Abstract
Purpose
Pyogenic spondylodiscitis (PS) is still burdened by a high rate of orthopedic and neurological complications. Despite the rising incidence, the choice of a proper orthopedic treatment is often delayed by the lack of clinical data. The aim of this study was to propose a clinical-radiological classification of pyogenic spondylodiscitis to define a standard treatment algorithm.
Methods
Based on data from 250 patients treated from 2008 to 2015, a clinical-radiological classification of pyogenic spondylodiscitis was developed. According to primary classification criteria (bone destruction or segmental instability, epidural abscesses and neurological impairment), three main classes were identified. Subclasses were defined according to secondary criteria. PS without segmental instability or neurological impairment was treated conservatively. When significant bone loss or neurological impairment occurred, surgical stabilization and/or decompression were performed. All patients underwent clinical and radiological 2-year follow-up.
Results
Type A PS occurred in 84 patients, while 46 cases were classified as type B and 120 as type C. Average time of hospitalization was 51.94 days and overall healing rate was 92.80%. 140 patients (56.00%) were treated conservatively with average time of immobilization of 218.17 ± 9.89 days. Both VAS and SF-12 scores improved across time points in all classes. Residual chronic back pain occurred in 27 patients (10.80%). Overall observed mortality was 4.80%.
Conclusions
Standardized treatment of PS is highly recommended to ensure patients a good quality of life. The proposed scheme includes all available orthopedic treatments and helps spine surgeons to significantly reduce complications and costs and to avoid overtreatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyogenic spondylodiscitis (PS) is a potentially life-threatening infectious disease involving the intervertebral disc and adjacent vertebral bodies. Previous studies estimated the annual incidence of pyogenic spondylodiscitis ranging from 0.4 to 2.4/100,000 inhabitants in Europe [1, 2]. The variability of available data about incidence depends on the inclusion criteria and statistical methods of the few existing studies covering limited geographical areas. PS occurs predominantly in elderly and chronically debilitated patients aged over 50 years and is more common in male patients, with a male to female ratio of 1.5–2:1 [1, 3, 4]. Pyogenic spondylodiscitis has always been considered a rare condition in Europe according to the few epidemiological reports available in the past. However, several recent studies reported an alarming increase of incidence in Europe in the last 20 years. A Danish 14-year population-based study showed that overall incidence of PS increased 2.2–5.8 cases per 100,000 person years [5]. Aging population and longer life expectancy of patients affected by chronic debilitating diseases and immunodeficiency led to an increased number of high-risk individuals, while the spread of invasive and minimally invasive diagnostic and surgical procedures exposed more patients to iatrogenic infections. The increase of incidence is also due to the higher diagnostic efficacy obtained with the spread of MRI. Estimated mortality is highly variable depending on geographical areas with reported peaks up to 11% [6–10].
Etiology and infection routes
According to etiology, pyogenic or non-specific spondylodiscitis can be differentiated into bacterial, fungal or parasitic infections. Among bacterial PS, Staphylococcus aureus is the most frequent causative microorganism accounting for half of the cases, while fungal and parasitic forms represent less than 2% of all cases [6]. PS is in most cases a hematogenous infection due to septic dissemination from distant infectious foci. The development of PS by direct inoculation is less common than hematogenous spread and can occur following spinal surgery, epidural procedures and lumbar puncture with a reported prevalence up to 18.8% [11]. Through the osteonecrosis, the infection can spread to paravertebral soft tissues and epidural space producing abscesses and leading to biomechanical instability and neurological impairment in about 1/3 of all cases [3, 6, 7].
Treatment goals
Treatment of pyogenic spondylodiscitis is a story of great successes which led from the mortality rate of about 70% observed by Kulowski in 1936 to nowadays optimal clinical outcomes [12]. First aim of the treatment strategy should be to eradicate the infection and prevent sepsis. However, while the antibiotic therapy greatly reduced mortality, PS is still burdened by a high rate of orthopedic and neurological complications with great impact on patients’ quality of life [9, 10]. The goals of orthopedic treatment are to preserve or establish spinal stability, to relieve pain, to prevent or reverse neurological deficits and to prevent recurrence [13–16].
