Abstract
Background
While the use of computer-assisted navigation systems in prosthetic implantation is steadily increasing, its utility in reverse shoulder arthroplasty (RSA) remains unclear. The purpose of this study was to evaluate the clinical utility of an intraoperative navigation system in patients undergoing RSA.
Materials and methods
Patients undergoing navigated or standard RSA at a single institution between September 2020 and December 2021 were prospectively enrolled. Exclusion criteria included noncompliance with study procedures or humeral fracture. Outcome measures included postoperative version and inclination, range of motion (ROM), complications, and patient-reported outcome measurements (PROMs: American Shoulder and Elbow Surgeons score [ASES], Disabilities of the Arm, Shoulder, and Hand score [DASH], Simple Shoulder Test [SST], and Visual Analog Scale [VAS]) at final follow-up.
Results
The final cohort contained 16 patients with navigation and 17 with standard RSA at a mean follow-up of 16 months (range 12–18 months). Average age was 72 years (range 66–80 years), 8 male (24%) and 25 female (76%). There were no differences in demographics between groups (p > 0.05). At baseline, the navigated group had a greater proportion of Walch B1 and B2 glenoids (p = 0.04). There were no differences between groups regarding baseplate type and native/planned/postoperative glenoid version and inclination. In both groups, planned and postoperative versions were not significantly different (p = 0.76). Patients who did not have navigation demonstrated significant differences between planned and postoperative inclination (p = 0.04), while those with navigation did not (p = 0.09). PROM scores did not differ between groups at final follow-up for SST (p = 0.64), DASH (p = 0.38), ASES (p = 0.77), or VAS (p = 0.1). No difference in final ROM was found between groups (p > 0.05). Over 50% of all screws in both groups were positioned outside the second cortex (p = 0.37), albeit with no complications.
Conclusions
There were no statistically significant differences in ROM, PROMs, and satisfaction between patients receiving computer-navigated and standard RSA at a short-term follow-up. Despite more severe preoperative glenoid erosion in the navigated group, all patients were able to achieve an appropriate neutral axis postoperatively. The cost effectiveness and appropriate use of computer-navigated RSA warrant specific investigation in future studies.
Level of evidence: II, prospective cohort study.
Trial registration: 9/1/2020 to 12/31/2021.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long-term survival of reverse shoulder arthroplasty (RSA) largely depends on stable bone fixation and accurate positioning of the glenoid component [1]. Either poor fixation or misplacement of the glenoid component can lead to early mobilization (cut-out), instability, scapular notching, and possible failure [2, 3]. Instability and mobilization constitute two of the most common causes of early revision surgery, at rates of 38.5% and 18.0%, respectively [4]. By contrast, aseptic loosening of the glenoid component is the leading cause of long-term clinical failure for both anatomic prosthesis and RSA; therefore, accurate positioning is paramount in preventing instability and ensuring postoperative survival [5, 6]. In doing so, this facilitates correct version and inclination, while avoiding excessive medialization which would otherwise impede an adequate bone stock for fixation. Proper attention to detail with regard to these elements is imperative in promoting adequate shoulder movement and avoiding rapid deterioration postoperatively.
While several authors have theorized the ideal position for the baseplate and screws when performing a RSA, accurate placement remains technically difficult for the surgeon given the relatively blind nature of the procedure coupled with the complex anatomy of the scapula [7,8,9]. Walch and Favard have individually developed two classification systems categorizing various gradations of glenoid wear in the axial and coronal planes [10, 11]. In instances of symmetrical glenoid wear (Walch type A1 and A2), the surface is well balanced and reamed to the subchondral bone, thereby maintaining individual retroversion. However, in cases of pathological retroversion with posterior wear (Walch type B1, B2, or C), the retroversion must be corrected utilizing asymmetrical reaming or posterior augmentation, with the extent of subsequent correction remaining an estimate. The use of computer-assisted navigation systems in prosthetic implantation is steadily increasing [12, 13]. Albeit promising, the utility of intraoperative computer-assisted navigation in handling shoulder prosthetics remains unclear [14].
Therefore, the purpose of this study was to evaluate the clinical utility of an intraoperative navigation system in patients undergoing RSA and how it may affect motion, patient-reported outcomes, and complications.
Materials and methods
Study design
This prospective cohort investigation was reviewed and approved by our Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Thirty-five consecutive patients scheduled to undergo RSA for rotator cuff tear arthropathy at our institution between September 2020 and December 2021 who provided their informed consent were enrolled in this prospective non-randomized study. Patients were excluded if they (1) had cognitive and/or neurological pathologies affecting their ability to follow study protocol, (2) were unwilling to comply with clinical or radiological evaluation, (3) were unavailable for preoperative radiographic examinations, or (4) possessed a humeral fracture. Enrolled patients either underwent preoperative planning and real-time intraoperative navigation (Group A) or only preoperative planning alone (Group B). A shoulder CT scan was performed on all patients to evaluate their joint anatomy, especially the glenoid component. Three-dimensional preoperative planning was conducted with the Equinoxe Planning App version 1.5 (Exactech Blue Ortho, Grenoble, France). The glenoid erosion pattern (based on the modified Walch classification) and relevant clinical factors were evaluated to determine the best operative plan and appropriately select the glenoid component [15]. Real-time intraoperative navigation, utilized only in Group A, was conducted with the ExactechGPS Total Shoulder V1.4.1 application (Exactech Blue Ortho, Grenoble, France). All procedures were performed by the senior surgeon.
