Abstract
Purpose
This study aimed to investigate the trends in infectious spondylitis over the past two decades.
Methods
We included 157 cases, from 2000 to 2020, of infectious spondylitis. The cases were divided into two groups: 00 (cases during 2000–2009; 82 cases:) and 10 (cases during 2010–2020; 75 cases) groups. Patients’ age, sex, causative organism, and localization were examined and compared between the two groups.
Results
The proportions of women in the 00 and 10 groups were 30.5% and 38.7%, respectively, with no significant difference (P = 0.28). The average age was significantly higher in the 10 group (72.6 years) than in the 00 group (68.8 years; P < 0.01). A compromised host was the cause of infection in 52.4% and 36.0% of the patients in the 00 and 10 groups, respectively, showing a significant difference. The bacterial identification rates were 70.1% and 77.3% in the 00 and 10 groups, respectively (P < 0.01), and the genus Staphylococcus was the most common bacteria. The proportions of resistant bacteria such as methicillin-resistant Staphylococcus aureus in the 00 and 10 groups were 27.3% and 6.7%, respectively (P < 0.01). Conversely, infectious diseases caused by indigenous bacteria in the oral cavity and intestines were more common in the 10group (37.8%) than in the 00 group (13.0%), showing a significant difference (P < 0.01).
Conclusion
Recently, infections caused by indigenous bacteria in the oral cavity and intestines have increased more than those caused by resistant bacteria over the past two decade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious spondylitis, which accounts for approximately 3–5% of all osteomyelitis cases in patients aged > 50 years [1], used to be considered a rare disease. However, as the life expectancy in Japan increases, the population is aging, and the number of immunocompromised patients is increasing. Infectious spondylitis has generally been associated with Staphylococcus aureus as the most common causative organism [2]. Nagashima et al. [3] reported that, in the 1950s, tuberculous spondylitis was the predominant type of infectious spondylitis and that the number of common bacteria, especially S. aureus, has been gradually increasing in addition to the increasing prevalence of methicillin-resistant S. aureus (MRSA). Although MRSA infections are a tremendous infectious disease, their incidence has been declining in recent years [4, 5], and the number of infectious spondylitis cases caused by resistant bacteria is assumed to decrease. Additionally, Japan has an aging population [6], and the aging trend is very evident. The causative agents of infectious spondylitis may have changed in recent years as demographic changes have altered the patients’ backgrounds. However, reports describing the recent trends in the pathogenesis of infectious spondylitis are lacking. In the present study, we aimed to investigate the trends of infectious spondylitis from 2000 to 2020.
Patients and methods
This retrospective single-center study enrolled all patients diagnosed with infectious spondylitis at our hospital from January 2000 to August 2020. Spinal infection was considered to be present if one of the following conditions was met: (1) the presence of a pathogen was demonstrated by microbiologic culture or Polymerase Chain Reaction; (2) infection was diagnosed based on the radiological or other imaging findings by at least two physicians; and (3) the patient had obvious symptoms of infection or obvious clinical effects of treatment with antimicrobial, antiviral, or antifungal agents. We diagnosed the infections by positive blood or local cultures. Patients with negative cultures were diagnosed after considering the clinical findings (recurrent symptoms, no improvement without antibiotics, and infectious inflammation on pathological examination). All patients in the blood culture-positive group also had the same bacteria detected in the local cultures; therefore, we concluded that there was no contamination in these cases. We excluded cases of postoperative infection from this study.
All procedures performed in the studies involving human participants were in accordance with the ethical standards, and this research was approved by the Ethics Committee of out institution (approval No. 21A142).
The present study was conducted in accordance with the guidelines stipulated in the Declaration of Helsinki, and data confidentiality was ensured. We did not directly obtain informed consent from the patients. We used the opt-out method to obtain consent from the patients to participate in this study. However, some patients died before the study. Hence, informed consent was not obtained. Ethics Committee of our institution has decided to exclude the data of the patients who died if the patients’ family refuses to provide consent based on the opt-out method.