Practical guidelines for the pharmacological management of PS have been proposed by the Infectious Diseases Society of America (IDSA) in 2015 [17]. On the other hand, the lack of large clinical studies and the variability of inclusion criteria of existing clinical series still hinder the development of a standard algorithm for orthopedic treatment.
Aim of this study is to propose a new classification of pyogenic spondylodiscitis based on clinical and radiological features to define a simple and reproducible treatment algorithm for spine surgeons.
Materials and methods
Based on data from 250 patients treated at our University Hospital from 2008 to 2015 with a 2-year follow-up, a clinical-radiological classification of pyogenic spondylodiscitis was developed. Both spine surgeons and infectious diseases specialists were involved in caring of all the patients establishing a multidisciplinary clinical management. Diagnostic approach included accurate anamnesis and physical examination, serial C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), complete blood count, and full spine contrast-enhanced MRI (or CT when contraindicated). Blood cultures were collected from all patients after adequate antibiotics interruption and just before and after every invasive procedure. Patients with negative blood cultures underwent CT-guided biopsy, while surgical transpedicular biopsy was used as third line isolation technique [13].
All patients received intravenous antibiotic therapy for at least 4 weeks followed by oral course until normalization of radiological and laboratory markers of infection [17]. Patients without etiological diagnosis even after surgical biopsy, received empiric broad-spectrum antibiotic therapy and underwent closer blood tests monitoring [17]. Conservative orthopedic treatment consisted of 24/7 immobilization with rigid orthosis until complete infection healing. Rigid orthosis consisted on hard cervical collar with proper chin support, or thoraco-lumbar rigid brace molded on plaster cast to avoid kyphosis. Posterior percutaneous screw-rod instrumentation bridging the infected level was used as alternative minimally invasive approach to orthosis in patients with single-level thoraco-lumbar PS and high functional demand. Open surgical treatment included debridement of necrotic tissues, decompression of neurological structures and spine instrumentation, avoiding infected vertebral bodies, through anterior or posterior approaches. The same orthopedic treatments were used in patients without microbiological diagnosis.
Physical examination, serial blood tests and targeted MRI were performed at 1, 3, 6, 12 and 24 months from diagnosis. At each follow-up visit, patients were also asked to fill in Visual Analogic Scale (VAS) and Short-Form 12 (SF-12) questionnaires.
Classification criteria
Classification criteria were selected among clinical and radiological factors with known prognostic value. Three main types (A, B and C) were defined depending on to the following primary criteria: bone destruction or segmental instability, epidural abscesses and neurological impairment (Table 1). Secondary criteria were: involvement of paravertebral soft tissues and intramuscular abscesses (Table 1). Biomechanical instability was defined as more than 25% change of segmental kyphosis at the infection level. Accurate physical examination including evaluation of deep tendon reflexes, sensibility, muscle strength and central pathological signs, was performed to exclude neurological impairment. Contrast-enhanced MRI was used to evaluate neurological structures, abscesses and involvement of paravertebral soft tissues.
Type A
All cases without biomechanical instability neither acute neurological impairment or epidural abscesses were included (Fig. 1). Depending on secondary criteria, subclasses were defined as follow:
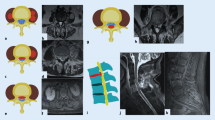
Magnetic resonance imaging (MRI) of type A pyogenic spondylodiscitis (PS). a Sagittal T1-weighted and axial T2-weighted MRI showing type A.1. post-operative L4–L5 discitis. b Sagittal T1-weighted and axial T2-weighted MRI of type A.2. PS with involvement of both L4 and L5 vertebral bodies. c Sagittal and coronal T1-weighted contrast-enhanced MRI showing type A.3. PS involving D7–D8 vertebral bodies with paravertebral abscess (arrows). d Coronal and axial contrast-enhanced MRI showing type A.4.2. PS with bilateral intra-psoas abscesses (arrows)
-
A.1: simple discitis without the involvement of vertebral bodies (Fig. 1a).
