Abstract
Introduction
Diagnosis of periprosthetic infections is challenging. The aim of this study was to compare the diagnostic accuracy of conventional periprosthetic tissue culture and culture of sonication fluid of the explanted prostheses.
Methods
We prospectively enrolled 114 patients undergoing revision hip or knee arthroplasty because of loosening of the prostheses, at our institution, between July 2012 and July 2016. Patients’ medical history and demographic characteristics were recorded. The explanted hardware was separated in sterile containers and sonicated under sterile conditions. At least five samples of periprosthetic tissue were sent for culture and histological examination. We compared the culture of samples obtained by sonication of explanted hip and knee prostheses with conventional culture of periprosthetic tissue for the microbiological diagnosis of prosthetic joint infection.
Results
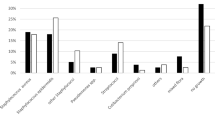
Infectious Diseases Society of America guidelines were used for the definition of prosthetic joint infection. Sixty-one patients had periprosthetic infection and 53 aseptic loosening (73 hip prostheses and 41 knee prostheses). The sensitivity of sonication fluid culture was 77.04%, and the sensitivity of conventional tissue cultures was 55.73% (p value = 0.012). The specificities of the two methods were 98.11 and 94.34%, respectively. The sensitivity of the histopathological examination of the periprosthetic tissue was 72.10%. There were 17 patients with PJI where the isolated pathogen was detected in SFC but not in PTC, while in five cases the pathogen was detected only in PTC. There were nine patients where no bacteria were detected by any microbiological method and the diagnosis was based on clinical and histological findings, according to the guidelines.
Conclusions
The sonication method represents a reliable test for the diagnosis of prosthetic joint infections with a greater sensitivity and specificity than the conventional periprosthetic tissue cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hip and knee arthroplasties are two of the most successful orthopedic surgical operations, relieving chronic pain and improving quality of life. Periprosthetic joint infection (PJI) is the most devastating complication of total joint arthroplasty, associated with high morbidity, prolonged hospitalization and substantial cost [1]. The expected increasing number of arthroplasties because of demographic changes will unavoidably lead to an increase in PJIs, in the next decades [2].
Differentiation of PJI from aseptic loosening remains a challenging diagnostic problem. As the application of anti-bacterial drugs is of paramount importance in the treatment of PJI, the isolation and identification of the responsible microorganism are pivotal. PJI is considered to be associated with the presence of bacterial glycocalyx biofilms attached to the implant, where the bacteria have changed their phenotypes to an extremely tough form of life with an increased antibiotic resistance [3]. Moreover, bacteria of the biofilm enter a stationary state in which they are less susceptible to growth-dependent antibiotics [4]. Conventionally, the periprosthetic tissue culture (PTC) is the gold standard in the microbiological diagnosis of PJI. However, due to previous use of antibiotics and the formation of biofilm which protects bacteria from detection and elimination, the sensitivity of conventional culture methods does not exceed 75% [3, 5]. Thus, the disaggregation of the biofilm from the surface of the explanted prostheses enables a sampling of vital bacteria, improving the results of the culture and the outcome of the treatment of the infection.
Sonication is one of the most promising methods for the diagnosis of PJI. The diagnostic value of the application of long-wave ultrasound before cultures for the disruption of the prostheses biofilm and the enhancement of the bacterial growth has been introduced by Trampuz et al. in 2007 [6]. The method dislodges the adherent bacteria, providing a significant better recovery of bacterial growth in culture than the conventional culture of periprosthetic tissue samples for the microbiological diagnosis of PJIs. The purpose of this prospective study was to compare the diagnostic accuracy of conventional periprosthetic tissue culture and culture of fluid derived from vortexing and bath sonication of the explanted total hip and knee prostheses, evaluating the usefulness of sonication fluid culture (SFC) in the diagnosis of PJI.
Materials and methods
Patient selection
Between July 2012 and July 2016, we investigated 114 patients, 29 men and 85 women, undergoing revision hip or knee arthroplasty because of loosening of the prostheses, at our hospital. Seventy-three patients had undergone total hip replacement and 41 total knee replacements. Preoperative measures of C-reactive protein (CRP) and erythrocyte sedimentation rates (ESR) were obtained in all subjects. The explanted hardware was separated in sterile containers and sonicated under sterile conditions. At least five samples of periprosthetic tissue were sent for culture and histological examination. Demographic characteristics, clinical, laboratory and microbiological data of the patients were recorded. The study design was assessed and approved by the institutional review board before the beginning of the study.
