Abstract
Introduction
Patients with pelvic and acetabular fractures often have considerable pain in the perioperative period. Regional anesthesia (RA) including peripheral nerve blocks and spinal analgesia may reduce pain. However, the real-world impact of these modalities on inpatient opioid consumption and outpatient opioid demand is largely unknown. The purpose of this study was to evaluate the impact of perioperative RA on inpatient opioid consumption and outpatient opioid demand.
Methods
This is a retrospective, observational review of inpatient opioid consumption and outpatient opioid demand in all patients ages 18 and older undergoing operative fixation of pelvic and acetabular fractures at a single Level, I trauma center from 7/1/2013–7/1/2018 (n = 205). Unadjusted and adjusted analyses were constructed to evaluate the impact of RA on inpatient opioid consumption and outpatient opioid demand while controlling for age, sex, race, body mass index (BMI), smoking, chronic opioid use, ASA score, injury mechanism, additional injuries, open injury, and additional inpatient surgery.
Results
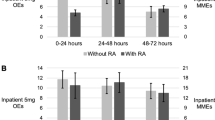
Adjusted models demonstrated increases in inpatient opioid consumption in patients with RA (12.6 estimated OE’s without RA vs 16.1 OE’s with RA from 48 to 72 h post-op, p < 0.05) but no significant differences at other timepoints (17.5 estimated OE’s without RA vs 16.8 OE’s with RA from 0 to 24 h post-op, 15.3 vs 17.1 from 24 to 48 h post-op, p > 0.05). Estimated cumulative outpatient opioid demand was significantly higher in patients with RA at discharge to 90 days post-op (and 156.8 vs 207.9 OE’s to 90 days, p < 0.05) but did not differ significantly before that time (121.5 OE’s without RA vs 123.9 with RA from discharge to two weeks, 145.2 vs 177.2 OE’s to 6 weeks, p > 0.05).
Discussion
In pelvis and acetabulum fracture surgery, RA was associated with increased inpatient and outpatient opioid demand after adjusting for baseline patient and treatment characteristics. Regional anesthesia may not be beneficial for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fractures of the pelvis and acetabulum are major, often life-threatening, orthopedic injuries with considerable radiographic and clinical heterogeneity [1,2,3,4]. These fractures typically result from high energy impact and are associated with significant blood loss, morbidity, and mortality [5, 6]. The incidence of pelvic and acetabular fractures is relatively low (approximately 7.1 per 100,000 person years for acetabular fractures and 56 per 100,000 person years for pelvic fractures) [7], but is rising with increased incidence of motor vehicle accidents [8]. Patients with pelvic ring injuries often present with additional injuries and may require damage control resuscitation, procedures for hemorrhage control, and early stabilization of the fracture prior to definitive fixation [9]. Not only are fractures of the pelvis and acetabulum associated with high morbidity and mortality, but also significant acute and chronic pain [10, 11]. In patients with AO class A, B, and C pelvic fractures, the prevalence of persistent posttraumatic pelvic pain is 38%, 67%, and 90%, respectively [12]. Patients with acetabular fractures may go on to develop posttraumatic arthritis or heterotopic ossification, causing pain, and limitations in function [13, 14].
Perioperative opioids are a mainstay in pain management for pelvic and acetabular fractures [15]. However, opioids are associated with significant risks. Up to 82% of patients experience moderate or severe opioid-related adverse effects [16]. These are dose dependent and include nausea, sedation, and respiratory depression [17]. Opioid use also increases the odds of poor orthopedic outcomes such as periprosthetic fracture, infection, and hardware loosening [18]. Additionally, persistent opioid use after surgery is a rising complication among both opioid naïve patients and those taking opioids preoperatively [19]. Chronic opioid use after acetabular fractures may be as high as 41% at 6 months and 33% at 12 months [20]. Fractures managed with external fixation are also associated with increased opioid demand up to a year postoperatively [21]. Further, patients with pre-existing mental health and opioid use disorders are at risk for increased postoperative opioid use [22,23,24]. These facts are especially concerning considering that 2/3 of prescription drug overdose deaths now involve an opioid [25]. Despite appropriate institutional, state, and federal efforts to limit opioid prescribing, long-term misuse continues to be problematic [26,27,28,29,30,31,32]. Developing perioperative opioid-sparing protocols that can adequately manage pain holds promise for reducing long-term opioid use [19, 33]. Consequently, a multimodal approach to analgesia has been advocated in the setting of orthopedic trauma.
Regional anesthesia (RA) has received increased attention in perioperative multimodal analgesia. RA, which has been used in orthopedic trauma, uses local anesthetics to prevent neural action potential propagation and the detection of nociceptive input by the central nervous system [34]. Various techniques have been developed to administer RA paravertebrally or in specific nerve distributions, and some modalities have demonstrated reductions in opioid consumption and adverse effects [35]. However, analgesic efficacy has been shown to vary across surgical procedures and the potential benefits of RA should be evaluated accordingly [36]. Only two studies have investigated the use of RA in pelvic and acetabular fractures with mixed results [37, 38], and the long-term impact of RA on opioid use in these patients remains to be elucidated. Given the knowledge gap regarding the impact of RA on longitudinal perioperative and postoperative opioid demand, the purpose of this study is to evaluate the impact of these modalities on inpatient opioid consumption and outpatient opioid demand in patients undergoing pelvis and acetabulum fracture surgery. The study hypothesis is that RA will be associated with a decrease in inpatient opioid consumption but have no impact on outpatient opioid demand.
