Abstract
Purpose
Bracing is the most commonly used treatment for scoliosis. But braces remain predominantly “handcrafted.” Our objective was to create a novel brace simulator using a high-fidelity 3D “avatar” of the patient’s trunk.
Methods
An observational cross-sectional study was constructed. The inclusion criteria were patients with a moderate idiopathic scoliosis (between 15° and 35° of Cobb angle) aged between 9 and 15 years old with an indication of brace treatment. Twenty-nine scoliotic patients, 25 girls and four boys, with a mean age of 12.4 years were included. Twenty right thoracic and 14 left lumbar were measured with a mean Cobb angle of 24°. 3D “avatars” were generated using a novel technology called the “anatomy transfer.” Biomedical simulations were conducted by engineers who were blinded to the clinical effect of the real patient brace. The in-brace Cobb angle effect (real effect) was compared with the virtual numeric in-brace Cobb angle observed using the blindly constructed avatar (simulation effect).
Results
Real and simulated in-brace Cobb angle were compared using a paired two-sided Student’s t test. The real mean Cobb angle was 11° and 17° in the simulation which was statistically significant. The strength of prediction of the simulation was assessed for each individual patient; 76% of the real in-brace Cobb angles had good and moderate prediction (± 10°).
Conclusions
Incorporating high-fidelity copy of the entire 3D shape of the patient’s trunk and multiple 3D-reconstructed bony images into an anatomical reference avatar resulted in moderate-to-good prediction of brace effect in three quarters of patients.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bracing is the most commonly used treatment for scoliosis during the entire growth period of children with progressive curves. Although previously a controversial topic, it is now well documented that brace therapy is effective in preventing curve progression [1,2,3,4]. Bracing has proved to be more beneficial for smaller curves, thus it should be instituted as soon as possible once progression is confirmed [5]. In the future, identifying predictive factors of curve progression should allow clinicians to decide, even in the earliest stages of curve progression, whether it is necessary to commence brace treatment [6, 7].
To achieve satisfactory long-term results, the brace must be mechanically effective from the outset. A number of parameters need to be considered when designing a brace, including curve severity, patient age and the flexibility and location of the curve. This explains why many clinicians support the notion of patient-specific evaluation of brace treatment [8, 9].
In addition to involving complex patient-specific parameters, braces remain predominantly “handcrafted” and as such, their fabrication is highly dependent on the skill of the individual brace manufacturer. This is also why manufacturing companies of braces are not equivalent and why re-adjustments are frequently required.
An effective brace simulator could aid brace manufacturers worldwide to produce an optimal patient-specific effective brace without delay. Brace simulators are already available on the market; however, the majority relies on finite element models (FEM) that have not been clinically evaluated or validated [10,11,12,13,14,15,16,17]. The pilot studies recently published employ simple FEM that cannot ensure real-life three-dimensional (3D) brace shapes since they have only taken into account the internal skeleton of the trunk in 3D without the non-skeletal components [18, 19].
Our objective was to create a 3D “avatar” of the patient’s trunk incorporating both the “internal” and “external” components from the skin to bone. The 3D avatar generated would subsequently be submitted to the mechanical action of the real patient brace’s 3D shape. In order to evaluate the effectiveness of the simulated trunk, we compared the brace effect experienced by the patient (real effect) and the virtual numeric effect observed using the blindly constructed avatar (simulation effect).
Materials and methods
Ethical approval for this study was obtained from the local ethics committee on February 20, 2015, (CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, IRB 5891).
Study design
This pilot study is prospective, non-randomized, monocentric, observational and controlled (simulation versus real brace effect). It was developed and implemented with the assistance of the local clinical study center specializing in innovative medical devices.
It should be noted that the brace simulations performed in this study did not modify the conception and fabrication of the real braces.
Patients
All patients in the study were recruited after obtaining informed and written parental consent between January 2016 and December 2017. Informed consent forms are available on request. Inclusion criteria were as follows:
-
Age 9–15 years old
-
Idiopathic scoliosis (lumbar and/or thoracic curve);
-
Moderate curve: Cobb angle of between 15° and 35° and
-
Brace treatment indicated
Few patients with progressive curves under 20° were included, which is not strictly in-line with the SRS guidelines. In our institution, we use nighttime bracing (with Chêneau or Charleston brace) for progressive small curves that appear very effective in preventing further progression [20].