-
A.2: spondylodiscitis involving the intervertebral disc and adjacent vertebral bodies (Fig. 1b).
-
A.3: spondylodiscitis with limited involvement of paravertebral soft tissues (Fig. 1c).
-
A.4: spondylodiscitis with unilateral (A.4.1) or bilateral (A.4.2) intramuscular abscesses (Fig. 1d).
Treatment of choice was 24/7 rigid orthosis immobilization until complete infection healing (Table 2). Patients with higher functional demand underwent minimally invasive posterior percutaneous stabilization (Table 2).
Type B
All cases with radiological evidence of significant bone destruction or biomechanical instability without acute neurological impairment or epidural abscesses were included (Fig. 2). Patients were treated according to the following subclasses:
MRI of type B destructive pyogenic spondylodiscitis (PS). a Sagittal T1-weighted and axial T2-weighted MRI showing type B.1 PS with partial destruction of L3 vertebral body. b Sagittal and coronal contrast-enhanced MRI of type B.2 destructive PS involving L4 and L5 vertebral bodies with paravertebral abscess (arrows). c Sagittal and axial T2-weighted MRI showing type B.3 thoracic PS with 46.71° kyphosis
-
B.1: destructive spondylodiscitis without segmental instability (Fig. 2a).
-
B.2: destructive spondylodiscitis extended to paravertebral soft tissues without segmental instability (Fig. 2b).
-
B.3: destructive spondylodiscitis with biomechanical instability and segmental kyphosis (B.3.1 < 25°; B.3.2 > 25°) (Fig. 2c).
Conservative treatment or percutaneous stabilization was indicated for destructive PS with preserved spine stability (B.1 and B.2) (Table 2). When segmental kyphosis or instability occurred (B.3), surgical stabilization was always performed (Table 2). Minimally invasive posterior percutaneous approach was considered an option to treat patients with mild kyphosis (B.3.1).
Type C
All cases with epidural abscesses or acute neurological impairment were included (Fig. 3).
MRI of type C pyogenic spondylodiscitis (PS). a Sagittal contrast-enhanced MRI and axial T2-weighted MRI of type C.1 lumbar PS with limited epidural abscess. b Sagittal and axial T2-weighted MRI showing type C.2 lumbar PS with limited epidural abscess (arrows) and significant bone destruction. c Sagittal and axial contrast-enhanced MRI showing type C.3 lumbar PS with extended epidural abscess (arrows) in a 53-year-old male patient presented with acute cauda equina syndrome. d Sagittal and axial T2-weighted MRI of type C.4 thoracic PS in a 68-year-old female patient presented with acute paraplegia
-
C.1: epidural abscess without neurological symptoms neither segmental instability (Fig. 3a).
-
C.2: epidural abscess and segmental instability without neurological impairment (Fig. 3b).
-
C.3: epidural abscess and acute neurological impairment without segmental instability (Fig. 3c).
-
C.4: epidural abscess and acute neurological impairment with segmental instability (Fig. 3d).
Patients without acute neurological symptoms and segmental instability (C.1) were treated conservatively and closely monitored to evaluate neurological anomalies (Table 2). Type C.2 spondylodiscitis were treated with surgical stabilization and abscess debridement to avoid impending neurological damages (Table 2). When neurological impairment occurred (C.3 and C.4), surgical decompression of neurological structures was always performed, combined with segmental stabilization in the same procedure if biomechanical stability was jeopardized (Table 2).
Statistical analysis
Baseline demographic and clinical onset characteristics were assessed using independent-sample t tests for continuous variables and χ 2 tests for frequency variables. Analysis of variance was used to assess differences in clinical outcomes across follow-up, and p values 0.05 or less were considered significant. Continuous variables are presented as mean ± standard error. Frequency data are presented as counts and percentages. Epi Info statistic software, version 7 (CDC, Atlanta, GA, USA), was used for data analysis.