Patient eligibility
For the definition of PJI, Infectious Diseases Society of America (IDSA) guidelines were used [7]. According to these guidelines, one of the following criteria is definitive evidence of PJI: (a) presence of sinus tract that communicates with the prosthesis, (b) presence of acute inflammation on the histopathological examination of the periprosthetic tissue, (c) presence of visible purulence surrounding the prosthesis and (d) two or more positive intraoperative PTCs or positive SFC. Aseptic failure was defined as loosening of the prosthesis in the absence of any the above criteria. SFC was considered positive when it yielded >50 colony-forming units (CFU)/ml of the same organism. Isolation of a bacterial species in a single tissue sample or yielding <50 CFU/ml in sonication fluid was classified as false positive. PJIs were classified according to the onset of symptoms, as early, delayed and late (less than 3 months, 3–24 months, more than 24 months, respectively) [8]. Inflammation in the histopathological examination was defined as >5 neutrophils per high-power field. Previous antimicrobial therapy was defined as administration of antimicrobial agents during the 14 days before removal of the prostheses.
Periprosthetic tissue cultures
For all patients, at least five intraoperative periprosthetic tissue specimens were collected from the bone–cement/bone–prosthesis interface, from capsule and from soft tissues with obvious inflammatory changes. Tissue specimens were located into sterile boxes and individually homogenized in 3 ml Trypticase soy broth for 1 min using mortar and pestle. Tissue homogenate samples were inoculated in 0.1 ml aliquots onto aerobic (SBA) and anaerobic sheep blood agar (ASBA) plates and in 1 ml aliquots into thioglycollate broth. The cultures were incubated at 37 °C for 10 days. A terminal subculture was performed from all thioglycollate broth specimens on blood agar plates and incubated at 37 °C for 5 more days. Positive tissue cultures were considered those with the same microorganism isolation of at least two periprosthetic tissue samples. Each unique colony of isolated microorganisms was identified, and their antimicrobial susceptibility was tested by using standard automatic methods (Vitek-2 system; bio Mérieux, Marcy L’Etoile, France). Two more periprosthetic tissue samples were sent for histological examination.
Sonication fluid cultures
The explanted prosthesis (or its components) was aseptically removed in the operating room and located in a widemouthed, sterile, solid airtight container (Lock & Lock; Vertrag AG, Stafa, Switzerland). The implant was transported to the microbiology laboratory and sonicated within 6 h, inside an ultrasound bath (Figs. 1, 2). Sonication of the implant was performed according to the method described by Trampuz et al. [6]. Aliquots of 0.1 ml sonicate fluid were inoculated onto sheep blood agar (SBA) and anaerobic sheep blood agar (ASBA) plates. Additionally, 1 ml of the remaining of sonication fluid was added in 10 ml thioglycollate broth (TGB). The SBA plates and TSB were incubated at 37 °C aerobically and the ASBA plates and TGB at 37 °C anaerobically and inspected daily for bacterial growth.
Statistical analysis
The sensitivities, specificities, positive predictive values and negative predictive values of the different methods were calculated with two-by-two contingency tables. Ninety-five percent confidence intervals (95% CI) were calculated as exact binomial confidence intervals. The sensitivity of the different culture methods was compared by McNemar’s test of paired proportions. A probability p value <0.05 was considered statistically significant. Statistical analysis was performed using the PASW 18 (SPSS release 18.0; SPSS Inc., Chicago, Illinois).
Results
During the study period, 114 patients who were operated because of loosening of total hip and knee arthroplasty were enrolled. Sixty-one patients were revised because of septic loosening and 53 because of aseptic loosening. Demographics, reason for arthroplasty, age of implants, preoperative and perioperative findings are shown in Table 1. The mean age of the patients was 70 years (range 45–88 years). Primary osteoarthritis was the most common cause for both total hip and knee arthroplasty. The mean time from implantation to revision or resection surgery was longer in patients with aseptic loosening than in patients with periprosthetic infection. There were 8 early PJIs, 17 delayed PJIs and 36 late PJIs. No patient received any previous antimicrobial therapy during the 14 days before removal of the prostheses.