Thirty patients were initially recruited into the study. One patient was subsequently excluded due to the late finding of six lumbar vertebrae. The 3D spinal reconstruction with the EOS® system is only validated at present for patient with the standard number of vertebrae.
Mean age was 12.4 years (range 9–15), 25 girls and 4 boys.
Mean Cobb angle before treatment was 24° (range 15°–35°).
3D reconstruction of bony structures
All patients underwent full spine X-rays with EOS® imaging in the free standing position with and without bracing.
The EOS® imaging system is a tridimensional imaging modality that is validated for 3D evaluation of spinal deformities. This low-dose imaging technique provides simultaneous AP and lateral views in the standing normal weight-bearing position. Computerized reconstructions with sterEOS® software based on identifiable anatomic points provide a 3D image of the entire spine, the pelvis and the thoracic cage.
3D shape acquisition of the trunk
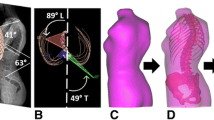
Computer-assisted brace fabrication relies on the acquisition of the 3D shape of the patient’s trunk. High-accuracy optical scans are now routinely used to obtain 3D body trunk shape (Fig. 1a). The resulting trunk shape is then modified manually with a specific software program (Fig. 1b).
For this study, optical scans were performed with the ScanGogh II, Vorum Research Corporation.
The scan of the external shape of the patient was acquired in the same free standing position used for EOS® imaging. The delay between EOS and optical scan acquisitions was less than 2 months in order to avoid any significant modification of height and trunk shape over time.
Avatar construction
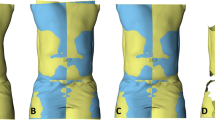
One of the challenges of this study was to combine the internal skeletal 3D patient shape obtained with EOS® with the corresponding external 3D shape resulting from the optical scan. For this step, we turned to the Anatoscope® technology known as “anatomy transfer,” which consists of constructing an animated anatomical avatar of a given patient by combining a 3D reference model (canonical avatar) with medical images of the patient. Biomedical simulation can then be performed on this personalized avatar, allowing 3D visualization and optimization of a treatment before it is applied to the patient in real life. The reference model exhibits standard human anatomy complemented with biomechanical data and parameters, such as landmarks and attachments.
In the initial stage “registration,” the reference model is deformed to fit the patient data in order to generate a patient-specific biomechanical avatar. This personalized avatar can then undergo biomedical simulations in the second stage.
For our brace simulation, the input data were:
-
General patient information (age, sex, weight, height)
-
Two standard EOS® X-ray views (AP and lateral) with generated semiautomatic bone reconstruction of spine (vertebra T1 to L5), pelvis S1 and rib cage (ribs 1 to 10)
-
Surface scan of the patient’s upper skin is captured by the brace manufacturer (Fig. 1a). (This scan is then modified by the brace manufacturer (Fig. 1b) in order to obtain the brace's 3D shape)
-
Computer-generated 3D model of the brace which would be used to construct the patient’s real brace (Fig. 1c)
The reference model contains all the large intra-cavity organs and soft tissues (skin, fat, muscles, ligaments, etc.) not inferred from the medical images of the patient, as well as the sternum and ribs 11 to 12.
Since the personalized rheological parameters of these anatomical structures were unknown, the organs and soft tissues were modeled as a global elastic continuum embedding the bones. The bones were considered as rigid bodies with connections between the vertebra and ribs, and ribs and sternum, modeled using 6D constraints (three translational and three rotational). Each constraint was characterized by specific nonlinear stiffness and deformation limits to represent the inherent nature of the spinal column.
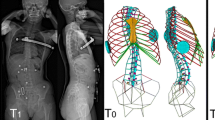
Once the patient’s bony imaging was integrated into the reference model in the final period of registration, the virtual patient trunk (chest and abdomen) was ready for simulation (Fig. 2).
The brace, modeled as a surface mesh, was positioned automatically on the personalized avatar using a least-squares fitting technique (Fig. 3). Contact was then simulated between the brace (a moving rigid body) and the deformable virtual body until a static equilibrium was reached. Biomechanical data generated by the simulation at this point included the predicted spinal curve, the forces applied to the bones and the pressure distribution on the skin.
Simulations of this type were undertaken for all patients. The 3D shape of the brace applied in the simulation was identical to the original shape used to create the real patient brace. All simulations were conducted by Anatoscope® engineers who were blinded to the clinical effect of the real patient brace. It should be stressed that the brace simulations had no impact on the patient and/or the design and fabrication of the brace. Simulations were performed after initiation of brace treatment.
