Abstract
Purpose
Accurate glenoid component placement in total shoulder arthroplasty (TSA) remains challenging even with preoperative planning, especially for variable glenoid erosion patterns in the coronal plane.
Methods
We retrospectively reviewed 170 primary TSAs in which preoperative planning software was used. After registration of intraoperative bony landmarks, surgeons were blinded to the navigation screen and attempted to implement their plan by simulating placement of a central-axis guide pin: 230 screenshots of simulated guide pin placement were included (aTSA = 66, rTSA = 164). Displacement, error in version and inclination, and overall malposition from the preoperatively-planned target point were stratified by the Favard classification describing superior-inferior glenoid wear: E0 (n = 89); E1 (n = 81); E2 (n = 29); E3(n = 29); E4(n = 2). Malposition was considered > 10° for version/inclination errors or > 4 mm displacement from the starting point.
Results
Mean displacement error was 3.5 ± 2.7 mm (aTSA = 2.7 ± 2.3 mm, rTSA = 3.8 ± 2.9 mm), version error was 5.7 ± 4.7° (aTSA = 5.8 ± 4.4°, rTSA = 5.7 ± 4.8°), inclination error was 7.1 ± 5.6 (aTSA = 4.8 ± 4.8°, rTSA = 8.1 ± 5.7°), and malposition rate was 53% (aTSA = 38%, rTSA = 59%). When compared by Favard classification, there were no differences in any measure; when stratified by TSA type, version error differed for rTSAs (P = .038), with E1 having the greatest version error (6.9 ± 5.2°) and E3 the least (4.2 ± 3.4°). When comparing glenoids without wear (E0) and glenoids with superior wear (E2 and E3), the only difference was greater version error in glenoids without wear (6.0 ± 4.9° vs. 4.6 ± 3.7°, P = .041).
Conclusions
Glenoid malposition did not differ based on coronal glenoid morphology. Although, malposition was relatively high, suggesting surgeons should consider alternate techniques beyond preoperative planning and standard instrumentation in TSA.
Level of evidence III
Retrospective Cohort Study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence and prevalence of anatomic and reverse total shoulder arthroplasty (aTSA and rTSA) have significantly increased within the past 10 years and are projected to continue to increase at an exponential rate [1]. It is well-known that glenoid component positioning is key for successful shoulder arthroplasty; hence, implant positioning remains an important surgeon-modifiable risk factor [2]. Appropriate version can decrease the incidence of loosening in both aTSA, and appropriate position in rTSA helps to prevent scapular notching, which can lead to superior clinical outcomes given the deleterious effects of loosening and notching [3,4,5,6].
Preoperative planning software is frequently used to improve glenoid component placement, although limited visualization intraoperatively can still make accurate placement a challenge [3]. For this reason, intraoperative computer navigation has gained popularity as an adjuvant for glenoid component positioning [3]. Prior cadaveric studies have demonstrated improved accuracy and precision of glenoid positioning with navigation compared to without, and additional studies have demonstrated that navigation allows for replication of the preoperatively-planned inclination and version [7,8,9]. However, the potential cost of navigation has remained an obstacle to widespread adoption [3, 10]. For this reason, further identification of patient risk factors for component malposition may allow surgeons to selectively utilize navigation [3]. Previously, Hao et al. [3] demonstrated that posterior glenoid bone loss (Walch [11] B2 or B3) more commonly resulted in glenoid version errors exceeding 10° and component malposition in rTSA. Even without significant posterior wear, malposition rates were relatively high for both aTSA (36%) and rTSA (53%) [3]. However, it is unclear whether supero-inferior glenoid morphology influences surgeons’ success in recreating their preoperative plan intraoperatively.
In this study, we sought to determine if surgeons' ability to accurately execute their preoperative plan varied based on the native inferior-to-superior glenoid morphology as determined using the Favard classification [12]. We hypothesized that greater coronal plane deformity would lead to increasing error rates of version, inclination, and malposition when only three-dimensional preoperative planning was used in vivo during shoulder arthroplasty.
Materials and methods
This study was first approved by our Institutional Review Board. A retrospective review was then conducted of 170 primary TSAs performed with computer navigation at a single institution between September 2017 and March 2020. Since 2017, three-dimensional preoperative planning software and intraoperative computer navigation (Equinoxe Planning App and Exactech GPS; Exactech, Gainesville, FL, USA) have been used for nearly all primary aTSAs and rTSAs performed at our institution. Subsequently, four fellowship-trained shoulder surgeons have been tracking their individual accuracy at placing the glenoid implant based on their preoperative plan. Cases of primary aTSA and rTSA within the study period were included, and cases for which navigation was unable to be performed or screenshots were not available for analysis were excluded.