Preoperative radiographic protocol
A preoperative non-contrast CT scan of the shoulder was performed in all study participants using the Siemens Somatom Perspective CT device (Siemens Medical Solutions USA, Malvern, PA). The scan was performed according to a specific standardized protocol provided by Exactech (Gainesville, FL, US): patients were in a supine position with the arm adducted on the side of the trunk, and the shoulders subsequently scanned to capture the entire scapula in the axial plane without rotation. The tube current was set to at least 120 kV (peak) with image reconstruction utilizing a convolutive bone core in a field of view of 154–410 mm and standard image matrix size of 512 × 512 pixels, resulting in 200 –450 images. Distance between the slices was 0.3–1 mm with a recommended distance of 0.625 mm. The subsequent CT file was then sent to the manufacturer to be loaded into the surgical planning software (Exactech). Images were then manually segmented by the manufacturer to reconstruct a three-dimensional visual model of the shoulder to facilitate preoperative planning.
Preoperative planning
After segmenting the scapula from the humerus, the images were used to three-dimensionally reconstruct the scapula. The Friedman axis was determined by drawing a line from the center of the glenoid to the crossing point of the “Y” of the scapula [16]. The center of the glenoid is determined by the intersection of the lines from the highest to lowest point and most anterior to posterior point of the glenoid. The “Y” of the scapula is determined by the average of 3 points along the medial margin of the scapula where the spines converge in a triangular shape.
For both groups, the planning software enabled the surgeon to virtually implant a glenoid component within the 3D model of the shoulder, allowing for an individualized assessment for each patient. This planning served two purposes: (1) to restore neutral alignment in the both the axial and coronal planes. In instances where this was unattainable, either non-neutral alignment or 8° posterior augmentation with asymmetric baseplates was used. The glenoid component had to be implanted to best assimilate with the glenoid surface, allowing for the highest possible sitting position and (2) to avoid leakage of the center pin and screws by maintaining an optimal position for the baseplate.
Standard surgical technique
All procedures were performed by the same senior surgeon with the patient in a beach chair position (30°–45°). Patients underwent reverse prosthesis surgery with a lateralizing humeral component (Equinoxe; Exactech, Gainesville, FL, USA). In all patients, a standard deltoid-pectoral access was performed, which allowed for an appropriate exposure of the glenoid. Once the deltoid-pectoral fascia was incised, the subscapularis tendon was visualized and, if present, a tenotomy was performed at the level of the myotendinous junction. The humeral head was resected with respect to the greater and lesser tuberosity and tenotomy was performed of the long head of the biceps tendon. At this point, the glenoid was exposed with subsequent excision of the labrum and capsule. In accord with the CT-based preoperative planning performed in both groups, neutral alignment was established based on the appearance of the glenoid and deformity measurements. The glenoid center was identified at the intersection of the vertical and perpendicular mid-glenoid lines. The orientation of the glenoid was determined visually, and the Kirschner wire was inserted in a neutral version. Degree of inclination was defined by the preoperative measurements on the central hole using a jig (Exactech) whose lower edge was oriented to the lower edge of the glenoid. Next, the cannulated drill perforates the slotted K wire to create the housing of the central taproot. The glenoid component is then inserted and screw holes are drilled at the 12 and 6 o'clock positions. The upper screw is oriented toward the coracoid, while the lower screw toward the body of the scapula. Following insertion of the glenosphere, the trial humeral shaft was replaced with the finale shaft, humeral footplate, and polyethylene component. The humeral stem was implanted with 10° of retroversion to weakly favor extra rotation.
Real-time, intraoperative navigation
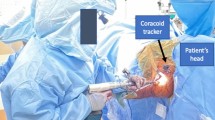
For the navigated group, a CT-based surgeon-controlled computerized surgical work system was used with the aid of optical active trackers to provide specific real-time guidance of the patient's scapula intraoperatively. Initially, the navigation system hardware and software are activated and the navigation trackers registered on the computer. The planning software, which was transferred to a sterile workstation (ExactechGPS; Exactech) in the operating room, is then accessed to finalize the individualized plan.