Judgement criteria
Given that the main objective was to evaluate the accuracy and clinical pertinence of the predictions furnished by the brace simulation; our primary criteria were the difference in degrees between the in-brace Cobb angle measured after the simulation and the “real” in-brace Cobb angle reported in the EOS® full spine X-rays. One Cobb angle was measured in patients with single curves and two for those with double curves.
The upper and lower end vertebrae for the Cobb angle measurement were semiautomatically defined by the SterEOS® software. The same two vertebrae were used for all Cobb angle measurements in the same patient whether it was measured following the simulation or after real in-brace 3D reconstruction.
The secondary objective was to assess the difference between the in-brace kyphosis and lordosis angles after simulation and the corresponding “real” in-brace angles reported in the EOS® full spine X-rays. Thoracic kyphosis was measured using perpendiculars drawn from T4 and T12, lordosis from perpendiculars at the level of L1 and L5.
Statistics
Statistical analysis was performed using the SPSS version 12.0.1 for Windows (SPSS inc. Chicago, IL, USA). Mean values of each parameter were compared with and without a brace using a paired two-sided Student’s t test. Differences were considered to be statistically significant at a p value cut-off = 0.05.
In addition to overall group statistics, the strength of prediction of the simulation (good, moderate or poor) was assessed for each individual patient. Strength of prediction was defined as following:
-
Good prediction difference between simulation and real in-brace Cobb angle < or = ± 6°.
-
Moderate prediction difference between simulation and real in-brace Cobb angle > 6° but < 10°.
-
Poor prediction difference between simulation and real in-brace Cobb angle ≥ 10°.
The admitted threshold of Cobb angle measurement error is 4° with the EOS® system after 3D reconstruction of the spine.
Results
Results of the simulation for each case are displayed in Table 1.
Variability of Cobb angle prediction
Thirty-four curves were measured in 29 patients. (Five patients possessed significant double curves necessitating two measurements.) Twenty curves were right thoracic and 14 were left lumbar. Good prediction was observed for 21 curves, moderate prediction for five curves and poor prediction for the remaining eight curves. 3D spine reconstructions (real out of brace, real in-brace and simulations) of case 8 and 14 are illustrated in Figs. 4 and 5.
Global statistics are displayed in Table 2
The mean Cobb angle without a brace was 24.5° (15°–35°). Although both real and simulated in-brace measurements showed a smaller Cobb angle with bracing (mean angle = 11° in EOS® X-rays and 17° in the simulation), the difference between means was statistically significant (p = 0.03).
Without bracing, the mean kyphosis angle was 22.5° (2°–43°). The mean real in-brace kyphosis angle was 18° (0°–42°). The mean simulated kyphosis angle was 24° (1°–41°), representing a significant difference between real and simulated values (p ≪ 0.05).
For the lumbar lordosis, the mean angle without a brace was 44° (21°–60°). No difference could be observed between the real in-brace lordosis angles (mean 41°, range 15°–58°) and the simulated in-brace lordosis angles (mean 39°, range 14°–58°).
Discussion
The principal finding is that this experimental brace simulation method accurately predicted 60% of the real in-brace Cobb angles of scoliotic curves. For the study population as a whole, the simulator underestimated the brace curve correction (in-brace Cobb angle 17° vs. 11°). However, this does not detract from the fact that the simulation had already provided a good estimation of the correction achieved with bracing. To our knowledge, it is the first study conducted in which the observers of the simulation, namely the Anatoscope® engineers, were blinded to the clinical effect of the brace. We considered study blinding to be mandatory given that the goal of developing this brace simulator is to facilitate the orthotists work.
Studies using FEM of brace action already exist in the literature [10,11,12,13, 15, 21,22,23,24]. However, model validation and simulation uncertainty have rarely been addressed, which has limited the clinical implementation of personalized models. In a recent study by Vergari et al. [19]. evaluating brace action in 42 patients, brace action was simulated by using soft cylindrical pads acting on the rib cage and spine combined with displacements applied to key vertebrae. The authors used the patient’s 3D geometry obtained from stereo-radiography but did not integrate the patient’s trunk surface or their internal organs in the simulation. Moreover, the brace itself was modeled via cylindrical pads, which is a simplification of the shape and functioning of an actual brace. Finally, an important limiting factor, notably in comparison with the current study, was using in-brace stereo-radiography images to define the positioning of the pads. In our study, the simulations were performed by blinded investigators without the use of in-brace stereo-radiography.