Results
A total of 250 patients (161 men and 89 women) were treated at our University Hospital from January 2008 to January 2015. Average age was 64.98 ± 0.97, moreover, age distribution showed a significant peak in the 60–75 years of age group. Average diagnostic delay was 49.90 ± 5.53 days from symptoms onset. Most common symptoms at diagnosis were localized spinal pain [236 patients; 94.40%; 95% CI (89.68–97.21)] and fever [159 patients; 63.60%; 95% CI (55.80–70.97)]. Limbs radiating pain and weight loss were referred less frequently [106 patients; 42.40%; 95% CI (35.04–50.43) and 84 patients; 26.30%; 95% CI (26.30–41.40)]. 56 patients presented with neurological deficits at diagnosis [22.40%; 95% CI (16.43–29.53)] (Fig. 4). Lumbar spine was the most commonly affected [184 patients; 73.60%; 95% CI (66.29–80.16)] with peaks at L3–L5 levels. Other frequently involved levels were C5–C6 for cervical spine and D8–D10 for thoracic spine. No localizations were observed at C0–C3 levels.
Etiological diagnosis was assessed in about ¾ of all patients [190 patients; 76.00%; 95% CI (68.91–82.21)]. Observed sensibility of blood culture, CT-guided biopsy and surgical biopsy was 57.64% [95% CI (49.13–65.82)], 46.27% [95% CI (34.00–58.88)] and 60.00% [95% CI (44.33–74.30)], respectively. Staphylococcus aureus was the most frequently isolated causative agent [70 patients; 28.00%; 95% CI (20.97–36.11)]. Other commonly isolated microorganisms were Staphylococcus epidermidis, Escherichia coli, and Klebsiella pneumoniae. 12 patients [4.80%; 95% CI (1.95–9.63)] were infected by Methicillin-resistant Staphylococcus aureus (MRSA). 19 patients [7.60%; 95% CI (3.75–13.69)] had polymicrobial infections (Table 3).
62 patients developed PS after surgical procedures [24.80%; 95% CI (18.61–31.98)]. Direct inoculation of causative agents during spine invasive procedures occurred in 45 patients [18.00%; 95% CI (12.51–24.46)].
Type A spondylodiscitis occurred in 84 cases (33.60%). Types A.1 and A.2 included 11 (13.09%) and 28 (33.33%) patients, respectively. Paravertebral abscesses (type A.3) were reported in 33 patients (39.29%), whereas intramuscular abscesses (type A.4) occurred in 12 patients (14.29%). Among type A cases, 74 patients (88.10%) were prescribed 24/7 rigid orthosis immobilization, whereas 10 patients (11.90%) underwent posterior percutaneous stabilization.
46 cases (18.40%) were classified as type B destructive spondylodiscitis. Segmental stability was preserved (types B.1 and B.2) in 17 cases (36.96%). 29 patients (63.04%) presented segmental instability (type B.3) and 20 of them needed open surgical fixation, whereas 9 patients underwent percutaneous stabilization.
120 cases (48.00%) were classified as type C spondylodiscitis with epidural abscesses or neurological impairment. Among type C cases, 49 patients (40.83%) had no neurological deficits neither segmental instability (type C.1), thus they were treated conservatively. Type C.2 included 15 patients (12.50%) that underwent surgical stabilization and abscess debridement. 30 patients (25.00%) with acute neurological deficits without segmental instability (type C.3) underwent surgical decompression of neurological structures. Open surgical stabilization was combined in the same procedure (type C.4) in 26 cases (21.67%).
Average time of hospitalization was 51.94 ± 3.00 days. Full healing from infection was achieved in 232 patients with an overall healing rate of 92.80% [95% CI (87.17–96.49)]. 14 relapses have been reported during follow-up with an overall recurrence rate of 5.60% [95% CI (2.67–10.67)].