The patient was placed in the standard beach chair position, reclined from 30° to 45° with the neck slightly flexed laterally to increase space for the coracoid tracker. The deltopectoral approach was used to expose the glenohumeral joint, with an emphasis on exposing the superior surface of the coracoid to mount a tracker using threaded screws to its inferolateral base. Subsequently, the glenoid articular cartilage was curetted to expose the underlying bone. A portable tracker was applied to the anatomical landmarks to register the scapula with the patient planning template. After registration, the software provided two- and three-dimensional guidelines for carrying out the preoperative planning. At the end of the recording, only registration with minimal error (< 1 mm) was validated by the surgeon. Once the registration was validated, the surgeon proceeded with identification of the glenoid pilot hole (according to the preoperative planning), glenoid reaming (guided according to the planned version, inclination, and depth of the reaming), and positioning and drilling of the central hole.
The ultimate goal with glenoid component placement was to correct the version to obtain between 5° of retroversion and 5° of anteversion, while also minimizing the extent of bone removal. The Exactech glenoid component has the central tap root positioned eccentrically with respect to the center of the glenoid and possesses 6 available holes for the screws. The 12 o’clock and 6 o’clock holes were consistently used, while additional screws may have been used based on qualitative testing of the stability and conformation of the glenoid vault. The screws were placed under image-guided navigation using tracked instruments to maximize length. Humerus preparation and fixation were not navigated for the standard group.
Functional evaluation
To evaluate the functional recovery of the upper limb, the evaluation forms administered to patients at the final follow-up were the DASH instrument, Simple Shoulder Test, and ASES [17].
Postoperative CT evaluation
At follow-up in the clinic, all patients underwent a postoperative CT evaluation to analyze the version and inclination of the baseplate relative to the axis of the scapula.
Using the Equinoxe version 1.5 planning system (Exactech Blue Ortho, Grenoble, France) in conjunction with the Friedman studies, which were subsequently standardized by Walch, the axis of the scapula was defined in terms of glenoid version (axial plane) and inclination (coronal plane) [16].
Because the glenosphere covered the baseplate, it was not possible to trace the axis using the four cardinal points of the baseplate; instead, it was performed with the upper and lower points of the taproot in the coronal plane and the most posterior and anterior points in the axial plane (Fig. 1). In doing so, this enabled a comparative assessment of the degree of version and inclination of the prosthetic glenoid component postoperatively with that during preoperative planning.
Positioning of the central tap and screws
Utilizing reconstructions in the axial, coronal, and sagittal planes, it was examined whether the proximal screw was in the direction of the coracoid and if both proximal and distal screws penetrated both cortices. Finally, the complete presence of the central taproot inside the glenoid and the glenoid vault was verified. The length of the implanted screws that were grouped is as follows:
-
Completely inside the glenoid vault
-
Outside 1/3 of the screw length (Fig. 2A)
-
2/3 off the length of the screw or with the wrong axis. (Fig. 2B, C)
Statistical analysis
Qualitative variables were described in terms of absolute numbers and percentages. Quantitative variables were described using mean and standard deviation when parametric and median and interquartile range when nonparametric. Comparisons between the qualitative variables were made using a Chi-square test for multiple groups or Fisher's exact test if comparing dichotomous variables. Given the nonparametric distribution of the data, the Wilcoxon–Mann–Whitney test was used to compare the quantitative variables between two analysis groups and the Kruskal–Wallis test for comparison between three or more groups. Alpha was set to a level of 0.05. Analyses were done using SAS software (Version 9.4).
Results
Of the 35 patients originally enrolled, 2 were excluded for inability to perform postoperative CT evaluation. The final cohort contained 16 patients with navigation (Group A) and 17 without (Group B). Eight patients were male, 25 female and had a mean age of 72 (range 66–80) years. There were no differences in demographics between groups (navigation group [A]: mean age = 73 years, sex: 3 males and 13 females; standard group [B]: mean age = 74 years, sex: 5 males and 12 females; age: p = 0.35, sex: p = 0.48). Median operating time from incision to skin closure was 104 min in the navigation group and 98 min in the standard group (p = 0.32). Clinical and radiographic evaluation was performed at the final follow-up at a mean of 16 (range 12–18) months (Group A: mean = 16 [range: 12–18] months, Group B: mean = 16 [range 13–18] months, p > 0.99).
Clinical and functional outcomes by navigation use
PROM score performance did not differ at final follow-up. The median Simple Shoulder Test was 9 if navigated and 8 if not navigated (p = 0.64). The median DASH score was 6.5 in the navigation group and 13.6 in the standard group (p = 0.38). The median ASES Score was 86 if navigated and 87 if standard (p = 0.77). The median postoperative pain via a 10-point visual analog scale (VAS) was 0 amongst patients with navigation and 1 amongst patients without navigation (p = 0.1). There was no difference in final range of motion between groups (Table 1).
Radiographic outcomes by navigation use
At baseline, Walch glenoid types differed between groups. The navigated group had 7 A1 glenoid, 2 A2, 5 B1, and 2 B2 glenoids. By contrast, the standard group had 12 type A1 glenoids, 3 A2, 2 B2 (p = 0.05). There were no differences between groups in terms of native, planned, and postoperative glenoid versions and inclination (Table 2). Baseplate types did not differ between navigated and standard groups (p = 0.35, Table 3).