For the experimental brace model described by Vergari et al. [19], root-mean-squared error (RMSE) was used to measure the differences between the Cobb angles predicted by the model and those observed with actual brace effect in in-brace stereo-radiographies. Simulation RMSEs of Cobb angle were lower than measurement uncertainty in 79% of the patients. If we encompass “good” and “moderate” predictions (i.e., difference between means < 10°) our results are almost equivalent (76%).
Vergari et al. [19] showed that brace simulation is possible with a personalized simplified FEM performed a posteriori. Our study confirms that it is now possible to use a brace simulator that can predict, a priori, the effect of bracing.
The method was not successful for all the patients studied; the simulation poorly predicted curve correction in eight (26%) of the 30 patients. This finding could be largely due to certain simulation parameters being standardized in advance for all patients regardless of age, gender and curve flexibility. Poor predictions were generally observed in the more “flexible” patients. For example, in cases 17 and 24, the real in-brace correction was very good, respectively, 3° and 2°, meaning that the curves were very flexible. On the other hand, the simulated Cobb angle was, respectively, 13° and 27°. The simulator was incapable of accounting for the variations in spine flexibility. It was not possible to adjust these prefixed simulation parameters in light of the desired blinded evaluation.
These findings are very encouraging because we feel that integrating other biomechanical characteristics, particularly curve “flexibility,” will help to improve the prediction capacity of the model. The importance of having good and reproducible clinical indicators of the biomechanical characteristics of scoliosis patients cannot be overstressed.
In conclusion, incorporating high-fidelity copy of the entire 3D shape of the patient’s trunk and multiple 3D reconstructed bony images (spine, ribs, pelvis) into an anatomical reference avatar resulted in moderate-to-good prediction of brace effect in three quarters of patients when combined with simple spine FEM. These findings open a new era of personalized FEM that will help the multidisciplinary clinical team to manage their patients with spinal deformities. More extensive clinical data are still necessary to promote the development and validation of personalized simulation models. We foresee that simulators are the future of rigid brace conception in the next decade.
References
Dolan LA, Wright JG, Weinstein SL (2014) Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med 370(7):681. https://doi.org/10.1056/NEJMc1314229
Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA (2008) Adolescent idiopathic scoliosis. Lancet 371(9623):1527–1537. https://doi.org/10.1016/S0140-6736(08)60658-3
Weinstein SL, Dolan LA, Wright JG, Dobbs MB (2013) Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med 369(16):1512–1521. https://doi.org/10.1056/NEJMoa1307337
Weinstein SL, Dolan LA (2015) The Evidence Base for the Prognosis and Treatment of Adolescent Idiopathic Scoliosis: the 2015 Orthopaedic Research and Education Foundation Clinical Research Award. J Bone Joint Surg Am 97(22):1899–1903. https://doi.org/10.2106/JBJS.O.00330
Courvoisier A, Vialle R, Skalli W (2014) EOS 3D Imaging: assessing the impact of brace treatment in adolescent idiopathic scoliosis. Expert Rev Med Devices 11(1):1–3. https://doi.org/10.1586/17434440.2014.848166
Courvoisier A, Drevelle X, Dubousset J, Skalli W (2013) Transverse plane 3D analysis of mild scoliosis. Eur Spine J 22(11):2427–2432. https://doi.org/10.1007/s00586-013-2862-x
Skalli W, Vergari C, Ebermeyer E, Courtois I, Drevelle X, Kohler R, Abelin-Genevois K, Dubousset J (2017) Early detection of progressive adolescent idiopathic scoliosis: a severity index. Spine 2(11):823–830. https://doi.org/10.1097/brs.0000000000001961
Courvoisier A, Drevelle X, Vialle R, Dubousset J, Skalli W (2013) 3D analysis of brace treatment in idiopathic scoliosis. Eur Spine J 22(11):2449–2455. https://doi.org/10.1007/s00586-013-2881-7