Clinical outcomes for each class are shown in Table 4. A total of 140 patients (56.00%) were treated conservatively. Average time of rigid orthosis immobilization was 218.17 ± 9.89 days. Among 110 cases (44.00%) treated surgically, 4 patients underwent procedure-related complications, however, none of them reported permanent damages.
Self-reported VAS scores consistently decreased across time points in all classes reaching an average value of 0.65 ± 0.21 at 2 years from diagnosis (Fig. 5). Similarly, mental and physical components of SF-12 questionnaire increased significantly (Fig. 6).
Residual chronic back pain occurred in 27 patients (10.80%), whereas 13 patients (5.20%) showed residual neurological deficits. Overall mortality was 4.80% [95% CI (3.33–6.48)].
Discussion
Despite considerably improved diagnostic efficiency, there are no standardized orthopedic treatment guidelines for pyogenic spondylodiscitis due to heterogeneous clinical data available in literature, often based on small patient populations [16]. Actually, few standardized treatments are suggested but without prospective randomized controlled trials the level of evidence for treatment recommendations remains low [18]. Therefore, a classification of PS based on clinical and radiological features with a standard treatment algorithm can be very useful to spine surgeons facing this pathology.
Clinical onset of pyogenic spondylodiscitis is typically nonspecific and paucisymptomatic. Thus, differential diagnosis with degenerative spine pathologies is often difficult. Different reviews reported a mean diagnostic delay of 90 days [6, 7]. For this reason, early diagnosis should be the first aim of clinical approach to PS. Early diagnosis is based on a high level of suspicion with emphasis on patients presenting acute and localized spinal pain combined with fever and known risk factors for infectious diseases. About 1/3 of all patients can be apyretic at clinical onset hindering the differential diagnosis with non-infectious spine pathologies. Contrast-enhanced MRI showed the highest diagnostic sensibility and provides a baseline picture on biomechanical stability and neurological structures [19, 20]. When MRI is promptly performed, impending neurological lesions or structural damages can be detected earlier allowing the choice of less invasive treatments. CT may be useful when there are doubts on osteonecrosis extension or biomechanical stability. There are no pathognomonic laboratory markers but CRP, ESR and WBC anomalies should raise the level of suspicious.
The high healing rate and low recurrence rate observed in this study first demonstrate that a multidisciplinary approach to PS is the best choice. Early administration of specific antibiotics prevents further damages and diffusion of causative agent. While waiting for the microbiological results, an empirical therapy can be started in severe cases [17]. According to 2015 IDAS guidelines, intravenous therapy should be administrated for at least 4 weeks followed by oral course until normalization of laboratory and radiological markers of infection [17]. On the other hand, surgical debridement may be necessary to eradicate the infection [9, 10]. The high efficiency of microbiological isolation techniques observed in this study (76.00%) also derives from the close collaboration with infectious diseases specialists. In fact, blood culture when adequately collected, especially before and after invasive procedures, showed higher sensibility then reported in literature data. 55 patients (22.00%) needed surgical biopsy.
When there are no neurological deficits and no significant instability, PS can be managed without surgical intervention (types A.1–4, B.1–2 and C.1). Lumbar epidural abscess may be approached with a conservative treatment only if there is no evidence for cauda equina or conus dysfunction [9, 22]. Immobilization is necessary to maintain spinal stability until bony ankylosis occurs [9, 10]. However, spontaneous ankylosis requires 6–24 months and may not take place at all [23]. In our population, average time of immobilization was about 7 months. Residual chronic back pain observed in 10.80% of our patients is mostly attributable to non-surgically treated patients with postinfection kyphosis or pseudoarthrosis.
Since January 2010, percutaneous posterior screw-rod instrumentation bridging the infected level has been used as safe and effective alternative approach to prolonged rigid bracing for single-level thoraco-lumbar PS. A retrospective cohort study demonstrated faster recovery and improved quality of life associated with minimally invasive treatment [24]. Posterior percutaneous stabilization is also indicated in patients with biomechanical instability and mild segmental kyphosis (type B.3.1).