Baseline measurements
We investigated the patients’ sex and age, identification method of the causative organism, presence or absence of an immunocompromised host, and high morbidity.
The patients were divided into the following two groups: the 00 group diagnosed from 2000 to 2009, the 10 group diagnosed from 2010 to 2020. The patients’ average age was 70.5 years (12–91 years).
A comparison was made between the two groups for each study item.
Statistical analysis
Mann–Whitney U test was used to analyze the categorical variables in the intergroup case, whereas the chi-square test was for the other variables. For cases with an expected frequency of ≤ 5, Fisher’s exact test was performed. SPSS version 27(IBM, Illinois, USA) was used in all statistical analyses, with a significance threshold set at p < 0.05.
Results
In the 00 group, 82 patients (57 men and 25 women) aged 68.8 years were included, whereas the 10 group comprised 75 cases (46 men, 29 women, average age of 72.6 years; hence, the patients in this group was significantly older than the patients in the 10 group. The proportion of susceptible hosts was significantly higher in the 00 group than in the 10 group. The proportion of susceptible hosts was not low (36.0%). The bacterial activity rate was ≥ 70% in both groups. In both groups, the highest prevalence of cases were located in the lumbar spine (Table 1).
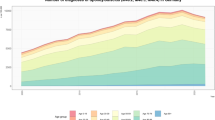
Figure 1 shows all bacteria detected from 2000 to 2020. Regarding the causative organisms, there were few cases of Mycobacteria tuberculosis and nontuberculous mycobacterial infections in both groups.
In fact, in the 00 group, there were four cases of M. tuberculosis infections and three cases of atypical M. tuberculosis infections, whereas in the 10 group, there was one case for each infection type, showing a decrease in the number of cases (Fig. 2).
The prevalence of resistant bacteria, such as MRSA, was significantly reduced from 23 (27.3%) in group 00 to only 5 (6.7%) in group 10 (Fig. 3). Conversely, the number of infections caused by oral and commensal bacteria increased significantly from 3 (13.0%) in group 00 to 17 (37.8%) in group 10 (Fig. 4).
Table 2 shows the causative bacteria detected in group 10, but not in group 00. Most of the causative bacteria were indigenous to the oral cavity and skin. They were not associated with the presence or absence of immunodeficiency, and most of the patients were aged > 70 years
Discussion
The present study demonstrated the trends in infectious spondylitis with a focus on the data from 2000. Nagashima et al. have been investigating trends in infectious spondylitis over the last 50 years, specifically from the 1950s to the 2000s [3]. Reports investigating trends in infectious spondylitis since the 2000s are lacking. A characteristic of the present study is the low number of cases of tuberculous spondylitis, which was particularly noticeable in the 10 group. A previous Japanese study has reported that the number of vascular infections peaked at 599.0 cases per 100,000 population in 1955 and decreased by 99% to 4.5 cases per 100,000 population in 2022 [7]. Despite a trend toward a younger age group, the overall downward trend in incidence rates has persisted [8], and the decrease in the number of tuberculous spondylitis cases may reflect this background.
As a result, although S. aureus was the most common organism causing the outbreak, the percentage of resistant bacteria, such as MRSA, decreased significantly in the 10 group, indicating a new change in the trend of the organism causing the outbreak. MRSA infections are known to be on a gradual downward trend [9], although outbreaks vary from region to region [10].
The trend in the 2000s was that MRSA outbreaks occurred worldwide [11,12,13], and since then, measures, such as the proper use of antibacterial agents against resistant bacteria, have been performed at each facility since 2010. It is believed that this strategy decreased the number of infectious spondylitis by resistant bacteria [9, 10]. Moreover, a previous nationwide study reported that the number of MRSA cases in Japan is decreasing [4].