Pre-operative planning
Commercially available preoperative planning software was used in all TSAs with multiplanar 2-dimensional computed tomography (CT) and a three-dimensional implant overlay. Participating surgeons (always at least one attending, fellowship-trained shoulder surgeon, sometimes with one or more upper-extremity fellows) collaborated to use the planning software to determine appropriate implant placement based on patient-specific glenoid morphology. In all cases, the final decision on implant placement was made by the attending surgeon. In some cases, full-wedge augments were planned based on surgeon discretion. The planned cases were then saved and uploaded to the operating room computer navigation unit for use during surgery.
Operative technique
Each arthroplasty was performed via the deltopectoral approach, and the incision was extended approximately 1–2 cm proximal to the coracoid tip, enabling placement of the coracoid tracker for computer navigation. Management of the subscapularis was per surgeon preference. The humerus was exposed with extension and external rotation after an inferior capsular release. For aTSA, the head was cut in its native retroversion. For rTSA, the head underwent osteotomy in either 20° of retroversion or its native retroversion, per surgeon preference, using an extramedullary guide. The glenoid was then exposed in a routine fashion. The biceps if present was routinely tenodesed to the pectoralis major tendon, and the remaining proximal stump and labral tissue were removed. Any remaining cartilage was carefully removed using a Cobb elevator. Soft tissue was released off the anterior glenoid neck. The superior aspect of the coracoid was exposed using electrocautery. The coracoacromial ligament was preserved. The tracker stand was then secured in place with two screws. The glenoid bony surface was registered according to the manufacturer's protocol to link the patient's CT scan and preoperative plan to the visualized anatomy.
Study protocol
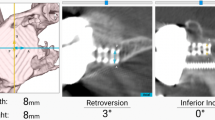
After registration, participating surgeons with knowledge of the preoperative plan were blinded to the navigation screen and attempted to implement their preoperative plan by simulating placement of a central-axis cage drill (Figs. 1 and 2). Two-hundred thirty screenshots of surgeons’ simulated guide pin placement were included (aTSA = 66, rTSA = 164). Displacement, error in version and inclination, and overall malposition from the preoperatively-planned target point were stratified by the Favard classification [12] describing superior-inferior glenoid wear: E0 (n = 89) = superior humeral migration with no glenoid erosion; E1 (n = 81) = concentric glenoid erosion; E2 (n = 29) = glenoid erosion predominantly in the superior pole; E3 (n = 29) = global glenoid erosion more severe in the superior pole; E4 (n = 2) = glenoid erosion predominantly in the inferior pole. All glenoids were classified according to the Favard classification [12] using preoperative CTs by a single fellowship-trained upper-extremity surgeon (R.C.S). Components were considered malpositioned for version/inclination errors > 10° or displacement from the starting point > 4 mm.
Example of a computer navigation screenshot showing surgeon-blinded simulated placement of the central-axis guide pin (yellow outline), attempting to match the preoperative plan (blue outline, 1° of retroversion and 3° of superior inclination); the values for the starting point location, version, and inclination for the simulation were then compared with the preoperatively planned component position to determine displacement, version and inclination error, and malposition. P, posterior; A, anterior; S, superior; I, inferior
Statistical analysis
Displacement, version error, and inclination error were compared based on specific glenoid Favard classification [12] (E0, E1, E2, E3, E4) using a one-way analysis of variance (ANOVA) test and stratified by procedure. Malposition rates were compared using Fisher’s Exact test. Additionally, we performed a sub-analysis comparing glenoids with no wear (E0) to glenoids with superior wear (E2 or E3). We also assessed the proportion of glenoids with version and inclination error < 5°, between 5 and 10°, and > 10°. All statistics were performed using R Software (version 4.2.0, R Core Team, Vienna, Austria), and the significance was set at a P value of 0.05.
Results
We included 230 simulated guide pin screen shots from 170 primary TSAs performed during the study period. Seventy percent of images were from rTSA cases. The mean age at surgery was 69.0 ± 9.1 years (range, 38–86 years), and 53% of included patients were female. The Favard classification [12] of included images were as follows: E0 in 89; E1 in 81; E2 in 29; E3 in 29; E4 in 2.