When acute neurological impairment or severe kyphosis occur (types B.3.2, C.3–4), open surgical approach is mandatory. Emergency decompression is indicated if complete plegia develops [16]. Decompression and instrumented spinal stabilization should be performed in the same procedure if neurological deficit exists (type C.4). On the contrary, if instability and pain are present in a patient without neurological impairment (types B.3 and C.2) [21], correction of deformity through instrumentation may be delayed until infection has been cleared [22, 25]. Debridement and arthrodesis may be sufficient in cases without instability (type C.3), combined with bed rest and bracing [17, 26]. When the bone loss created by debridement leads to potential instability, surgical reconstruction through interbody fusion or posterior stabilization is necessary [23].
The choice of surgical techniques and appropriate approaches and instrumentation to treat PS is still a matter of controversy. Pyogenic infections predominantly involve the vascularized vertebral body with involvement of the posterior elements in only 5% of the cases [26]. Thus, anterior surgical approach is most often used when aggressive debridement is indicated. The gold standard for anterior column reconstruction is the use of structural bone auto- or allografting [25, 27]. An effective alternative to structural bone autograft is the titanium mesh or PEEK cage. When cage is combined with anterior and/or posterior instrumentation in a single stage procedure, this technique ensures immediate stability [15, 27–29].
Observed surgical complication rate was very low with no permanent disability. Among 56 patients with neurological deficits at diagnosis, reverse of neurological deficits was achieved in 43 cases (76.79%).
Improvement in VAS and SF-12 scores across time points demonstrate that an adequate treatment algorithm ensures effective pain relief and good quality of life.
In conclusion, standardized treatment of pyogenic spondylodiscitis based on clinical-radiological classification is highly recommended. The proposed scheme includes all available orthopedic treatments and helps spine surgeons to significantly reduce complications and avoid overtreatment. The use of a common language to treat pyogenic spondylodiscitis allows shorter hospitalization with reduced costs. This simple and reproducible scheme is suitable also for spine surgeons with less experience in treating PS.
References
Grammatico L, Baron S, Rusch E, Lepage B, Surer N, Desenclos JC, Besnier JM (2008) Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002–2003. Epidemiol Infect 136:653–660. doi:10.1017/s0950268807008850
Beronius M, Bergman B, Andersson R (2001) Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis 33:527–532
Carragee EJ (1997) Pyogenic vertebral osteomyelitis. J Bone Jt Surg Am 79:874–880
Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, Sabbagh AS, Kanafani ZA, Kanj SS (2010) Pyogenic spondylodiscitis: an overview. J Infect Public Health 3:5–16. doi:10.1016/j.jiph.2010.01.001
Kehrer M, Pedersen C, Jensen TG, Lassen AT (2014) Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect 68:313–320. doi:10.1016/j.jinf.2013.11.011
Fantoni M, Trecarichi EM, Rossi B, Mazzotta V, Di Giacomo G, Nasto LA, Di Meco E, Pola E (2012) Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci 16(Suppl 2):2–7
Duarte RM, Vaccaro AR (2013) Spinal infection: state of the art and management algorithm. Eur Spine J 22:2787–2799. doi:10.1007/s00586-013-2850-1
Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM (2006) Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine (Phila Pa 1976) 31:2695–2700. doi:10.1097/01.brs.0000244662.78725.37
Rutges JP, Kempen DH, van Dijk M, Oner FC (2016) Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: a systematic literature review. Eur Spine J 25:983–999. doi:10.1007/s00586-015-4318-y
Aagaard T, Roed C, Dahl B, Obel N (2016) Long-term prognosis and causes of death after spondylodiscitis: a Danish nationwide cohort study. Infect Dis (Lond) 48:201–208. doi:10.3109/23744235.2015.1103897