Post M, Verdun S, Roussouly P, Abelin-Genevois K (2018) New sagittal classification of AIS: validation by 3D characterization. Eur Spine J. https://doi.org/10.1007/s00586-018-5819-2
Clin J, Aubin CE, Parent S, Ronsky J, Labelle H (2006) Biomechanical modeling of brace design. Stud Health Technol Inform 123:255–260
Clin J, Aubin CE, Labelle H (2007) Virtual prototyping of a brace design for the correction of scoliotic deformities. Med Biol Eng Comput 45(5):467–473. https://doi.org/10.1007/s11517-007-0171-4
Clin J, Aubin CE, Parent S, Labelle H (2010) A biomechanical study of the Charleston brace for the treatment of scoliosis. Spine 35(19):940–947. https://doi.org/10.1097/brs.0b013e3181c5b5fa
Clin J, Aubin CE, Parent S, Sangole A, Labelle H (2010) Comparison of the biomechanical 3D efficiency of different brace designs for the treatment of scoliosis using a finite element model. Eur Spine J 19(7):1169–1178. https://doi.org/10.1007/s00586-009-1268-2
Clin J, Aubin C, Sangole A, Labelle H, Parent S (2010) Correlation between immediate in-brace correction and biomechanical effectiveness of brace treatment in adolescent idiopathic scoliosis. Spine 35(18):1706–1713. https://doi.org/10.1097/brs.0b013e3181cb46f6
Clin J, Aubin C, Parent S, Labelle H (2011) Biomechanical modeling of brace treatment of scoliosis: effects of gravitational loads. Med Biol Eng Comput 49(7):743–753. https://doi.org/10.1007/s11517-011-0737-z
Grant CA, Johnston M, Adam CJ, Little JP (2019) Accuracy of 3D surface scanners for clinical torso and spinal deformity assessment. Med Eng Phys 63:63–71. https://doi.org/10.1016/j.medengphy.2018.11.004
Pasha S, Flynn J (2018) Data-driven Classification of the 3D Spinal Curve in Adolescent Idiopathic Scoliosis with an Applications in Surgical Outcome Prediction. Sci Rep 8(1):16296. https://doi.org/10.1038/s41598-018-34261-6
Vergari C, Courtois I, Ebermeyer E, Bouloussa H, Vialle R, Skalli W (2015) Simulation of orthotic treatment in adolescent idiopathic scoliosis using a subject-specific finite element model. Comput Methods Biomech Biomed Eng 18(Suppl 1):2076–2077. https://doi.org/10.1080/10255842.2015.1069629
Vergari C, Courtois I, Ebermeyer E, Bouloussa H, Vialle R, Skalli W (2016) Experimental validation of a patient-specific model of orthotic action in adolescent idiopathic scoliosis. Eur Spine J 25(10):3049–3055. https://doi.org/10.1007/s00586-016-4511-7
Lateur G, Grobost P, Gerbelot J, Eid A, Griffet J, Courvoisier A (2017) Efficacy of nighttime brace in preventing progression of idiopathic scoliosis of less than 25. Orthop Traumatol Surg Res 103(2):275–278. https://doi.org/10.1016/j.otsr.2016.10.022
Clin J, Aubin C, Lalonde N, Parent S, Labelle H (2011) A new method to include the gravitational forces in a finite element model of the scoliotic spine. Med Biol Eng Comput 49(8):967–977. https://doi.org/10.1007/s11517-011-0793-4
Cobetto N, Aubin CE, Clin J, Le May S, Desbiens-Blais F, Labelle H, Parent S (2014) Braces optimized with computer-assisted design and simulations are lighter, more comfortable, and more efficient than plaster-cast braces for the treatment of adolescent idiopathic scoliosis. Spine Deform 2(4):276–284. https://doi.org/10.1016/j.jspd.2014.03.005
Desbiens-Blais F, Clin J, Parent S, Labelle H, Aubin CE (2012) New brace design combining CAD/CAM and biomechanical simulation for the treatment of adolescent idiopathic scoliosis. Clin Biomech 27(10):999–1005. https://doi.org/10.1016/j.clinbiomech.2012.08.006
Sattout A, Clin J, Cobetto N, Labelle H, Aubin CE (2016) Biomechanical assessment of providence nighttime brace for the treatment of adolescent idiopathic scoliosis. Spine Deform 4(4):253–260. https://doi.org/10.1016/j.jspd.2015.12.004
Acknowledgements
We acknowledge Dr Kelly Dilworth for the manuscript English review.
Funding
Grenoble Alps University Hospital Innovation Grant 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Courvoisier, A., Nesme, M., Gerbelot, J. et al. Prediction of brace effect in scoliotic patients: blinded evaluation of a novel brace simulator—an observational cross-sectional study. Eur Spine J 28, 1277–1285 (2019). https://doi.org/10.1007/s00586-019-05948-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-05948-9