Postoperative version and inclination were compared with the preoperative planning. In both groups, the planned and postoperative versions were not statistically significant (p = 0.76). By contrast, patients who did not have navigation demonstrated significant differences between planned and postoperative inclination (p = 0.03), while patients with navigation did not (p = 0.1) (Table 4, Fig. 3).
Radiographic outcomes by preoperative glenoid type
As expected, patients with Walch type B1 and B2 glenoids were more likely to receive baseplates with 8° of posterior augmentation and patients with type A1 glenoids were more likely to receive standard baseplates (p = 0.03, Table 5).
The pre- and postoperative version and inclination were compared by preoperative Walch glenoid classification (Table 6). Preoperatively, there was no statistically significant difference in version between the 4 groups (p = 0.05), with the greatest retroversion in type B2 glenoids. There were no significant preoperative differences in inclination between Walch glenoid types (p = 0.09).
Postoperatively, version was not statistically different depending on the type of glenoid (p = 0.24), including amongst patients with a preoperative B1 and B2 glenoid. There were also no differences in postoperative inclination (p = 0.11), including between the glenoid type A1 and B1 (p = 0.07).
Complications by navigation use
There were no differences in the proportions of screws that had backed out of the glenoid vault between navigated and standard groups (p = 0.57, Table 7). There were no reported cases of periprosthetic infection, periprosthetic fracture, dislocation, or axillary nerve paralysis in either group. In patients who received navigation and required placement of the tracker on the coracoid, no cases of intraoperative and immediate postoperative fracture were reported. There were also no cases of acromion stress fractures.
Discussion
In this prospective cohort study of patients receiving RSA for eccentric shoulder arthropathy, the principal findings were: (1) patients with navigated or standard RSA had comparable patient-reported outcomes, pain, and range of motion at a mean of 16 months after surgery; (2) in patients with navigation or without navigation, there was neutral correction of glenoid orientation in the axial plane, even in patients with more complex glenoid types at baseline (B1 and B2); (3) postoperative inclination differed from the planned inclination in standard patients but not navigated patients; (4) there were no differences in complication rates and screw back-out between navigated and standard groups.
The aim of this study was to additionally assess whether the use of computer navigation may help restore a neutral axis in both the coronal and axial planes after RSA. The ability to correct the glenoid version is influenced by the implant design, bone geometry (glenoid area and central tap volume), and available bone stock [18]. In a case–control study of 33 patients with navigated TSA and 27 patients with standard TSA, Nashikkar et al. demonstrated that computer-assisted navigation successfully reduced the standard deviation of the inclination with respect to the neutral axis, as well as the degree of version in the axial plane [12]. Similarly, in the current study, despite more severe glenoid defects at baseline in the navigated group, both navigated and standard groups had excellent correction of axial orientation, with more than 80% of the cohort demonstrating postoperative version within 3° of the planned position. However, this was not true within the coronal plane, as 40% of the standard group demonstrated a postoperative inclination more than 3° varied from the planned angulation. Therefore, navigation may confer greater benefits for optimizing inclination than version.
As described by Nguyen et al., it is likely that the major errors in the positioning of the glenoid implant were observed during drilling [13]. In 4 of the 16 cases treated with real-time intraoperative navigation, it was necessary to implant baseplates with an 8° posterior augment. Three patients had an initial retroversion of 11°, 12°, and 14° with a type B2 glenoid, while the fourth patient a retroversion of 7° with a type B1 glenoid configuration. An asymmetrical reaming was preferred in these three patients in order to reduce the need for bone grafts, which are known to be both technically demanding, at risk of inconsistent graft osseointegration, and associated with an increased risk of aseptic loosening of the glenoid component [19,20,21]. Based on these prior studies, many shoulder surgeons follow a strict treatment paradigm based on the degree of glenoid retroversion. Posteriorly worn glenoids with retroversion less than 10° can be treated with eccentric glenoid reaming and not augmented glenoid components; however, glenoid with retroversion greater than 10° would require other treatment options such as bone grafting and/or a posteriorly augmented baseplate, which both demonstrate improvements in ROM and PROM scores [22,23,24].
Indeed, most studies report increased use of augmented implants when computer navigation is utilized, which may underlie the improved ROM reported by some studies of navigated RSA [25]. In contrast to the current study, a recent 2-year multicenter outcome report by Youderian et al. comprising of more than 500 RSAs found that patients who underwent navigated RSA had improvements in internal rotation and external rotation [25]. This finding is particularly important for RSA, as there are well-described limitations in postoperative to internal and external rotation; with the exception of subscapularis repair and preservation of the rotator cuff tendons, there are few surgical or patient factors that can improve both IR and ER after lateralizing RSA [26,27,28]. However, there were no differences between groups for final ROM in the current study. The Youderian et al. study has a larger sample size, and therefore larger statistical power to detect smaller differences in internal and external rotation. It is possible that the current study was underpowered to detect these differences, and future prospective randomized studies are required to elucidate whether navigation may improve preoperative planning, lateralization of the joint line, and range of motion.