Table 2 demonstrates the sensitivity of the culture of sonication fluid versus the culture of periprosthetic tissue in different study groups. The overall sensitivity of SFC was 77.05% (95% CI 0.65–0.87), and the sensitivity of PTC was 55.74% (95% CI 0.42–0.68, p value = 0.01). Moreover, SFC had a higher specificity (98.11%) in comparison with PTC (94.34%). The sensitivity of the histopathological examination of the periprosthetic tissue was 72.10%. As shown in Table 2, sensitivity of SFC is superior to the sensitivity of PTC in all study groups, with a statistical significance in periprosthetic hip infections (p value = 0.02). Table 3 compares specificity, positive predictive value and negative predictive value between the two methods. There was one case of aseptic loosening with a false-positive SFC that yielded 10 CFU/ml considered as contamination by a coagulase-negative staphylococcus. Respectively, we observed three cases with a false-positive PTC, where bacteria were detected in only one sample of periprosthetic tissue. There were 17 patients with PJI where the isolated pathogen was detected in SFC but not in PTC, while in five cases the pathogen was detected only in PTC. There were nine patients where no bacteria were detected by any microbiological method and the diagnosis was based on clinical and histological findings, according to IDSA guidelines. In cases of infection, coagulase-negative staphylococci were isolated in 42.3% of patients; staphylococcus aureus was isolated in 13.5% of patients, Gram-negative bacteria in 32.7% of patients and other bacteria in 11.5% of patients. PTC was positive in 65.7% of Gram (+) infections and in 64.7% of Gram (−) infections, while the corresponding rates for SFC were 85.7 and 94.1%, respectively.
Discussion
PJI is one of the most demanding complications in reconstructive hip and knee surgery. Early and reliable diagnosis is important, as it allows selection of the best antimicrobial therapy. Understanding the etiology of infection is significant. In PJIs, bacteria have the capacity to tightly adhere to artificial surfaces, forming protective biofilms, which make them resistant to antibiotics and difficult to detect with conventional tissue cultures [6, 9]. Dapunt et al. [10] have supported the theory than the presence of biofilm elicits an inflammatory response which triggers the generation of bone-resorbing osteoclasts, leading to osteolysis and loosening. Classical culture methods often fail to detect the pathogen, and considerable effort has been focused on developing alternative approaches of bacteria identification. Sonication dislodges these bacteria from the prosthesis allowing them to be cultured, without disturbing their viability [6]. In the present study, we compared the results of microbiological cultures from sonication fluid with classical microbiological cultures of periprosthetic tissues.
The results of our study demonstrate that in our group of hip and knee PJIs, SFC had an overall sensitivity of 77.04% in comparison with 55.73% sensitivity of PTC. This difference was found to be statistically significant (p value = 0.012). In 17 patients, the diagnosis of PJI was based only on the SFC, as no bacteria were detected on the PTC, supporting the hypothesis that sonication increases the chance of bacteria isolation in culture-negative PJIs. Our results are in agreement with the study by Trampuz et al. [6] with similar numbers of reported sensitivity for SFC (78.5%) and PTC (60.8%). Furthermore, the higher sensitivity of sonication method, ranging from 67 to 91%, as opposed to tissue cultures, has been confirmed by many previous studies [11,12,13,14,15,16,17,18,19]. Two recent meta-analyses showed that SFC is of great value for the diagnosis of PJI with a greater sensitivity than PTC (79%), especially for patients with previous antibiotic treatment [20, 21]. On the contrary, Kempthorne et al. [22] have found that SFC is less sensitive than PTC, but the lack of use of histopathological examination may have underestimated the true incidence of PJI.
In our study, no patient received antibiotics for at least 14 days prior to revision surgery. In the large prospective study of Trampuz et al. [6] including 331 cases, the sensitivity of SFC was statistically significant over standard tissue cultures in cases where antibiotics have been administered within 14 days from revision surgery. A further study of 112 PJIs also commented on the negative effect of recent antibiotic administration on the sensitivity of PTC but not on the sensitivity of the SFC [23]. Scorzolini et al. [18] found that the sensitivity of SFC was not affected by the timing of antibiotic interruption before surgery. On the contrary, the study by Puig-Verdie et al. [9] of 317 PJIs did not confirm these findings; nevertheless, the authors acknowledged that only 19 patients had antibiotics within 14 days from revision surgery.
Our methodology aimed to ensure maximum sensitivity of both SFC and PTC. At least five tissue specimens were collected intraoperatively as this has been shown to increase the sensitivity of PTC at its maximum [6, 24]. Before sonication, the specimens were vortexed for 30 s. Vortexing has been shown to increase the positive cultures following sonication of prosthesis [6, 25]. Vortexing alone has been shown to have an inferior result as opposed to vortexing and subsequent sonication [26, 27]. All the prostheses were sonicated within solid containers in order to avoid the possibility of contamination as shown in studies where plastic bags have been used. In our study, we performed standard culture of the sonication fluid. Moreover, the high cutoff value of 50 CFU/ml for the SFC was set to avoid false-positive results due to contamination.