Methods
Study design
This is a retrospective, observational study of inpatient opioid consumption and outpatient opioid demand in all patients age 18 years and older undergoing pelvis and acetabulum fracture surgery at a single institution from 7/1/2013–7/1/2018. This study is designed and reported in accordance with the STROBE statement on reporting observational studies [39].
Variables and data sources
Patients ages 18 and older with operatively treated pelvis and acetabular fractures (CPT codes 27215, 27216, 27217, 27218, 27226, 27227, 27228) at a single, Level I trauma center between 7/1/2013 and 7/1/2018 were included. Inpatient opioid consumption (0–24 h, 24–48 h, and 48–72 h post-op) and outpatient opioid prescribing (discharge to 2 weeks, 6 weeks, and 90 days) were recorded in oxycodone 5-mg equivalents (OE’s) [40]. Additional baseline and operative characteristics include RA usage, age, sex, race, body mass index (BMI), smoking status, American Society of Anesthesiologists (ASA) score (measure of operative risk based on patient physiologic status [41]), injury mechanism, additional injuries, open fracture, additional surgery. Preoperative opioid usage, which was defined as patients with 1 or more opioid prescriptions within 6-months to 1-month preoperative, was also recorded, in line with the definition recommended by the Centers for Disease Control (CDC) [42]. General 90 day postoperative complications were also recorded through chart review including mortality, surgical site infection, compartment syndrome, loss of fixation, deep vein thrombosis (DVT), pulmonary embolism (PE), falls, delirium, and ileus.
As shown in Table 1, patients without RA tended to be non-Caucasian and had higher rates of high energy mechanism of injury. Spinal analgesia in the form of continuous (34/67, 50.7%) and single-shot epidurals (5/67, 7.5%) was the most common type of block. Fascia iliaca (12/67, 17.9%) and femoral (8/67, 11.9%) blocks were the most common peripheral nerve blocks.
Pain protocol
Our institution’s multimodal pain regimen generally includes oral opioids administered according to a visual analog scale (VAS) pain scale (generally 5–15 mg oxycodone every 4 h as needed for pain), intravenous (IV) opioids (typically hydromorphone) for breakthrough pain, and scheduled acetaminophen. Adjunctive oral and IV non-steroidal anti-inflammatory pain medications (NSAID’s) are not frequently used after pelvis and acetabulum fracture surgery at our institution. Patients are considered for RA by their treating anesthesiologist. Anesthetic treatment was performed by the anesthesiologist assigned to the individual case, which is generally provided by one of five core anesthesiologists within the regional anesthesia division. Discharge pain medications are prescribed by the treating team, and opioids are commonly prescribed. While the decision to discharge patients with opioids is made on a case-by-case basis by the primary team, 184 of 205 (89.8%) of patients in this series received a discharge opioid prescription.
Surgical technique
Open reduction and internal fixation of pelvis and acetabular injuries are complex and surgeon and case dependent. Generally, fixation of pelvic ring injuries is accomplished with percutaneously applied sacroiliac fixation using cannulated lag screws supplemented when needed by anterior symphyseal plating. Acetabular fracture fixation is largely dependent on fracture location and typically involves plate and screw fixation. Percutaneous acetabular fixation through appropriate osseous fixation pathways is sometimes utilized as an adjunct or as a stand-alone fixation strategy when indicated. There were four orthopedic traumatologists at our institution that performed pelvis and acetabular fracture fixation during the study time period. Operating times were case-dependent, and there were insufficient data to determine case times in this retrospective review.
Missing data
All patients ages 18 and older and undergoing the fracture fixation CPT’s previously listed were considered for inclusion (n = 206). For one patient of 206 (0.5%), BMI could not be determined. Overall results were evaluated with and without these patients and found to be similar. In order to adjust for the potential impact of this characteristic, this patient was excluded from the multivariable analyses leaving 205 patients for analysis. Pelvic external fixation is only performed as needed for pelvic and acetabular fractures. Patients were not excluded if they had previously received this treatment.
Statistical analysis
Medians with quartiles and proportions with percentages were used to display descriptive statistics. Fisher’s exact test and Wilcoxon rank sum were used to evaluate unadjusted outcomes. When considering adjusted models, histograms were first created of study outcomes and demonstrated positive skew. The top 2% of opioid utilizers were excluded due to their outlier status. Since treatment with RA was not randomly assigned, the propensity to have received the treatment was considered. Propensity score weights were determined including age, sex, race, BMI, smoking, pre-op opioid usage, ASA score (binarized to 1 to 2 vs 3 or more), injury energy (binarized to high vs low energy), presence of additional injuries, open injury, and additional surgery within 7 days post-fracture surgery as model covariates. Generalized linear modeling including propensity score weighting and the factors listed above was then carried out in a “doubly robust” fashion [43, 44]. The negative binomial distribution and log link function were used based on the data distribution. Incident rate ratios from adjusted modeling for each outcome were obtained. The impact of treatment vs no treatment was simulated within the dataset to provide medians and 95% confidence intervals of simulated treatment effect. Histograms displaying the study outcomes were also created. R and R Studio (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2020) were used for statistical calculations. P-values less than 0.05 were considered significant.