The results of the current study also suggest good short-term PROM scores and low complication rates following RSA using computer navigation. Youderian et al. also found equivalent or better outcomes and lower postoperative complication and dislocation rates with navigated RSA, regardless of glenoid morphology [25]. However, for both the current study (18 months) and their study (2 years), it is possible that there was insufficient follow-up to identify complications, especially in patients with more severe glenoid defects. Reassuringly, in patients with Walch B2, B3 and C glenoids, the recently published findings of Virk et al. suggest that RSA using eccentric reaming and augmented glenoid components has good long-term outcomes [29]. They found that the use of a baseplate with 8° posterior augment was associated with excellent clinical and radiographic findings and low complication rate at a mean follow-up of 40 months. There were no cases of aseptic loosening of the glenoid component with 8° augmented baseplate, despite the patients having a mean native glenoid retroversion of approximately 21°. Based on our experience and data in the literature, we conclude that posterior augmented baseplates in RSA may be a viable treatment option for Walch glenoids B2, B3, and C with retroversion greater than 10°, especially if positioned via intraoperative navigation. However, large, long-term clinical and radiographic follow-up would be needed to confirm these promising short-term results.
This study also found that there were no differences in screw migration between navigated and standard groups. Several authors suggested screw placement is crucial for initial fixation, implant stability, and prevention of complications such as soft tissue damage or impingement [1, 7, 8]. In particular, the inferior screw is crucial for early fixation. It is recommended to place it in correspondence with the abutment of the scapula, an area with excellent grip and bone stock [7]. In cases of difficult glenoid exposure or bone deficiency of the inferior margin of the glenoid, the screws can be misplaced and even be outside the cortex. In accordance with the findings of Verborgt et al., in both navigated and standard patients of the current study, the inferior screw always remained within the glenoid vault [30]. Due to a relative small sample size, this study was likely underpowered to determine if navigation plays a role in appropriate inferior screw fixation, within the glenoid vault. However, a larger study by Nashikkar et al., suggests that intraoperative navigation allows for easier positioning of the central taproot within the glenoid vault [12].
In the current study, in over 60% of the cases at least one screw was had at least 1/3 of the screw length outside of the glenoid, without a statistically significant difference between the two groups. Therefore, in the patients with real-time intraoperative navigation, an attempt was made to position the screw long enough to anchor the second cortex, thus obtaining greater stability. In one patient with posterosuperior glenoid deficiency (Walch B2, Favard E2) who received intraoperative navigation, the superior screw was positioned more than 1/3 outside the glenoid vault but with the correct angulation and axis. In the standard group, even though 19 patients (57.6%) had Walch type A1 glenoids, it was not possible to position the screws completely inside the glenoid vault, probably due to limited visibility of the scapula during surgery. In two patients from the standard group, the superior screw was positioned with a completely wrong axis such that 50% of the screw was found outside the glenoid vault. The design and placement of the baseplate may also have played a role, as prior biomechanical studies suggest differences in screw micromotion and fixation strength based on glenoid tilt, oval versus circular baseplate frames, and curved back versus flat back fixation stems [31, 32]. A malpositioned screw can also cause damage to surrounding soft tissues, such as the axillary nerve (inferior screw), suprascapular nerve (superior screw), or blood vessels [33]. Notably, despite the radiographic evidence of screw malposition reported within the study cohort, no short-term complications were found on the adjacent peripheral nervous and vascular structures.
One potential concern involved with intra-operative navigation includes the possibility of fracturing of the tracker mounting site and loosening of the tracker itself. Although no coracoid fractures were reported in this cohort, the current study may have been underpowered to detect the effect of navigation on this complication. As reported by Nashikkar et al., the incidence of coracoid fracture and tracker mobilization is low (2 cases out of 35), but still highlights the importance of considering bone quality when choosing the most appropriate surgical approach [12]. Further investigation is warranted to better assess the risk of this complication in large cohorts and determine how surgeon experience or bone quality may play a role in safe patient selection for navigated procedures.
Another concern for widespread adoption of navigated RSA is the additional time and costs associated with this newer technology. In the current study, navigation only took 6 min longer than standard screw placement. This is similar to the findings of a prior clinical trial of navigation, which demonstrated similar or reduced surgical time with navigation, depending on the surgeon’s familiarity with the system [34]. The Youderian et al. multicenter study further validates this finding [25]. Although this may seem counterintuitive given the additional time needed to set up the navigation system, retrospective studies of navigated RSA suggests fewer and longer screws are used than in standard RSA, which may account for the minimal difference in operating time [25, 35, 36]. Unfortunately there is a paucity of data regarding the cost effectiveness of computer navigation in RSA [37], nor were these data assessed in the current analysis. Future cost-effectiveness studies are warranted to help determined the meaningful use of navigation technology in RSA.