Sonication with subsequent polymerase chain reaction (PCR) of the cultured fluid has been shown to have improved results [15, 28]. A further study demonstrated that combination of sonication and PCR can identify microorganisms in presumed aseptic loosening with negative PTC [29]. Nevertheless, the widely reported range of specificity of this technique has raised concerns as to its broad application [24]. We feel that, at present, there is no definitive evidence for the routine use of PCR of the sonicate fluid of hip and knee prostheses. A few studies have been published, reporting that the use of dithiothreitol is more sensitive than the sonication method, but its diagnostic value needs to be further investigated [30,31,32].
In our study, SFC demonstrated a better sensitivity of 80.6% in patients with late infections as opposed to 61.1% of PTC. In agreement is a recent study of 317 PJIs where SFC was found to have higher diagnostic accuracy in patients with late infections. The theory behind this is that in acute infections the microorganisms have not formed biofilms as yet, and therefore, tissue culture has also good sensitivity [9]. Nevertheless, in our study, SFC demonstrated higher sensitivity in early infections as well (87.5%) as opposed to PTCs (62.5%). However, these differences in sensitivity are not statistically significant. In 2013, the International Consensus Meeting on Periprosthetic Joint Infection advocated against the routine sonication of explanted prosthesis. The group concluded that SFC should be used in cases of suspected or proven prosthetic joint infections in which preoperative aspirates have failed to reveal any pathogens and in cases where antibiotics have been administered within 2 weeks from revision surgery [24]. The study by Puig-Verdie et al. [9] declared that the SFC is recommended only in delayed implant failures. On the other hand, other published studies have come to conclusion that the sonication method is quite reliable and sufficient for pathogen detection in the clinical diagnostic routine [33,34,35]. We believe that the use of sonication is a cheap and useful test, with high sensitivity in all patient subcategories and should be applied as a routine in PJI investigation.
Table 2 shows statistically significant superiority of the SFC in periprosthetic hip infections but not in knee infections. A possible explanation would be the wider use of antibiotic-loaded cement in total knee arthroplasties, which could affect the structure of the biofilm, decreasing the sensitivity of SFC. Moreover, the absolute number of periprosthetic knee infections in this study is relatively low, reducing the possibility of statistically significant results.
Limitations of our study include the relatively small number of patients as well as that only hip and knee arthroplasties were included. No molecular diagnostic method, such as PCR analysis of the sonicate fluid, was used to confirm bacterial isolation. Furthermore, none of the patients received antibiotics within 2 weeks from surgery which did not give us the opportunity to assess the effect of this parameter in the comparison between sonicate fluid and tissue cultures.
The results of our study demonstrate that SFC has a statistically higher sensitivity than PTC for PJIs around knee and hip arthroplasties. The technique is simple and can be performed in most microbiological laboratories. Taking into consideration that in patients with PJIs the tissue cultures are occasionally negative, we advocate the broad use of sonication as a particularly valuable diagnostic tool. Our findings need to be confirmed in a larger patient series and in other joint arthroplasties.
References
Bozic KJ, Kurtz SM, Lau E et al (2010) The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468:45–51
Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J (2012) Economic burden of periprosthetic joint infection in the United States. J Arthroplast 27(61–65):e61
Costerton JW, Montanaro L, Arciola CR (2005) Biofilm in implant infections: its production and regulation. Int J Artif Organs 28:1062–1068
Trampuz A, Zimmerli W (2005) Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly 135:243–251
Buret A, Ward KH, Olson ME, Costerton JW (1991) An in vivo model to study the pathobiology of infectious biofilms on biomaterial surfaces. J Biomed Mater Res 25:865–874
Trampuz A, Piper KE, Jacobson MJ et al (2007) Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663
Osmon DR, Berbari EF, Berendt AR et al (2013) Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis 56:e1–e25
Zimmerli W, Ochsner PE (2003) Management of infection associated with prosthetic joints. Infection 31:99–108
Puig-Verdie L, Alentorn-Geli E, Gonzalez-Cuevas A et al (2013) Implant sonication increases the diagnostic accuracy of infection in patients with delayed, but not early, orthopaedic implant failure. Bone Joint J 95-B:244–249