Displacement error
Mean displacement error was 3.5 ± 2.7 mm (aTSA = 2.7 ± 2.3 mm, rTSA = 3.8 ± 2.9 mm) (Table 1). Displacement error did not differ based on Favard classification [12] overall (P = 0.829; E0 = 3.5 ± 3.0 mm, E1 = 3.4 ± 2.8 mm, E2 = 3.2 ± 1.9 mm, E3 = 3.8 ± 2.4 mm, E4 = 2.0 ± 0.4 mm) nor when stratified based on aTSA versus rTSA. There was no difference in displacement error between glenoids without wear (E0) and glenoids with superior wear (E2 or E3) (3.5 ± 3.0 vs. 3.5 ± 2.2, P = 0.979) (Table 2).
Version error
Mean version error was 5.7 ± 4.7° (aTSA = 5.8 ± 4.4°, rTSA = 5.7 ± 4.8°). Mean version error did not differ based on Favard classification [12] overall (P = 0.297; E0 = 6.0 ± 4.9°, E1 = 6.2 ± 5.0°, E2 = 4.6 ± 3.7°, E3 = 4.6 ± 3.7°, E4 = 4.5 ± 4.9°) nor when stratified by aTSA versus rTSA. No differences in version error were found when grouped (Fig. 3A; P = 0.574). Glenoids without wear had greater version error compared to superiorly-worn glenoids (6.0 ± 4.9 vs. 4.6 ± 3.7, P = 0.041).
Inclination error
Mean inclination error was 7.1 ± 5.6 (aTSA = 4.8 ± 4.8°, rTSA = 8.1 ± 5.7°). Mean inclination error did not differ based on Favard classification [12] overall (P = 0.764; E0 = 7.2 ± 5.6°, E1 = 6.6 ± 5.7°, E2 = 7.4 ± 5.6°, E3 = 8.2 ± 5.7°, E4 = 6.0 ± 5.7°) nor when stratified by aTSA versus rTSA. No differences in inclination error were found when grouped (Fig. 3B; P = 0.833). There was no difference in inclination error between glenoids without wear and glenoids with superior wear (7.2 ± 5.6 vs. 7.8 ± 5.6, P = 0.527) (Figs. 2 and 3).
Glenoid malposition
The malposition rate was 53% (aTSA = 38%, rTSA = 59%). Although the malposition rate was mildly greater in E3 glenoids, there were no statistically significant differences found based on Favard classification overall (P = 0.381; E0 = 53%, E1 = 51%, E2 = 48%, E3 = 66%, E4 = 0%) nor when stratified by aTSA versus rTSA. There was no difference in the malposition rate between glenoids without superior wear and those with (53% vs. 57%, P = 0.735).
Discussion
Preoperative planning software has been increasingly used to improve glenoid component placement [3]. Given these reported benefits, we sought to determine if surgeons' ability to accurately execute their preoperative plan varied based on the native coronal plane glenoid morphology as determined using the Favard classification [12]. Our findings suggest that increasing coronal plane deformity does not reduce surgeon’s ability to replicate their preoperative plan when standard instrumentation is used, as glenoid component displacement, version error, inclination error, and overall malposition did not differ based on coronal glenoid morphology. Still, malposition rates were relatively high in this cohort (53%).
Proper glenoid implant position remains critical as failure rates are increased with component malposition [3, 7]. Hussey et al. [13] in their study of 344 TSAs found that native glenoids with eccentric wear preoperatively are associated with a greater than twofold increased rate of glenoid component loosening compared with glenoids with concentric wear in aTSA. Subsequently, Hao et al. [3] demonstrated in 170 primary TSAs that an increased error in version from the preoperative plan for glenoids with posterior wear compared to those without for rTSA (8.1° ± 5.6° vs. 4.7° ± 4.0°; P < 0.001), but not aTSA (5.7° ± 3.8° vs. 5.8° ± 5.0°, P = 0.875) when the baseplate was placed with preoperative planning and standard instrumentation alone. If glenoid loosening is further substantiated to result from glenoid malpositioning, technologies such as patient-specific instrumentation and intraoperative navigation may be beneficial for optimal glenoid placement to improve implant survivorship [13, 14]. While the optimal implant positioning remains debated, superior placement or improper tilt of the glenosphere has been associated with increased rates of scapular notching in rTSA [5, 6, 15], which in-turn may lead to less favorable patient outcomes and decreased range of motion in flexion and abduction [4]. In patients with difficult surgical exposure or complex glenoid morphology, intraoperative navigation in addition to preoperative planning may provide a substantial benefit [9, 16,17,18]. However, our results suggest supero-inferior glenoid morphology alone does not confer a differential rate of component placement error or overall malposition.
