Health Aging. 2018:22, 1148–1161. This paper details the first global clinical guidelines for sarcopenia that cover the continuum of care – screening, diagnosis, first-line and second-line treatment, and research.
Alley DE, Shardell MD, Peters KW, McLean RR, Dam TTL, Kenny AM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–66.
• Cawthon PM, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al.; Sarcopenia Definition and Outcomes Consortium Conference participants. Establishing the link between lean mass and grip strength cut-points with mobility disability and other health outcomes: proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J Gerontol A Biol Sci Med Sci 2019 14. pii: glz081. doi: https://doi.org/10.1093/gerona/glz081. Using data from several large, longitudinal studies in older adults, this paper summarizes findings to suggest that grip strength is an important discriminator of mobility disability and other endpoints.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94.
Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, Alfaro-Acha A, Rodriguez-Mañas L, Ara I, et al. The sit-to-stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Exp Gerontol. 2018;112:38–43.
• Richardson E, Burnell J, Adams HR, Bohannon RW, Bush EN, Campbell M, et al. Developing and implementing performance outcome assessments: evidentiary, methodologic, and operational considerations. Ther Innov Regul Sci. 2019;53:146–53. This paper addresses the challenges and clarifies a path forward for using performance measures to assess physical and cognitive function in the clinic and research studies.
Cesari M. Role of gait speed in the assessment of older patients. JAMA. 2011;305:93–4.
Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68:39–46.
Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8.
Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58:S329–36.
Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–9.
Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748–59.
WHO Clinical Consortium on healthy ageing 2017 – report of consortium meeting, 21–22 November 2017 in Geneva, Switzerland. Geneva: world health Organization; 2018.
Beaudart C, Rizzoli R, Bruyère O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72:45.
Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017;28:2781–90.
Bianchi L, Volpato S. Muscle dysfunction in type 2 diabetes: a major threat to patient's mobility and independence. Acta Diabetol. 2016;53:879–89.
Buch A, Carmeli E, Boker LK, Marcus Y, Shefer G, Kis O, et al. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age--an overview. Exp Gerontol. 2016;76:25–32.
Fukushima H, Koga F. Impact of sarcopenia in the management of urological cancer patients. Expert Rev Anticancer Ther. 2017;17:455–66.
Ryan AS, Ivey FM, Serra MC, Hartstein J, Hafer-Macko CE. Sarcopenia and physical function in middle-aged and older stroke survivors. Arch Phys Med Rehabil. 2017;98:495–9.
Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Hayashida I, Kusabiraki T, et al. Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European working group on sarcopenia in older people. Arch Gerontol Geriatr. 2014;59:295–9.
• Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0169548. This paper summarizes the health-related consequences of people with sarcopenia, using studies with an appropriate definition to select participants.
Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. BMC Geriatr. 2018;18:188. https://doi.org/10.1186/s12877-018-0878-0.
Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. J Am Med Dir Assoc. 2015;16:607–13.
Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36.
Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–30.
Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–44.
•• Borde R, Hortobágyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45:1693–720. This paper described the dose of resistance training exercise associated with increases in skeletal muscle strength and mass reported in randomized clinical trials involving healthy older adults.
Lai CC, Tu YK, Wang TG, Huang YT, Chien KL. Effects of resistance training, endurance training and whole-body vibration on lean body mass, muscle strength and physical performance in older people: a systematic review and network meta-analysis. Age Ageing. 2018;47:367–73.
Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–34.
Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60:1425–31.
Lozano-Montoya I, Correa-Pérez A, Abraha I, Soiza RL, Cherubini A, O'Mahony D, et al. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: a systematic overview - the SENATOR Project ONTOP Series. Clin Interv Aging. 2017;12:721–40.
Lopez P, Pinto RS, Radaelli R, Rech A, Grazioli R, Izquierdo M, et al. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin Exp Res. 2018;30:889–99.
Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175:959–67.
WHO World Report on Ageing and Health. World Health Organization; 2015.
Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18:248–53.
Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, et al. Protein intake and mobility limitation in community-dwelling older adults: the health ABC study. J Am Geriatr Soc. 2017;65:1705–11.
Bradlee ML, Mustafa J, Singer MR, Moore LL. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J Gerontol A Biol Sci Med Sci. 2017;73:88–94.
Nowson C, O’Connell S. Protein requirements and recommendations for older people: a review. Nutrients. 2015;7:6874–99.
Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2018;37:1121–32.
Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, Huang G, et al. Effect of protein intake on lean body mass in functionally limited older men. JAMA Int Med. 2018;178:530–41.
Verlaan S, Maier AB, Bauer JM, Bautmans I, Brandt K, Donini LM, et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults - the PROVIDE study. Clin Nutr. 2018;37:551–7.
• Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int. 2016;98:319–33. This paper provides a broad overview of sarcopenia and the various pharmacotherapeutic options being evaluated.
Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, et al. Treatment of sarcopenia with bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017;65:1988–95.
Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–57.
Papanicolaou DA, Ather SN, Zhu H, Zhou Y, Lutkiewicz J, Scott BB, et al. A phase IIA randomized, placebo controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17:533–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Daniel Rooks has a patent for the use of bimagrumab in sarcopenia pending. In addition, he is a full time employee of the Novartis Institutes of BioMedical Research. However, Dr. Rooks reports that no recommendation or data regarding any drug developed by or marketed by Novartis is included in the manuscript, and that the focus of the paper is non-drug approaches to care for sarcopenia.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Geriatric Rehabilitation
Rights and permissions
About this article
Cite this article
Rooks, D. Sarcopenia: a Muscle Disease with Decreased Functional Capacity and an Increased Risk of Adverse Health Outcomes. Curr Phys Med Rehabil Rep 7, 290–296 (2019). https://doi.org/10.1007/s40141-019-00236-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-019-00236-5