Results
After adjustment for baseline patient and treatment factors, RA had no effect on opioid consumption at 0–24 h or 24–48 h but was associated with a significant increase in opioid consumption from 48–72 h post-op (Table 2 and Fig. 1). Similarly, RA was associated with increased outpatient opioid demand from discharge to 90 days post-op (Table 3 and Fig. 2). Adjusted analyses also demonstrated that RA was associated with increased opioid refills from two weeks to 90 days post-op (Table 4). As shown in Table 5, general 90-day outcomes did not differ significantly between groups between groups.
Appendix Tables 6, 7, 8 display complete results of multivariable modeling. For inpatient opioid consumption, adjusted models demonstrated significant decreases with increased age, female sex, and additional surgery (from 0 to 24 h post-op) and significant increases with increased BMI, smoking, increased ASA, high energy injury, additional injury, additional surgery (24–72 h post-op), and RA (Appendix Table 6). For outpatient opioid prescribing, adjusted models demonstrated significant decreases with increased age and significant increases with additional surgery and RA (Appendix Table 7). Odds of opioid fill or refill were significantly decreased with increased age, Caucasian race, increased ASA, and additional injury while it was significantly increased with unknown smoking status, open fracture, additional surgery, and RA.
As shown in Appendix Tables 9, 10, 11, there were no significant differences between groups in unadjusted analyses of inpatient opioid consumption, outpatient opioid demand, and rates of opioid fill and refill. As previously demonstrated in the adjusted models, age, sex, and additional surgery were important drivers of opioid demand.
Discussion
In this study of perioperative opioid demand in patients undergoing fixation of pelvis and acetabulum fractures with and without RA, there were significant increases in inpatient opioid consumption and outpatient opioid demand after adjusting for baseline patient characteristics. Age, sex, race, BMI, smoking, ASA score, additional injuries, open fracture, and additional surgery were significant drivers of inpatient and outpatient opioid demand metrics.
A limited number of prior studies have examined the effects of regional anesthesia in fractures of the acetabulum. Chelly et al. carried out a prospective case–control study with 26 patients to compare postoperative pain control with a continuous lumbar plexus block to patient-controlled morphine analgesia following surgical fixation of isolated acetabular fractures [38]. Patients in the lumbar plexus catheter group consumed significantly less morphine in the first 48 h postoperatively and were able to ambulate unassisted earlier. No significant differences in opioid-induced adverse effects or benefits associated with early ambulation, including mortality, were observed. Strauss et al. conducted a retrospective case–control study of 138 patients with surgically managed posterior wall fractures, comparing short-term outcomes in patients treated with a general anesthesia alone to those receiving a postoperative epidural catheter and general anesthesia [37]. There was no difference in postoperative pain scores or time to mobilization. However, pain scores were only analyzed until discharge from the post-anesthesia care unit and opioid use was not included in the outcome measures.
This study is the first to our knowledge to examine the impact of RA in fractures of the pelvis in addition to the acetabulum. We found no significant difference in opioid consumption from 0 to 48 h postoperatively in patients receiving RA. While we could not measure pain in this study, VAS pain score has previously been found to predict postoperative opioid requirements [45] and this score is the basis for oral opioid dosing at our institution. Therefore, there was likely no difference in pain levels between groups. Fracture pain is largely due to afferent signals from the disrupted periosteum, which is innervated by nerves supplying the overlying muscles [46, 47]. The pelvis and acetabulum are mainly innervated by the lumbar plexus [38], though sacral rami may also contribute to postsurgical fracture pain, particularly if fixation of the sacroiliac joint is involved [48]. Consequently, some patients with pelvic fractures receiving RA targeted to branches of the lumbar plexus alone may have experienced incomplete analgesia. Given our larger sample size and greater number of longitudinal data points compared to prior studies, we must also consider that RA may not provide superior pain relief in patients with acetabular fractures. In addition to periosteal disruption, trauma to the labrum can contribute to pain in these fractures [49]. The extent to which RA targets labral free nerve endings is unknown, though infiltration of local anesthetics into the joint and surrounding soft tissues has been proposed [50]. Lastly, both pelvic and acetabular fractures are often associated with polytrauma. In this study, 75% of patients had at least one additional injury. It is possible that while RA provided adequate analgesia to the fracture sites studied, pain from other injuries led to no significant difference in opioid use overall. Previous studies of RA in acetabular fractures excluded patients with multiple fractures, nerve injuries, and those requiring laparotomy or intubation. By including patients with additional injuries, we were able to capture data across a range of injury severity that more closely replicates the actual clinical effects of RA in these fractures.