Nasto LA, Colangelo D, Rossi B, Fantoni M, Pola E (2012) Post-operative spondylodiscitis. Eur Rev Med Pharmacol Sci 16(Suppl 2):50–57
Kulowski J (1936) Pyogenic osteomyelitis of the spine: an analysis and discussion of 102 cases. J Bone Jt Surg Am 18:343–364
Quiñones-Hinojosa A, Jun P, Jacobs R, Rosenberg WS, Weinstein PR (2004) General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus 17(6):E1. doi:10.3171/foc.2004.17.6.1
Acosta FL Jr, Chin CT, Quiñones-Hinojosa A, Ames CP, Weinstein PR, Chou D (2004) Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus 17(6):E2. doi:10.3171/foc.2004.17.6.2
Ruf M, Stoltze D, Merk HR, Ames M, Harms J (2007) Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine (Phila Pa 1976) 32:E275–E280. doi:10.1097/01.brs.0000261034.83395.7f
Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P (2007) Suggestion for managing pyogenic (nontuberculous) discitis in adults. Jt Bone Spine 74:133–139
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM 3rd, Petermann GW, Osmon DR (2015) Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis 61:e26–e46. doi:10.1093/cid/civ482
Homagk L, Homagk N, Klauss JR, Roehl K, Hofmann GO, Marmelstein D (2016) Spondylodiscitis severity code: scoring system for the classification and treatment of non-specific spondylodiscitis. Eur Spine J 25:1012–1020. doi:10.1007/s00586-015-3936-8
Leone A, Dell’Atti C, Magarelli N, Colelli P, Balanika A, Casale R, Bonomo L (2012) Imaging of spondylodiscitis. Eur Rev Med Pharmacol Sci 16(Suppl 2):8–19
Tins BJ, Cassar-Pullicino VN (2004) MR imaging of spinal infection. Semin Musculoskelet Radiol 8:215–229. doi:10.1055/s-2004-835362
Chen WH, Jiang LS, Dai LY (2007) Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J 16:1307–1316. doi:10.1007/s00586-006-0251-4
Hsieh PC, Wienecke RJ, O’Shaughnessy BA, Koski TR, Ondra SL (2004) Surgical strategies for vertebral osteomyelitis and epidural abscess. Neurosurg Focus 17(6):E4
Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ (2000) Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 25:1668–1679
Nasto LA, Colangelo D, Mazzotta V, Di Meco E, Neri V, Nasto RA, Fantoni M, Pola E (2014) Is posterior percutaneous screw-rod instrumentation a safe and effective alternative approach to TLSO rigid bracing for single-level pyogenic spondylodiscitis? Results of a retrospective cohort analysis. Spine J 14:1139–1146. doi:10.1016/j.spinee.2013.07.479
Przybylski GJ, Sharan AD (2001) Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg 94(Suppl 1):1–7
Pola E, Logroscino CA, Gentiempo M, Colangelo D, Mazzotta V, Di Meco E, Fantoni M (2014) Medical and surgical treatment of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci 16(Suppl 2):35–49
Strowitzki M, Vastmans J, Vogel M, Jaksche H (2011) Complex 360°-reconstruction and stabilization of the cervical spine due to osteomyelitis. Eur Spine J 20(Suppl 2):S248–S252. doi:10.1007/s00586-010-1645-x
Korovessis P, Petsinis G, Koureas G, Iliopoulos P, Zacharatos S (2006) Anterior surgery with insertion of titanium mesh cage and posterior instrumented fusion performed sequentially on the same day under one anesthesia for septic spondylitis of thoracolumbar spine: is the use of titanium mesh cages safe? Spine (Phila Pa 1976) 20:1014–1019. doi:10.1097/01.brs.0000215049.08622.9d
Fukuta S, Miyamoto K, Masuda T, Hosoe H, Kodama H, Nishimoto H, Sakaeda H, Shimizu K (2003) Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine (Phila Pa 1976) 1:E302–E308. doi:10.1097/01.brs.0000083318.40123.5e
Acknowledgements
The authors declare that they received no grants or funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pola, E., Autore, G., Formica, V.M. et al. New classification for the treatment of pyogenic spondylodiscitis: validation study on a population of 250 patients with a follow-up of 2 years. Eur Spine J 26 (Suppl 4), 479–488 (2017). https://doi.org/10.1007/s00586-017-5043-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5043-5