Although the number of MRSA infections decreased in the present review, the number of oral and indigenous skin bacterial infections increased after 2010. Romanga et al. [14] reported that 29% of 41 patients with spinal infections had concomitant bacterial oral infections, although only 25% of these patients had oral bacteria that matched the causative bacteria detected from disk biopsies. The oral microbiota comprises many bacterial species. The detection of pathogenic bacteria has long been attempted but remains challenging, with many bacteria still uncharacterized [15, 16]. There are two main reasons for the increasing detection of these bacteria. First, the oral cavity is inherently a site with a strong biological defense. However, it is hypothesized that bacterial breakthrough of the oral defense mechanisms occurs because of a decline in host immunity. The oral hygiene status of the elderly in particular is poor [17, 18]. Klotz et al. have reported that oral hygiene in patients in their 50s and 70s was not related to sex, but that patients in their 70s had poorer oral hygiene [19]. In the present study, the number of immunocompromised patients decreased significantly in the 10 group, but the patients’ age increased, which may have strongly reflected the influence of age factors. Additionally, as previously mentioned, oral bacteria have predominantly low virulence. Traditionally, the detection of causative bacteria in tissues such as intervertebral disks or blood was primarily conducted through a biochemical analysis based on the observation of the bacterial characteristics and morphology. However, mass spectrometry has become more prevalent in recent years, potentially leading to the detection of previously undetected low-virulence bacteria [20, 21]. Since 2014, the analytical method has been modified, and it appears that it reflects the results. However, it is necessary to consider that the number of infectious diseases that are caused by bacteria that are indigenous to the oral cavity is increasing.
Regarding the increasing proportion of pyogenic spondylitis caused by oral commensal bacterial infections, especially those caused by indigenous oral bacteria as described in various case reports [22, 23], pathogenic infections due to indigenous bacterial infections have been reported in all regions in the last 10 years; therefore, this hospital alone is not sufficient. It can be considered as an unrestrained or global trend.
Infectious spondylitis is generally considered a hematogenous infection [24], and the presence of a prior infection is a factor in the development of this type of an infection. Infectious endocarditis is a well-known complication, and echocardiography and other tests are recommended to investigate the source of infection if the organism causing pyogenic spondylitis is the same as the organism that causes endocarditis.
Given the increasing incidence of oral infections, oral hygiene should also be checked when treating patients with infectious spondylitis. In fact, Gordon et al. [25]‘s study involving postoperative knee arthroplasty patients reported that a history of dental caries and dental implants correlated with postoperative complications, indicating the importance of a detailed history taking of dental treatments before arthroplasty. Although bacteremia due to an infection with oral commensal bacteria is not common, it should be considered a possible source of the initiating organism. Management of oral hygiene may also be useful in preventing flare-ups of spondylitis. The strength of the present study is that the trend in the incidence of pyogenic spondylitis has changed over the past decade, with previously undetectable organisms, such as oral commensal bacteria, now being found to be the causative organisms. Although the identification of the causative organisms is considered essential [24, 26, 27], in cases wherein the causative organisms cannot be identified, empiric antimicrobial therapy may have to considered. However, the administration of antibiotics without the identification of the causative organisms is contrary to the proper use of drugs and is a breeding ground for the development of new resistant bacteria; hence, this should be avoided whenever possible. The identification rate may be improved if we are aware of the possibility that spondylitis can develop from an oral infection.
There are several limitations to this study. First, it was a single-center study. Second, some cases were not identified. However, even though the present investigation is a single-center study, long-term reports on the recent trends in infectious spondylitis to identify its causative organisms are lacking. The identification rate of the causative organisms in our department is > 70%, with the detection rate being better in group 10 than in group 00; thus, we do not believe that we are far from the actual clinical situation.
Conclusion
Our survey of the trends in infectious spondylitis since 2000 revealed that the organisms responsible for spondylitis have changed over time. Continued research is needed to identify the most common organisms that cause spondylitis, as they will continue to change depending on the patients’ backgrounds and infection control practices.
Data availability
The data that support the findings of this study are available from the corresponding author, Shinji Tanishima, upon reasonable request.