The significant increase in opioid demand at 48–72 h postoperatively in this study likely reflects the occurrence of rebound pain. This phenomenon that has been described with RA use for other fractures as the nerve block dissipates and has been associated with high levels of opioid use [51,52,53]. While the pathophysiology of rebound pain remains debated, theories include hyperalgesia [54,55,56], anesthetic-induced nerve damage [57], and upregulation of inflammatory mediators secondary to the presence of local anesthetics [58, 59]. The onset of rebound pain varies with block technique and the type and concentration of local anesthetic used. Prior studies have reported rebound pain 12–24 h after administration of single-shot blocks [51, 60, 61]. Continuous RA catheters have been shown to delay and attenuate rebound pain, with reported onset at 24–59 h after initiation of the block [62,63,64]. In the present study, 60% of patients received continuous RA catheters, likely delaying the onset of rebound pain. Further, the heterogeneity in block locations and local anesthetics in this study could account for a longer time to observed onset of rebound pain.
While RA has the theoretical potential to prevent the development of chronic pain by blocking early nociceptive input, evidence supporting the long-term benefits of RA is weak at present. A recent systematic review reported moderate and low-quality evidence that RA could reduce chronic postoperative pain after non-orthopedic surgeries [65]. However, the conclusions were based on a limited number of small studies and these findings cannot be extended to other surgical interventions. Evidence from prior retrospective studies has indicated that RA may not reduce chronic postoperative opioid use. In patients undergoing abdominal surgery, total knee arthroplasty, and shoulder arthroplasty, no association has been found between use of RA and the risk of persistent postoperative opioid use [66,67,68].
With respect to the long-term impacts of RA on opioid use in pelvic and acetabular fractures, we found that RA was associated with increased outpatient opioid demand. This difference was evident at 90 days postoperatively but not at the earlier cumulative timepoints, suggesting that pain was high for longer in the RA group while it diminished over time in the group without RA. Patients treated with RA also had significantly higher odds of opioid refill from two weeks to 90 days postoperatively, further suggesting prolonged postsurgical pain in this group. It is possible that these results represent the consequences of postoperative rebound pain. Several studies have demonstrated that acute postoperative pain and opioid demand predict the development of long-term pain and opioid use [19, 69, 70]. Given the finding of increased opioid consumption at 48–72 h in patients with RA, it would stand to reason these patients experienced an acute rise in postoperative pain, leading to increased likelihood of developing prolonged postsurgical pain. Persistent neuropathic pain might also contribute to these findings, as it is one of the proposed mechanisms behind rebound pain and a cause of persistent maladaptive plasticity [69]. However, these inferences are limited by our inability to measure pain retrospectively and warrant further investigation.
Considering that RA is associated with increased postoperative and long-term opioid use, the potential risks and benefits of RA unique to the pelvis and acetabulum merit discussion. The addition of epidural anesthesia (EA) to general anesthesia has been shown to significantly reduce intraoperative blood loss in total hip arthroplasty [71]. A small retrospective study by Acan et al. [72] and the aforementioned study by Strauss et al. [37] found similar results in acetabular fracture surgery. Minimizing intraoperative blood loss should be weighed against the risks from EA-induced hypotension. In particular, perineural lumbar plexus blocks carry the risk of accidental intrathecal injection or epidural spread and subsequent conversion to an epidural block, causing unanticipated hemodynamic instability [73]. While thoracic EA reduces both myocardial oxygen supply via hypotension and oxygen demand through blockade of cardiac sympathetic nerves, EA isolated to the lumbar plexus is unable to offset the reduced supply with a lower oxygen requirement [74]. Thus, lumbar epidural analgesia alone can be especially problematic in procedures with high intraoperative blood loss. Patients anticoagulated with low molecular weight heparin are also at increased risk for developing an epidural hematoma after EA administration [75]. Additionally, pre-existing nerve damage may predispose to further anesthesia-related nerve injury [76]. This is an important as 12.2% of acetabular fracture patients may have injury to the sciatic nerve, particularly in fracture dislocations of the affected hip [6].
Several additional clinical considerations apply to the use of RA in fractures of the pelvis and acetabulum. The location and severity of these fractures often prevent patients from RA administration outside of the operating room. Early administration of RA would be expected to block peripheral pain signals from reaching the spinal cord, decreasing central sensitization to pain. As definitive fixation is secondary to resuscitation and stabilization, patients with pelvic and acetabular fractures are more likely to receive RA at a later timepoint and not obtain this benefit. Additional benefits of EA, including blunted sympathetic and neuroendocrine stress responses, may not be seen when implemented postoperatively [77]. While rare, complications of RA procedures include peripheral nerve injury, infection, and local anesthetic systemic toxicity [78]. Finally, nerve blockade often adds time to the perioperative experience for the patient, surgeon, and anesthesiologist, incurs additional cost to the healthcare system, and represents an additional procedure for the patient.