Three-dimensional imaging provides a clear advantage over two-dimensional radiographs for assessment of glenoid morphology, extent and location of glenoid bone loss, and planning of ideal implant geometry and placement [19]. These factors facilitate improved placement of the glenoid component in the desired location [19, 20]. Neutral version of the glenoid implant in aTSA and avoidance of retroversion of the glenoid in rTSA can decrease the incidence of loosening [3, 4]. Previously, utilizing the Walch classification [11] for glenoid morphology and comparisons, Hao et al. [3] following their findings of significantly greater version error in rTSA for glenoids with posterior wear than for those without, concluded that in order to limit potential complications such as implant loosening, use of intraoperative navigation or patient-specific instrumentation should be strongly considered in rTSA cases with posterior glenoid bone loss. Further, Sadoghi et al. [10] in a meta-analysis including 247 shoulders from five studies found that glenoid version was significantly more accurate when computer navigation was used compared with standard instrumentation alone.
Version error has been shown to be significantly higher for retroverted glenoids (Walch B2/B3) [3]. Contrarily, in the coronal plane, glenoids without superior wear (E0) had greater version error compared to superiorly-worn glenoids (E2, E3) (P = 0.041), but no difference in displacement error, inclination error, or malposition rate. It is possible this has to do with easier access for placement of the guide pin with the approach and exposure if there is superior wear. Unfortunately, bone loss is a continuum and involves complex multiplanar patterns than do not always fit uniformly into the Walch [11] or Favard [12] classification. Even in the hands of high volume fellowship-trained surgeons, malpositioning can occur in more than one third of cases [7]. It is in these cases of significantly altered anatomy that surgeons may consider the use of navigation or patient-specific guides in order to minimize component malposition and the subsequent long term sequalae that may lead to poorer outcomes and/or revision surgery. In the case of glenoid deformity and bone loss, these advantages may be more important.
However, there are limitations to the current study. It is possible that selection bias exists in the dataset, as cases with difficult exposure, limiting visualization, may have limited the use of navigation or caused data collection steps to be missed. While the single institution design with all surgeons following the same protocol allows for the generation of a homogenous dataset but may limit generalizations outside the institution. In addition, both fellowship-trained attending surgeons and current fellow trainees were involved in the study, which may have contributed to elevated malposition rates, but also represents real world practice where low volume surgeons perform the majority of TSA in the United States [21]. Additionally, non-fellowship trained surgeons also routinely perform this procedure and are at increased risk of malposition errors compared to attending surgeons (58% vs. 38%, P = 0.047) [7]. Of note, the evaluation of surgeon ability to replicate the preoperative plan was based on hand position in space at the time of implant central-axis boss preparation, not on final implant position; however, this replicates many current systems that use a central-axis guide pin for glenoid preparation. Postoperative CT scans to evaluate final glenoid implant position were not obtained. However, prior CT studies have demonstrated excellent accuracy of the navigation system with final implant position within 5° of the preoperative plan [9]. Finally, the current study did not assess clinical outcomes such as implant survivorship. Hence, conclusions regarding the clinical benefit of intraoperative navigation during shoulder arthroplasty could not be made.
Conclusion
Glenoid component displacement, inclination error, and overall malposition did not differ based on coronal plane glenoid morphology as defined by the Favard classification. Version error was higher in glenoids without superior wear compared to those with superior wear. Malposition was relatively high in our cohort, suggesting that surgeons should consider alternate techniques beyond preoperative planning and standard instrumentation when performing shoulder arthroplasty. Further studies are needed to determine the clinical benefit of the improved glenoid position obtained via intraoperative navigation.