References
Jensen AG, Espersen F, Skinhøj P, Rosdahl VT, Frimodt-Møller N (1997) Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980–1990. J Infect 34:113–118. https://doi.org/10.1016/s0163-4453(97)92395-1
Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A (2009) Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 39:10–17. https://doi.org/10.1016/j.semarthrit.2008.03.002
Nagashima H, Yamane K, Nishi T, Nanjo Y, Teshima R (2010) Recent trends in spinal infections: retrospective analysis of patients treated during the past 50 years. Int Orthop 34:395–399. https://doi.org/10.1007/s00264-009-0741-1
Tsuzuki S, Matsunaga N, Yahara K, Gu Y, Hayakawa K, Hirabayashi A, Kajihara T, Sugai M, Shibayama K, Ohmagari N (2020) National trend of blood-stream infection attributable deaths caused by Staphylococcus aureus and Escherichia coli in Japan. J Infect Chemother 26:367–371. https://doi.org/10.1016/j.jiac.2019.10.017
Wyllie DH, Walker AS, Miller R, Moore C, Williamson SR, Schlackow I, Finney JM, O’Connor L, Peto TE, Crook DW (2011) Decline of meticillin-resistant Staphylococcus aureus in Oxfordshire hospitals is strain-specific and preceded infection-control intensification. BMJ Open 1:e000160. https://doi.org/10.1136/bmjopen-2011-000160
Iijima K, Arai H, Akishita M, Endo T, Ogasawara K, Kashihara N, Hayashi YK, Yumura W, Yokode M, Ouchi Y (2021) Toward the development of a vibrant, super-aged society: the future of medicine and society in Japan. Geriatr Gerontol Int 21:601–613. https://doi.org/10.1111/ggi.14201
Ota M, Hirao S, Uchimura K (2023) Age-period-cohort analysis on tuberculosis cases in Japan, 1953–2022. Int J Mycobacteriol 12:486–490. https://doi.org/10.4103/ijmy.ijmy_188_23
Hagiya H, Koyama T, Zamami Y, Minato Y, Tatebe Y, Mikami N, Teratani Y, Ohshima A, Shinomiya K, Kitamura Y, Sendo T, Hinotsu S, Tomono K, Kano MR (2018) Trends in incidence and mortality of tuberculosis in Japan: a population-based study, 1997–2016. Epidemiol Infect 147:e38. https://doi.org/10.1017/S095026881800290X
Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. https://doi.org/10.1128/CMR.00134-14
Hassoun A, Linden PK, Friedman B (2017) Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care 21:211. https://doi.org/10.1186/s13054-017-1801-3
Allard C, Carignan A, Bergevin M, Boulais I, Tremblay V, Robichaud P, Duperval R, Pepin J (2008) Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991–2005. Clin Microbiol Infect 14:421–428. https://doi.org/10.1111/j.1469-0691.2008.01965.x
El Atrouni WI, Knoll BM, Lahr BD, Eckel-Passow JE, Sia IG, Baddour LM (2009) Temporal trends in the incidence of Staphylococcus aureus bacteremia in Olmsted county, Minnesota, 1998 to 2005: a population-based study. Clin Infect Dis 49:e130–e138. https://doi.org/10.1086/648442
Laupland KB, Ross T, Gregson DB (2008) Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis 198:336–343. https://doi.org/10.1086/589717
Romagna A, Troeltzsch M, Birkenmaier C, Schwartz C, Suchorska B, Zausinger S (2018) Oral cavity infection: an underestimated source of pyogenic spondylodiscitis? J Neurol Surg A Cent Eur Neurosurg 79:218–223. https://doi.org/10.1055/s-0037-1608823
Nakamizo S, Egawa G, Honda T, Nakajima S, Belkaid Y, Kabashima K (2015) Commensal bacteria and cutaneous immunity. Semin Immunopathol 37:73–80. https://doi.org/10.1007/s00281-014-0452-6
Devine DA, Marsh PD, Meade J (2015) Modulation of host responses by oral commensal bacteria. J Oral Microbiol 7:26941. https://doi.org/10.3402/jom.v7.26941