This study has several limitations. First, adjunctive non-opioid perioperative analgesia was not evaluated. However, it is unlikely that the adjunctive pain management strategies differed between groups. Secondly, as this was a retrospective study, we did not evaluate pain, since this would have been collected at non-standardized time intervals. Further, opioid prescribing rather than opioid consumption was measured in the outpatient setting given the retrospective nature of the study. However, we included information on opioid refills which correlates well to patient opioid demand. Of note, our study only evaluated adult patients and cannot provide insight into cases of pediatric pelvis and acetabulum trauma, which is rare and may have unique challenges with regard to pain control and operative treatment [79]. Lastly, our data included patients that received RA at a variety of anatomic locations and with varying medication type, rate, and quantity. While this heterogeneity may decrease the specificity of our results to one particular technique, we believe that analyzing the data in this fashion broadens their clinical interpretation since it more closely matches the scenario encountered in clinical practice.
In conclusion, perioperative RA in pelvis and acetabulum fracture surgery was associated with small increases in inpatient opioid consumption and outpatient opioid demand. This is important information when considering the utility of perioperative nerve blockade in this patient population.
Appendix 1
See Table 6.
Appendix 2
See Table 7.
Appendix 3
See Table 8.
Appendix 4
See Table 9.
Appendix 5
See Table 10.
Appendix 6
See Table 11.
References
Langford JR, Burgess AR, Liporace FA, Haidukewych GJ (2013) Pelvic fractures: part 1 Evaluation, classification, and resuscitation. J Am Acad Orthop Surg 21(8):448–457. https://doi.org/10.5435/JAAOS-21-08-448
Langford JR, Burgess AR, Liporace FA, Haidukewych GJ (2013) Pelvic fractures: part 2 Contemporary indications and techniques for definitive surgical management. J Am Acad Orthop Surg 21(8):458–468. https://doi.org/10.5435/JAAOS-21-08-458
Tornetta P 3rd (2001) Displaced acetabular fractures: indications for operative and nonoperative management. J Am Acad Orthop Surg 9(1):18–28. https://doi.org/10.5435/00124635-200101000-00003
Fritz A, Gericke L, Hoch A, Josten C, Osterhoff G (2020) Time-to-treatment is a risk factor for the development of pressure ulcers in elderly patients with fractures of the pelvis and acetabulum. Injury 51(2):352–356. https://doi.org/10.1016/j.injury.2019.12.007
Demetriades D, Karaiskakis M, Toutouzas K, Alo K, Velmahos G, Chan L (2002) Pelvic fractures: epidemiology and predictors of associated abdominal injuries and outcomes. J Am Coll Surg 195(1):1–10. https://doi.org/10.1016/s1072-7515(02)01197-3
Letournel É, Judet R (1993). Fractures of the Acetabulum. 2nd edn. Springer-Verlag
Rinne PP, Laitinen MK, Huttunen T, Kannus P, Mattila VM (2017) The incidence and trauma mechanisms of acetabular fractures: a nationwide study in Finland between 1997 and 2014. Injury 48(10):2157–2161. https://doi.org/10.1016/j.injury.2017.08.003
Tarabadkar N, Alton T, Gorbaty J, Nork S, Taitman L, Kleweno C (2018) Trends in orthopedic fracture and injury severity: a level i trauma center experience. Orthopedics 41(2):e211–e216. https://doi.org/10.3928/01477447-20180103-01
Marmor M, El Naga AN, Barker J, Matz J, Stergiadou S, Miclau T (2020) Management of pelvic ring injury patients with hemodynamic instability. Front Surg 7:588845. https://doi.org/10.3389/fsurg.2020.588845
Suzuki T, Shindo M, Soma K, Minehara H, Nakamura K, Uchino M, Itoman M (2007) Long-term functional outcome after unstable pelvic ring fracture. J Trauma 63(4):884–888. https://doi.org/10.1097/01.ta.0000235888.90489.fc
Lefaivre KA, Slobogean GP, Valeriote J, O’Brien PJ, Macadam SA (2012) Reporting and interpretation of the functional outcomes after the surgical treatment of disruptions of the pelvic ring: a systematic review. J Bone Joint Surg Br 94(4):549–555. https://doi.org/10.1302/0301-620X.94B4.27960
Gerbershagen HJ, Dagtekin O, Isenberg J, Martens N, Ozgur E, Krep H, Sabatowski R, Petzke F (2010) Chronic pain and disability after pelvic and acetabular fractures–assessment with the mainz pain staging system. J Trauma 69(1):128–136. https://doi.org/10.1097/TA.0b013e3181bbd703
Schafer SJ, Schafer LO, Anglen JO, Childers M (2000) Heterotopic ossification in rehabilitation patients who have had internal fixation of an acetabular fracture. J Rehabil Res Dev 37(4):389–393
Stibolt RD Jr, Patel HA, Huntley SR, Lehtonen EJ, Shah AB, Naranje SM (2018) Total hip arthroplasty for posttraumatic osteoarthritis following acetabular fracture: a systematic review of characteristics, outcomes, and complications. Chin J Traumatol 21(3):176–181. https://doi.org/10.1016/j.cjtee.2018.02.004