This study has several strengths. Firstly, all surgeries were performed by the same experienced shoulder surgeon, maximizing the internal validity of these findings. Secondly, this study provides comprehensive radiographic and clinical assessment of a prospective cohort of patients receiving navigated versus standard RSA, including patients with posteriorly augmented baseplates. Finally, all patients were assessed via three-dimensional (3D) CT assessments intraoperatively and at follow-up, with the same device, to identify appropriate reference points for the version and tilt angles. While the authors of the current study propone the use of 3D imaging to provide as detailed information as possible about component positioning, it is important to note that the superiority of 3D imaging over two-dimensional (2D) imaging is debated. Some authors have shown that 3D reconstructions increase agreement among raters and questioned the accuracy of 2D measurements, while others have reported that 3D reconstructions are not advantageous [38,39,40].
Limitations
There are several limitations that affect this study. Since the outcomes of interest were component positioning, screw fixation, early complications, and clinical outcomes, this cohort was only followed for an average of 16 months following surgery. Studies assessing the time to achievement of key outcomes following RSA suggest that over 80% of the improvement in pain occurs within 6 weeks and over 85% of patients plateau in improvements on common PROM instruments after 6 months [41]. In fact, multiple studies suggest that the treatment of effects of RSA on PROM scores change little between 1 and 5 years following surgery, with the greatest improvements in the first 3 months [42, 43]. Therefore, the authors argue that the follow-up, albeit short, is sufficient to capture the outcomes of interest. Another limitation consists in the non-randomized study design and the risk of treatment bias between groups. There were differences in baseline Walch glenoid classifications between groups, in part related to the lack of randomization. However, there were a greater number of B1 and B2 glenoids in the navigation group, which may have artificially suppressed the treatment effect of navigation on postoperative inclination and version. The authors argue these baseline differences in fact strengthen the argument that navigation may assist in restoring appropriate joint positioning. Finally, the sample size of this study was relatively small (33 patients). Therefore, this study was likely underpowered to detect significant differences between groups. However, this is not a routine surgery and accrual rates for eligible patients were low. While patients receiving RSA for other indications (e.g., fractures) could have been included to expand the sample size, it would be complex to apply computer navigation technology to these cases. Therefore, the non-significant results between groups should be interpreted with caution, and future work should use the information presented here to design adequately powered comparisons.
Conclusions
There were no statistically significant differences in range of motion or patient reported outcomes at a short-term follow-up between the group of patients receiving RSA with real-time intraoperative navigation and standard procedure. Both groups of patients expressed a high degree of postoperative satisfaction. Despite a greater number of Walch B1 and B2 or Favard E2 and E3 glenoids in the navigated group, all patients were able to achieve an appropriate neutral axis following surgery. Navigation use was associated with greater accuracy of screw placement in the coronal plane, demonstrating smaller differences between planned and final inclination angles. Although over half of all screws in both navigated and standard RSA were positioned outside the second cortex, there were no complications related to impingement or soft tissue structural damage. These results are encouraging for the continued validation of intraoperative navigation in RSA, but cost effectiveness studies are warranted to improve the meaningful use of this technology.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- RSA:
-
Reverse shoulder arthroplasty
- PROMs:
-
Patient-reported outcome measurements
- ASES:
-
American shoulder and elbow surgeons
- DASH:
-
Disabilities of the arm, shoulder, and hand
- SST:
-
Simple shoulder test
- VAS:
-
Visual analog scale
- IR:
-
Internal rotation
- ER:
-
External rotation
- 3D:
-
Three-dimensional
- 2D:
-
Two-dimensional
References
Codsi MJ, Iannotti JP (2008) The effect of screw position on the initial fixation of a reverse total shoulder prosthesis in a glenoid with a cavitary bone defect. J Shoulder Elbow Surg 17(3):479–486. https://doi.org/10.1016/j.jse.2007.09.002
Harman M, Frankle M, Vasey M, Banks S (2005) Initial glenoid component fixation in “reverse” total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg 14(1):162S-S167. https://doi.org/10.1016/j.jse.2004.09.030
Wagner ER, Muniz AR, Chang MJ, Hunt T, Welp KM, Woodmass JM et al (2021) Neuroapraxia and early complications after reverse shoulder arthroplasty with glenoid bone grafting. J Shoulder Elbow Surg 30(2):258–264. https://doi.org/10.1016/j.jse.2020.05.004