Dapunt U, Lehner B, Burckhardt I, Zimmermann S, Hansch GM, Ewerbeck V (2014) Evaluation of implant sonication as a diagnostic tool in implant-associated infections. J Appl Biomater Funct Mater 12:135–140
Esteban J, Gomez-Barrena E, Cordero J, Martin-de-Hijas NZ, Kinnari TJ, Fernandez-Roblas R (2008) Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J Clin Microbiol 46:488–492
Evangelopoulos DS, Stathopoulos IP, Morassi GP et al (2013) Sonication: a valuable technique for diagnosis and treatment of periprosthetic joint infections. Sci World J 2013:375140
Portillo ME, Salvado M, Alier A et al (2014) Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J Infect 69:35–41
Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S, Perka C (2013) Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int Orthop 37:931–936
Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A (2010) Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol 48:1208–1214
Vergidis P, Greenwood-Quaintance KE, Sanchez-Sotelo J et al (2011) Implant sonication for the diagnosis of prosthetic elbow infection. J Shoulder Elbow Surg 20:1275–1281
Bogut A, Niedzwiadek J, Koziol-Montewka M et al (2014) Sonication as a diagnostic approach used to investigate the infectious etiology of prosthetic hip joint loosening. Pol J Microbiol 63:299–306
Scorzolini L, Lichtner M, Iannetta M et al (2014) Sonication technique improves microbiological diagnosis in patients treated with antibiotics before surgery for prosthetic joint infections. New Microbiol 37:321–328
Hischebeth GT, Randau TM, Molitor E et al (2016) Comparison of bacterial growth in sonication fluid cultures with periprosthetic membranes and with cultures of biopsies for diagnosing periprosthetic joint infection. Diagn Microbiol Infect Dis 84:112–115
Zhai Z, Li H, Qin A et al (2014) Meta-analysis of sonication fluid samples from prosthetic components for diagnosis of infection after total joint arthroplasty. J Clin Microbiol 52:1730–1736
Liu H, Zhang Y, Li L, Zou HC (2017) The application of sonication in diagnosis of periprosthetic joint infection. Eur J Clin Microbiol Infect Dis 36:1–9
Kempthorne JT, Ailabouni R, Raniga S, Hammer D, Hooper G (2015) Occult infection in aseptic joint loosening and the diagnostic role of implant sonication. Biomed Res Int 2015:946215
Sampedro MF, Huddleston PM, Piper KE et al (2010) A biofilm approach to detect bacteria on removed spinal implants. Spine 35:1218–1224 (Phila Pa 1976)
Zmistowski B, Della Valle C, Bauer TW et al (2014) Diagnosis of periprosthetic joint infection. J Arthroplast 29:77–83
Larsen LH, Lange J, Xu Y, Schonheyder HC (2012) Optimizing culture methods for diagnosis of prosthetic joint infections: a summary of modifications and improvements reported since 1995. J Med Microbiol 61:309–316
Kobayashi H, Oethinger M, Tuohy MJ, Procop GW, Bauer TW (2009) Improved detection of biofilm-formative bacteria by vortexing and sonication: a pilot study. Clin Orthop Relat Res 467:1360–1364
Portillo ME, Salvado M, Trampuz A et al (2013) Sonication versus vortexing of implants for diagnosis of prosthetic joint infection. J Clin Microbiol 51:591–594
Rak M, KavcIc M, Trebse R, Co RA (2016) Detection of bacteria with molecular methods in prosthetic joint infection: sonication fluid better than periprosthetic tissue. Acta Orthop 87:339–345
Bereza PL, Ekiel A, Augusciak-Duma A et al (2013) Identification of silent prosthetic joint infection: preliminary report of a prospective controlled study. Int Orthop 37:2037–2043
Drago L, Romano CL, Mattina R, Signori V, De Vecchi E (2012) Does dithiothreitol improve bacterial detection from infected prostheses? A pilot study. Clin Orthop Relat Res 470:2915–2925
Drago L, Signori V, De Vecchi E et al (2013) Use of dithiothreitol to improve the diagnosis of prosthetic joint infections. J Orthop Res 31:1694–1699
De Vecchi E, Bortolin M, Signori V, Romano CL, Drago L (2016) Treatment with dithiothreitol improves bacterial recovery from tissue samples in osteoarticular and joint infections. J Arthroplast 31:2867–2870
Janz V, Wassilew GI, Hasart O, Tohtz S, Perka C (2013) Improvement in the detection rate of PJI in total hip arthroplasty through multiple sonicate fluid cultures. J Orthop Res 31:2021–2024
Lass R, Giurea A, Kubista B et al (2014) Bacterial adherence to different components of total hip prosthesis in patients with prosthetic joint infection. Int Orthop 38:1597–1602
Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E (2011) Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res 29:617–622
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tani, S., Lepetsos, P., Stylianakis, A. et al. Superiority of the sonication method against conventional periprosthetic tissue cultures for diagnosis of prosthetic joint infections. Eur J Orthop Surg Traumatol 28, 51–57 (2018). https://doi.org/10.1007/s00590-017-2012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-017-2012-y