Kessler ER, Shah M, Gruschkus SK, Raju A (2013) Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy 33(4):383–391. https://doi.org/10.1002/phar.1223
Gan TJ, Lubarsky DA, Flood EM, Thanh T, Mauskopf J, Mayne T, Chen C (2004) Patient preferences for acute pain treatment. Br J Anaesth 92(5):681–688. https://doi.org/10.1093/bja/aeh123
Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM (2010). Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010 (1):CD006605. https://doi.org/10.1002/14651858.CD006605.pub2
Vakharia RM, Sabeh KG, Vakharia AM, Damodar DM, Law TY, Roche MW (2019) Comparison of implant related complications amongst patients with opioid use disorder and non-users following total knee arthroplasty. World J Orthop 10(3):137–144. https://doi.org/10.5312/wjo.v10.i3.137
Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E (2017) Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg 125(5):1733–1740. https://doi.org/10.1213/ANE.0000000000002458
Weiss RJ, Montgomery SM, Stiller C-O, Wick MC, Jansson K-Å (2012) Long-term follow-up of opioid use in patients with acetabular fractures. Injury Extra 43(7):49–53. https://doi.org/10.1016/j.injury.2012.03.027
Cunningham D, LaRose M, Yoon RS, Gage MJ (2020) Factors associated with perioperative opioid demand in lower extremity fractures: does consumption vary by anatomic location? Injury. https://doi.org/10.1016/j.injury.2020.10.038
Cunningham DJ, LaRose MA, Gage MJ (2020) The impact of mental health and substance use on opioid demand after hip fracture surgery. J Am Acad Orthop Surg. https://doi.org/10.5435/JAAOS-D-20-00146
Cunningham D, LaRose M, Gage M (2020) The impact of substance use and abuse on opioid demand in lower extremity fracture surgery. J Orthop Trauma. https://doi.org/10.1097/BOT.0000000000001958
Cunningham DJ, LaRose MA, Klifto CS, Gage MJ (2020) Mental health and substance use affect perioperative opioid demand in upper extremity trauma surgery. J Shoulder Elbow Surg. https://doi.org/10.1016/j.jse.2020.06.024
Hedegaard H, Warner M, Minino AM (2017) Drug overdose deaths in the united states, 1999–2016. NCHS Data Brief 294:1–8
Hsu JR, Mir H, Wally MK, Seymour RB, Task OTAMP, F (2019) Clinical practice guidelines for pain management in acute musculoskeletal injury. J Orthop Trauma 33(5):e158–e182. https://doi.org/10.1097/BOT.0000000000001430
Manchikanti L, Helm S, 2nd, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV (2012) Opioid epidemic in the United States. Pain Physician 15 (3 Suppl):ES9–38
Morris BJ, Mir HR (2015) The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg 23(5):267–271. https://doi.org/10.5435/JAAOS-D-14-00163
Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR (2011) Characteristics of opioid prescriptions in 2009. JAMA 305(13):1299–1301. https://doi.org/10.1001/jama.2011.401
Opioid prescription limits and policies by state. (2020). https://ballotpedia.org/Opioid_prescription_limits_and_policies_by_state. Accessed 5/25/2020 2020
Prevention CfDCa (2020) Understanding the Epidemic.
Services USDoHaH (2018) Strategy to Combat Opioid Abuse, Misuse, and Overdose.
Flanagan CD, Wysong EF, Ramey JS, Vallier HA (2018) Understanding the opioid epidemic: factors predictive of inpatient and postdischarge prescription opioid use after orthopaedic trauma. J Orthop Trauma 32(10):e408–e414. https://doi.org/10.1097/BOT.0000000000001256
Gadsden J, Warlick A (2015) Regional anesthesia for the trauma patient: improving patient outcomes. Local Reg Anesth 8:45–55. https://doi.org/10.2147/LRA.S55322
Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, McGready J, Cohen SR, Wu CL (2006) Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis Anesth Analg 102(1):248–257. https://doi.org/10.1213/01.ANE.0000181289.09675.7D
Gray A, Kehlet H, Bonnet F, Rawal N (2005) Predicting postoperative analgesia outcomes: NNT league tables or procedure-specific evidence? Br J Anaesth 94(6):710–714. https://doi.org/10.1093/bja/aei144
Strauss JE, O’Toole RV, Pollak AN (2012) Does supplemental epidural anesthesia improve outcomes of acetabular fracture surgery? J Orthop Trauma 26(2):67–72. https://doi.org/10.1097/BOT.0b013e31821cfc5b
Chelly JE, Casati A, Al-Samsam T, Coupe K, Criswell A, Tucker J (2003) Continuous lumbar plexus block for acute postoperative pain management after open reduction and internal fixation of acetabular fractures. J Orthop Trauma 17(5):362–367. https://doi.org/10.1097/00005131-200305000-00007
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2014) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 12(12):1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013
National Center for Injury Prevention and Control CfDCaP (2018). CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors.