Grönbeck Lindén I, Hägglin C, Gahnberg L, Andersson P (2017) Factors affecting older persons’ ability to manage oral hygiene: a qualitative study. JDR Clin Trans Res 2:223–232. https://doi.org/10.1177/2380084417709267
Morishita S, Watanabe Y, Ohara Y, Edahiro A, Sato E, Suga T, Hirano H (2016) Factors associated with older adults’ need for oral hygiene management by dental professionals. Geriatr Gerontol Int 16:956–962. https://doi.org/10.1111/ggi.12585
Klotz AL, Grill SK, Hassel AJ, Rammelsberg P, Ze Nthöfer A (2020) Differences between the oral health of people aged 50 and 70 years—an exploratory cohort study. Oral Health Prev Dent 18:239–243. https://doi.org/10.3290/j.ohpd.a43363
Sauer S, Kliem M (2010) Mass spectrometry tools for the classification and identification of bacteria. Nat Rev Microbiol 8:74–82. https://doi.org/10.1038/nrmicro2243
Lavigne JP, Espinal P, Dunyach-Remy C, Messad N, Pantel A, Sotto A (2013) Mass spectrometry: a revolution in clinical microbiology? Clin Chem Lab Med 51:257–270. https://doi.org/10.1515/cclm-2012-0291
Tatara Y, Niimura T, Mihara H (2020) Paraparesis and bilateral pulmonary abscesses secondary to pyogenic spondylodiscitis caused by Streptococcus anginosus Group bacteria. Spine Surg Relat Res 4:190–191. https://doi.org/10.22603/ssrr.2019-0069
Quast MB, Carr CM, Hooten WM (2017) Multilevel lumbar spine infection due to poor dentition in an immunocompetent adult: a case report. J Med Case Rep 11(1):328. https://doi.org/10.1186/s13256-017-1492-z
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM, Petermann GW, Osmon DR, Infectious Diseases Society of America (2015) 2015 Infectious diseases society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46. https://doi.org/10.1093/cid/civ482
Gordon AM, Ng MK, Erez O, Wong CH, Mont MA (2023) The importance of oral history: does dental implant placement or caries one year before or after primary total knee arthroplasty increase medical complications and periprosthetic joint infections? J Arthroplasty 38:476–483. https://doi.org/10.1016/j.arth.2022.10.013
Gouliouris T, Aliyu SH, Brown NM (2010) Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 65(suppl 3):iii11–iii24. https://doi.org/10.1093/jac/dkq303
Nagashima H, Tanishima S, Tanida A (2018) Diagnosis and management of spinal infections. J Orthop Sci 23:8–13. https://doi.org/10.1016/j.jos.2017.09.016
Funding
This study doesn’t have any Fundings.
Author information
Authors and Affiliations
Contributions
S.T. designed the study, the main conceptual ideas, and the proof outline. C.T, T.M. and S.F and collected the data. T.M, C.T. and H.N. aided in interpreting the results and worked on the manuscript. H.N. supervised the project. S.T. wrote the manuscript with support from T.M. and C.T. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
There are no competing interests.
Ethics approval
The Ethics Committee of the Faculty of Medicine of Tottori University (approval No. 21A142).
Consent
We used the opt-out method to obtain consent from the patients to participate in this study. However, some patients died before the study. Hence, informed consent was not obtained. The Tottori University Ethics Committee has decided to exclude the data of the patients who died if the patients’ family refuses to provide consent based on the opt-out method. In fact, there were no cases waived by the families’ offer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanishima, S., Mihara, T., Takeda, C. et al. Trends in infectious spondylitis from 2000 to 2020. Eur Spine J 33, 3154–3160 (2024). https://doi.org/10.1007/s00586-024-08286-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-024-08286-7