Bohsali KI, Bois AJ, Wirth MA (2017) Complications of shoulder arthroplasty. J Bone Jt Surg Am 99(3):256–269. https://doi.org/10.2106/jbjs.16.00935
Iannotti JP, Weiner S, Rodriguez E, Subhas N, Patterson TE, Jun BJ et al (2015) Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Jt Surg Am 97(8):651–658. https://doi.org/10.2106/jbjs.N.00493
Ho JC, Thakar O, Chan WW, Nicholson T, Williams GR, Namdari S (2020) Early radiographic failure of reverse total shoulder arthroplasty with structural bone graft for glenoid bone loss. J Shoulder Elbow Surg 29(3):550–560. https://doi.org/10.1016/j.jse.2019.07.035
Gutierrez S, Comiskey CAT, Luo ZP, Pupello DR, Frankle MA (2008) Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Jt Surg Am 90(12):2606–2615. https://doi.org/10.2106/JBJS.H.00012
Humphrey CS, Kelly JD 2nd, Norris TR (2008) Optimizing glenosphere position and fixation in reverse shoulder arthroplasty, part two: the three-column concept. J Shoulder Elbow Surg 17(4):595–601. https://doi.org/10.1016/j.jse.2008.05.038
Middernacht B, De Roo PJ, Van Maele G, De Wilde LF (2008) Consequences of scapular anatomy for reversed total shoulder arthroplasty. Clin Orthop Relat Res 466(6):1410–1418. https://doi.org/10.1007/s11999-008-0187-6
Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D (2004) Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Jt Surg Br 86(3):388–395. https://doi.org/10.1302/0301-620x.86b3.14024
Walch G, Badet R, Boulahia A, Khoury A (1999) Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 14(6):756–760. https://doi.org/10.1016/s0883-5403(99)90232-2
Nashikkar PS, Scholes CJ, Haber MD (2019) Computer navigation re-creates planned glenoid placement and reduces correction variability in total shoulder arthroplasty: an in vivo case-control study. J Shoulder Elbow Surg 28(12):e398–e409. https://doi.org/10.1016/j.jse.2019.04.037
Nguyen D, Ferreira LM, Brownhill JR, King GJ, Drosdowech DS, Faber KJ et al (2009) Improved accuracy of computer assisted glenoid implantation in total shoulder arthroplasty: an in-vitro randomized controlled trial. J Shoulder Elbow Surg 18(6):907–914. https://doi.org/10.1016/j.jse.2009.02.022
Theopold J, Pieroh P, Henkelmann R, Osterhoff G, Hepp P (2019) Real-time intraoperative 3D image intensifier-based navigation in reversed shoulder arthroplasty-analyses of image quality. BMC Musculoskelet Disord 20(1):262. https://doi.org/10.1186/s12891-019-2657-2
Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G (2016) A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg 25(10):1601–1606. https://doi.org/10.1016/j.jse.2016.03.010
Friedman RJ, Hawthorne KB, Genez BM (1992) The use of computerized tomography in the measurement of glenoid version. J Bone Jt Surg Am 74(7):1032–1037
Lippitt SB, Harryman DT, Matsen FA, Lippitt S, Lippit S (1993) A practical tool for evaluating function: the simple shoulder test
Hoenecke HR Jr, Hermida JC, Dembitsky N, Patil S, D’Lima DD (2008) Optimizing glenoid component position using three-dimensional computed tomography reconstruction. J Shoulder Elbow Surg 17(4):637–641. https://doi.org/10.1016/j.jse.2007.11.021
Hill JM, Norris TR (2001) Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Jt Surg Am 83(6):877–883
Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH (2008) Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res 466(3):579–583. https://doi.org/10.1007/s11999-007-0104-4
Steinmann SP, Cofield RH (2000) Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg 9(5):361–367. https://doi.org/10.1067/mse.2000.106921
Kersten AD, Flores-Hernandez C, Hoenecke HR, D’Lima DD (2015) Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg 24(7):1135–1141. https://doi.org/10.1016/j.jse.2014.12.007
Knowles NK, Ferreira LM, Athwal GS (2015) Augmented glenoid component designs for type B2 erosions: a computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg 24(8):1218–1226. https://doi.org/10.1016/j.jse.2014.12.018
Nabergoj M, Neyton L, Bothorel H, Ho SWL, Wang S, Chong XL et al (2021) Reverse shoulder arthroplasty with bony and metallic versus standard bony reconstruction for severe glenoid bone loss. A retrospective comparative cohort study. J Clin Med 10:22. https://doi.org/10.3390/jcm10225274
Youderian AR, Greene AT, Polakovic SV, Davis NZ, Parsons M, Papandrea RF et al (2023) Two-year clinical outcomes and complication rates in anatomic and reverse shoulder arthroplasty implanted with Exactech GPS© intraoperative navigation. J Shoulder Elbow Surg. https://doi.org/10.1016/j.jse.2023.05.021
Rol M, Favard L, Berhouet J, la Societe d’orthopedie de lO (2019) Factors associated with internal rotation outcomes after reverse shoulder arthroplasty. Orthop Traumatol Surg Res 105(8):1515–1519. https://doi.org/10.1016/j.otsr.2019.07.024
Al Yaseen M, Smart YW, Seyed-Safi P, Abdelmonem AH, Makki D, Morgan B et al (2022) Effect of implant size, version and rotator cuff tendon preservation on the outcome of reverse shoulder arthroplasty. Cureus 14(6):e25741. https://doi.org/10.7759/cureus.25741
Cheung EV, Sarkissian EJ, Sox-Harris A, Comer GC, Saleh JR, Diaz R et al (2018) Instability after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 27(11):1946–1952. https://doi.org/10.1016/j.jse.2018.04.015
Virk M, Yip M, Liuzza L, Abdelshahed M, Paoli A, Grey S et al (2020) Clinical and radiographic outcomes with a posteriorly augmented glenoid for Walch B2, B3, and C glenoids in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 29(5):e196–e204. https://doi.org/10.1016/j.jse.2019.09.031
Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F (2011) Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg 20(1):21–26. https://doi.org/10.1016/j.jse.2010.07.014
Roche CP, Stroud NJ, Flurin PH, Wright TW, Zuckerman JD, DiPaola MJ (2014) Reverse shoulder glenoid baseplate fixation: a comparison of flat-back versus curved-back designs and oval versus circular designs with 2 different offset glenospheres. J Shoulder Elbow Surg 23(9):1388–1394. https://doi.org/10.1016/j.jse.2014.01.050
Chae SW, Lee J, Han SH, Kim SY (2015) Inferior tilt fixation of the glenoid component in reverse total shoulder arthroplasty: a biomechanical study. Orthop Traumatol Surg Res 101(4):421–425. https://doi.org/10.1016/j.otsr.2015.03.009
Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G (2011) Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res 469(9):2512–2520. https://doi.org/10.1007/s11999-010-1695-8
Wang AW, Hayes A, Gibbons R, Mackie KE (2020) Computer navigation of the glenoid component in reverse total shoulder arthroplasty: a clinical trial to evaluate the learning curve. J Shoulder Elbow Surg 29(3):617–623. https://doi.org/10.1016/j.jse.2019.08.012
Hones KM, King JJ, Schoch BS, Struk AM, Farmer KW, Wright TW (2021) The in vivo impact of computer navigation on screw number and length in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 30(10):e629–e635. https://doi.org/10.1016/j.jse.2021.01.017
Velasquez Garcia A, Abdo G (2023) Does computer-assisted navigation improve baseplate screw configuration in reverse shoulder arthroplasty? A systematic review and meta-analysis of comparative studies. J Orthop 36:29–35. https://doi.org/10.1016/j.jor.2022.12.008
Jahic D, Suero EM, Marjanovic B (2021) The use of computer navigation and patient specific instrumentation in shoulder arthroplasty: everyday practice, just for special cases or actually teaching a surgeon? Acta Inform Med 29(2):130–133. https://doi.org/10.5455/aim.2021.29.130-133
Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D’Lima DD (2010) Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg 19(2):166–171. https://doi.org/10.1016/j.jse.2009.08.009
Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G (2010) Glenoid version: how to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg 19(8):1230–1237. https://doi.org/10.1016/j.jse.2010.01.027
Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP (2008) The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Jt Surg Am 90(11):2438–2445. https://doi.org/10.2106/JBJS.G.01341
Grubhofer F, Muniz Martinez AR, Ernstbrunner L, Haberli J, Selig ME, Yi K et al (2021) Speed of recovery of the most commonly performed shoulder surgeries. JSES Int 5(4):776–781. https://doi.org/10.1016/j.jseint.2021.03.007
Cabarcas BC, Gowd AK, Liu JN, Cvetanovich GL, Erickson BJ, Romeo AA et al (2018) Establishing maximum medical improvement following reverse total shoulder arthroplasty for rotator cuff deficiency. J Shoulder Elbow Surg 27(9):1721–1731. https://doi.org/10.1016/j.jse.2018.05.029
Huber J, Irlenbusch U, Kääb MJ, Reuther F, Kohut G, Judge A (2020) Treatment effects of reverse total shoulder arthroplasty—a simple method to measure outcomes at 6, 12, 24 and 60 months for each patient. BMC Musculoskelet Disord 21(1):397. https://doi.org/10.1186/s12891-020-03427-7
Acknowledgements
We would like to thank Exactech for making the intraoperative navigation system available to us free of charge.
Funding
This study was conducted without funds.
Author information
Authors and Affiliations
Contributions
EG cooperated to the conception, design of the work, and interpretation of data, approved the submitted version, and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. SMP contributed to the acquisition and analysis, drafted the work or substantially, revised it AND approved the submitted version, and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. OP interpreted the data, approved the submitted version, and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. EJB. drafted the work or substantially revised it, approved the submitted version, and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. HHP drafted the work or substantially revised it, approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. AR drafted the work or substantially revised it, approved the submitted version, and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. ADC drafted the work or substantially revised it, approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This prospective cohort investigation was reviewed and granted ethics approval by Sapienza University of Rome institutional Review Board (05/2020) and conducted in accordance with the Declaration of Helsinki. For all participants, informed consent was obtained prior to enrollment.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gaj, E., Pagnotta, S.M., Berlinberg, E.J. et al. Intraoperative navigation system use increases accuracy of glenoid component inclination but not functional outcomes in reverse total shoulder arthroplasty: a prospective comparative study. Arch Orthop Trauma Surg 144, 91–102 (2024). https://doi.org/10.1007/s00402-023-05038-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-05038-y