Doyle DJ, Goyal A, Bansal P, Garmon EH (2021). American Society of Anesthesiologists Classification. In: StatPearls. Treasure Island (FL)
Prevention. CfDCa (2016) Definitions Relevant to the Clinical QI Opioid Measures. https://www.cdc.gov/drugoverdose/pdf/prescribing/CDC-DUIP-FactSheet-At-A-Glance_Opioid-Measures-508.pdf. Accessed 5/28 2020
Glynn AN, Quinn KM (2010) An introduction to the augmented inverse propensity weighted estimator. Polit Anal 18(1):36–56. https://doi.org/10.1093/pan/mpp036
Antonio Olmos PG (2015) A practical guide for using propensity score weighting in R. Pract Assess Res Eval 20:13
Aubrun F, Langeron O, Quesnel C, Coriat P, Riou B (2003) Relationships between measurement of pain using visual analog score and morphine requirements during postoperative intravenous morphine titration. Anesthesiology 98(6):1415–1421. https://doi.org/10.1097/00000542-200306000-00017
Nencini S, Ivanusic JJ (2016) The physiology of bone pain how much do we really know? Front Physiol 7:157. https://doi.org/10.3389/fphys.2016.00157
McGlone R, Sadhra K, Hamer DW, Pritty PE (1987) Femoral nerve block in the initial management of femoral shaft fractures. Arch Emerg Med 4(3):163–168. https://doi.org/10.1136/emj.4.3.163
Vleeming A, Schuenke MD, Masi AT, Carreiro JE, Danneels L, Willard FH (2012) The sacroiliac joint: an overview of its anatomy, function and potential clinical implications. J Anat 221(6):537–567. https://doi.org/10.1111/j.1469-7580.2012.01564.x
Gerhardt M, Johnson K, Atkinson R, Snow B, Shaw C, Brown A, Vangsness CT Jr (2012) Characterisation and classification of the neural anatomy in the human hip joint. Hip Int 22(1):75–81. https://doi.org/10.5301/HIP.2012.9042
Langworthy MJ, Sanzone AG (2018) Multimodal pain strategies including liposomal bupivacaine for isolated acetabular fracture surgery. J Orthop Trauma 32(Suppl 2):S11–S15. https://doi.org/10.1097/BOT.0000000000001228
Sort R, Brorson S, Gogenur I, Nielsen JK, Moller AM (2019) Rebound pain following peripheral nerve block anaesthesia in acute ankle fracture surgery: an exploratory pilot study. Acta Anaesthesiol Scand 63(3):396–402. https://doi.org/10.1111/aas.13290
Dada O, Gonzalez Zacarias A, Ongaigui C, Echeverria-Villalobos M, Kushelev M, Bergese SD, Moran K (2019) Does rebound pain after peripheral nerve block for orthopedic surgery impact postoperative analgesia and opioid consumption? A narrative review. Int J Environ Res Public Health 16(18):3257. https://doi.org/10.3390/ijerph16183257
Henningsen MJ, Sort R, Moller AM, Herling SF (2018) Peripheral nerve block in ankle fracture surgery: a qualitative study of patients’ experiences. Anaesthesia 73(1):49–58. https://doi.org/10.1111/anae.14088
Kolarczyk LM, Williams BA (2011) Transient heat hyperalgesia during resolution of ropivacaine sciatic nerve block in the rat. Reg Anesth Pain Med 36(3):220–224. https://doi.org/10.1097/AAP.0b013e3182176f5a
Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R (2010) Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med 35(5):427–431. https://doi.org/10.1097/AAP.0b013e3181ef4cf0
Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R (2009) Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology 111(5):1111–1119. https://doi.org/10.1097/ALN.0b013e3181bbcc26
An K, Elkassabany NM, Liu J (2015) Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS ONE 10(4):e0123459. https://doi.org/10.1371/journal.pone.0123459
Gordon SM, Chuang BP, Wang XM, Hamza MA, Rowan JS, Brahim JS, Dionne RA (2008) The differential effects of bupivacaine and lidocaine on prostaglandin E2 release, cyclooxygenase gene expression and pain in a clinical pain model. Anesth Analg 106 (1):321–327, table of contents. doi:https://doi.org/10.1213/01.ane.0000296474.79437.23
Kroin JS, Buvanendran A, Watts DE, Saha C, Tuman KJ (2006) Upregulation of cerebrospinal fluid and peripheral prostaglandin E2 in a rat postoperative pain model. Anesth Analg 103 (2):334–343, table of contents. doi:https://doi.org/10.1213/01.ane.0000223674.52364.5c
Goldstein RY, Montero N, Jain SK, Egol KA, Tejwani NC (2012) Efficacy of popliteal block in postoperative pain control after ankle fracture fixation: a prospective randomized study. J Orthop Trauma 26(10):557–561. https://doi.org/10.1097/BOT.0b013e3182638b25
Abdallah FW, Halpern SH, Aoyama K, Brull R (2015) Will the real benefits of single-shot interscalene block please stand up? a systematic review and meta-analysis. Anesth Analg 120(5):1114–1129. https://doi.org/10.1213/ANE.0000000000000688