References
Wagner ER, Farley KX, Higgins I et al (2020) The incidence of shoulder arthroplasty: rise and future projections compared with hip and knee arthroplasty. J Shoulder Elbow Surg 29:2601–2609. https://doi.org/10.1016/j.jse.2020.03.049
Zhang M, Junaid S, Gregory T et al (2019) Effect of baseplate positioning on fixation of reverse total shoulder arthroplasty. Clin Biomech 62:15–22. https://doi.org/10.1016/j.clinbiomech.2018.12.021
Hao KA, Sutton CD, Wright TW et al (2022) Influence of glenoid wear pattern on glenoid component placement accuracy in shoulder arthroplasty. JSES Int 6:200–208. https://doi.org/10.1016/j.jseint.2021.11.021
Ho JC, Sabesan VJ, Iannotti JP (2013) Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg-Am Vol 95(12):e82-1-8. https://doi.org/10.2106/JBJS.L.00336
Jang YH, Lee JH, Kim SH (2020) Effect of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: a meta-analysis. Bone Jt J 102:1438–1445. https://doi.org/10.1302/0301-620X.102B11.BJJ-2020-0449.R1
Patel M, Martin JR, Campbell DH et al (2021) Inferior tilt of the glenoid leads to medialization and increases impingement on the scapular neck in reverse shoulder arthroplasty. J Shoulder Elbow Surg 30:1273–1281. https://doi.org/10.1016/j.jse.2020.09.023
Schoch BS, Haupt E, Leonor T et al (2020) Computer navigation leads to more accurate glenoid targeting during total shoulder arthroplasty compared with 3-dimensional preoperative planning alone. J Shoulder Elbow Surg 29:2257–2263. https://doi.org/10.1016/j.jse.2020.03.014
Jones RB, Greene AT, Polakovic SV et al (2020) Accuracy and precision of placement of the glenoid baseplate in reverse total shoulder arthroplasty using a novel computer assisted navigation system combined with preoperative planning: a controlled cadaveric study. Semin Arthroplasty JSES 30:73–82. https://doi.org/10.1053/j.sart.2020.05.004
Nashikkar PS, Scholes CJ, Haber MD (2019) Computer navigation re-creates planned glenoid placement and reduces correction variability in total shoulder arthroplasty: an in vivo case-control study. J Shoulder Elbow Surg 28:e398–e409. https://doi.org/10.1016/j.jse.2019.04.037
Sadoghi P, Vavken J, Leithner A, Vavken P (2015) Benefit of intraoperative navigation on glenoid component positioning during total shoulder arthroplasty. Arch Orthop Trauma Surg 135:41–47. https://doi.org/10.1007/s00402-014-2126-1
Walch G, Badet R, Boulahia A, Khoury A (1999) Morphologic study of the Glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 14:756–760. https://doi.org/10.1016/S0883-5403(99)90232-2
Sirveaux F, Favard L, Oudet D et al (2004) Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 86:388–395. https://doi.org/10.1302/0301-620X.86B3.14024
Hussey MM, Steen BM, Cusick MC et al (2015) The effects of glenoid wear patterns on patients with osteoarthritis in total shoulder arthroplasty: an assessment of outcomes and value. J Shoulder Elbow Surg 24:682–690. https://doi.org/10.1016/j.jse.2014.09.043
McLendon PB, Schoch BS, Sperling JW et al (2017) Survival of the pegged glenoid component in shoulder arthroplasty: part II. J Shoulder Elbow Surg 26:1469–1476. https://doi.org/10.1016/j.jse.2016.12.068
Friedman RJ, Barcel DA, Eichinger JK (2019) Scapular notching in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 27:200–209. https://doi.org/10.5435/JAAOS-D-17-00026
Hones KM, King JJ, Schoch BS et al (2021) The in vivo impact of computer navigation on screw number and length in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 30:e629–e635. https://doi.org/10.1016/j.jse.2021.01.017
Nashikkar PS, Scholes CJ, Haber MD (2019) Role of intraoperative navigation in the fixation of the glenoid component in reverse total shoulder arthroplasty: a clinical case-control study. J Shoulder Elbow Surg 28:1685–1691. https://doi.org/10.1016/j.jse.2019.03.013
Holzgrefe RE, Hao KA, Panther EJ et al (2023) Early clinical outcomes following navigation-assisted baseplate fixation in reverse total shoulder arthroplasty: a matched cohort study. J Shoulder Elbow Surg 32:302–309. https://doi.org/10.1016/j.jse.2022.07.007
Iannotti JP, Weiner S, Rodriguez E et al (2015) Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Jt Surg 97:651–658. https://doi.org/10.2106/JBJS.N.00493
Scalise JJ, Codsi MJ, Bryan J et al (2008) The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Jt Surg-Am 90:2438–2445. https://doi.org/10.2106/JBJS.G.01341
Hammond JW, Queale WS, Kim TK, McFarland EG (2003) Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. JBJS 85:2318
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mr. Hao has a consultancy agreement with LinkBio Corp. Dr. Thomas Wright is a consultant and receives royalties from Exactech, Inc. Dr. King is a consultant for Exactech, Inc and LinkBio Corp. Dr. Schoch is a paid consultant for Exactech. He receives royalties from Exactech, Innomed and Responsive Arthroscopy. Dr. Farmer is consultant for Exactech, Inc and Arthrex Inc. Dr. Srinivasan is a paid consultant for Acumed, Exsomed LLC, Turner Imaging, and Hinge Health. The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hagan, D.P., Hao, K.A., Hones, K.M. et al. Glenoid component placement accuracy in total shoulder arthroplasty with preoperative planning and standard instrumentation is not influenced by supero-inferior glenoid erosion. Eur J Orthop Surg Traumatol 33, 3159–3165 (2023). https://doi.org/10.1007/s00590-023-03546-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-023-03546-6