Capdevila X, Pirat P, Bringuier S, Gaertner E, Singelyn F, Bernard N, Choquet O, Bouaziz H, Bonnet F, French Study Group on Continuous Peripheral Nerve B 2005 Continuous peripheral nerve blocks in hospital wards after orthopedic surgery: a multicenter prospective analysis of the quality of postoperative analgesia and complications in 1,416 patients Anesthesiology 103 5 1035 1045 https://doi.org/10.1097/00000542-200511000-00018
Williams BA, Bottegal MT, Kentor ML, Irrgang JJ, Williams JP (2007) Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med 32(3):186–192. https://doi.org/10.1016/j.rapm.2006.10.011
Ding DY, Manoli A 3rd, Galos DK, Jain S, Tejwani NC (2015) Continuous popliteal sciatic nerve block versus single injection nerve block for ankle fracture surgery: a prospective randomized comparative trial. J Orthop Trauma 29(9):393–398. https://doi.org/10.1097/BOT.0000000000000374
Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, Hall CB, Andreae MH (2018). Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. 6 (6):CD007105. doi:https://doi.org/10.1002/14651858.CD007105.pub4
Ladha KS, Patorno E, Liu J, Bateman BT (2016) Impact of perioperative epidural placement on postdischarge opioid use in patients undergoing abdominal surgery. Anesthesiology 124(2):396–403. https://doi.org/10.1097/ALN.0000000000000952
Sun EC, Bateman BT, Memtsoudis SG, Neuman MD, Mariano ER, Baker LC (2017) Lack of association between the use of nerve blockade and the risk of postoperative chronic opioid use among patients undergoing total knee arthroplasty: evidence from the marketscan database. Anesth Analg 125(3):999–1007. https://doi.org/10.1213/ANE.0000000000001943
Mueller KG, Memtsoudis SG, Mariano ER, Baker LC, Mackey S, Sun EC (2017) Lack of association between the use of nerve blockade and the risk of persistent opioid use among patients undergoing shoulder arthroplasty: evidence from the marketscan database. Anesth Analg 125(3):1014–1020. https://doi.org/10.1213/ANE.0000000000002031
Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. Lancet 367(9522):1618–1625. https://doi.org/10.1016/S0140-6736(06)68700-X
Hsia HL, Takemoto S, van de Ven T, Pyati S, Buchheit T, Ray N, Wellman S, Kuo A, Wallace A, Raghunathan K (2018) Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med 43(7):705–711. https://doi.org/10.1097/AAP.0000000000000831
Dauphin A, Raymer KE, Stanton EB, Fuller HD (1997) Comparison of general anesthesia with and without lumbar epidural for total hip arthroplasty: effects of epidural block on hip arthroplasty. J Clin Anesth 9(3):200–203. https://doi.org/10.1016/s0952-8180(97)00035-4
Acan AE, Kilinc CY, Gultac E, Altiparmak B, Uysal AI, Aydogan NH (2020) Effects of different anesthesia techniques on intraoperative blood loss in acetabular fractures undergoing the modified stoppa approach. Ulus Travma Acil Cerrahi Derg 26(3):445–452. https://doi.org/10.14744/tjtes.2019.09294
Bendtsen TF, Pedersen EM, Haroutounian S, Soballe K, Moriggl B, Nikolajsen L, Hasselstrom JB, Fisker AK, Strid JM, Iversen B, Borglum J (2014) The suprasacral parallel shift vs lumbar plexus blockade with ultrasound guidance in healthy volunteers–a randomised controlled trial. Anaesthesia 69(11):1227–1240. https://doi.org/10.1111/anae.12753
Grass JA (2000) The role of epidural anesthesia and analgesia in postoperative outcome. Anesthesiol Clin North Am 18(2):407–428. https://doi.org/10.1016/s0889-8537(05)70170-x
Lumpkin MM (1998) FDA public health advisory. Anesthesiology 88(2):27A-28A. https://doi.org/10.1097/00000542-199802000-00001
Neal JM, Hebl JR, Gerancher JC, Hogan QH (2002) Brachial plexus anesthesia: essentials of our current understanding. Reg Anesth Pain Med 27(4):402–428. https://doi.org/10.1053/rapm.2002.34377
Moraca RJ, Sheldon DG, Thirlby RC (2003) The role of epidural anesthesia and analgesia in surgical practice. Ann Surg 238(5):663–673. https://doi.org/10.1097/01.sla.0000094300.36689.ad
Jeng CL, Torrillo TM, Rosenblatt MA (2010) Complications of peripheral nerve blocks. Br J Anaesth 105(Suppl 1):i97-107. https://doi.org/10.1093/bja/aeq273
Karunakar MA, Goulet JA, Mueller KL, Bedi A, Le TT (2005) Operative treatment of unstable pediatric pelvis and acetabular fractures. J Pediatr Orthop 25(1):34–38. https://doi.org/10.1097/00004694-200501000-00009
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no competing interest.
Ethics Approval
Yes, study approved by Institutional Review Board.
Informed consent
Not applicable for retrospective, observational study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cunningham, D.J., Robinette, J.P., Paniagua, A.R. et al. Regional anesthesia does not decrease opioid demand in pelvis and acetabulum fracture surgery. Eur J Orthop Surg Traumatol 32, 1357–1370 (2022). https://doi.org/10.1007/s00590-021-03114-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-021-03114-w