Abstract
African animal trypanosomosis is a debilitating tsetse-transmitted parasitic disease of sub-Saharan Africa. Therapeutic and prophylactic drugs were introduced more than 50 years ago, and drug resistance is increasingly reported. In a cross-sectional study, 467 cattle were microscopically screened for trypanosomes. Samples were collected in May–July 2014 from five villages (Botao, Mungama, Zalala-Electrosul, Zalala-Madal, and Namitangurine) in Nicoadala district, Zambezia province. To evaluate treatment efficacy, trypanosome-positive animals in each village were randomly assigned to two groups, one treated with 0.5 mg/kg b.w. isometamidium (Inomidium®), the second with 3.5 mg/kg b.w. diminazene (Inomazene®). Cattle were microscopically monitored at days 0, 14, and 28 post-treatment. At day 28, trypanocides were swapped to investigate single or multiple resistance. Microscopically negative samples from the monitoring days were tested using 18S-PCR-RFLP. 22.9% (107/467) was found positive on day 0. On day 14, nine animals in Botao and seven in Mungama were positive. On day 28, in Botao, four animals from the diminazene group and four from the isometamidium group were positive. In Mungama, four animals from the diminazene group were positive on day 28. On day 42, six animals (9%) in Botao and two (9.5%) in Mungama remained positive after drug swap. No relapses occurred in Namitangurine. The 18S-PCR-RFLP consistently detected more positive than microscopy: indeed, positives reached 12, 13, and 8 in Botao and 9, 7, and 4 in Mungama, at days 14, 28, and 42, respectively. Single- and multi-drug resistance in Nicoadala district, Zambezia province, is thus here confirmed. This should be considered when choosing control options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
African animal trypanosomosis (AAT) is a tsetse-transmitted, debilitating parasitic disease affecting both domestic and wild vertebrates (Barret et al. 2003). This disease is caused by protozoa, belonging to the genus Trypanosoma (Shah-Fischer & Say, 1981). In domestic animals, trypanosomosis is a disease with a great economic impact, affecting not only the well-being of the livestock population, but also efficient food production in crop-livestock production systems (Davila et al. 2003; Shaw et al. 2014). Among all African pathogenic trypanosome species, Trypanosoma congolense is arguably the one causing major losses in southern Africa (Sigauque et al. 2000; Sinyangwe et al. 2004; Specht 2008). The human form of the disease, known as sleeping sickness, is also sporadically detected in Mozambique, although disease surveillance is weak and under detection and underreporting are possible (Büscher et al. 2017; Franco et al. 2017).
The treatment and prevention of trypanosomosis in cattle are carried out using three drugs, namely isometamidium chloride (ISM), diminazene aceturate (DA), and homidium bromide. In the last 50 years, no new trypanocides have been made available in cattle and drug resistance has been reported in several countries (Delespaux et al. 2008). In trypanosomosis, drug resistance is usually the result of the loss of capacity for drug uptake by the parasite, an alteration in drug-target interaction, or even a change in the efflux mechanism (Baker et al. 2013), as it is suggested for ISM (Sutherland et al. 1992).
Drug resistance is often associated to areas with high drug usage (Delespaux and De Koning 2007). This is compounded by the lack of new drugs and the incorrect utilization of existing ones (Geerts and Holmes 1998). Precise mechanisms of drug resistance are still undeciphered. However, in T. brucei, DA resistance was found to be associated with a mutation in a P2-type purine transporter, encoded by the TbAT1 gene, which is responsible for the uptake of the drug by the parasite (Matovu et al. 2003; De Koning et al. 2004). In T. congolense, an orthologue gene of TbAT1, the TcoAT1 gene, seemed to play the same role (Delespaux et al. 2006). However, Munday et al. (2013) found that for this particular species, TcoAT1 acts in the transportation of inosine (P1-type purine transporter), as does the syntenic gene TbNT10 in T. brucei. The observed mutation in the TcoAT1 gene does not change the DA sensitivity of the parasite but is associated to the phenotype as a genetic marker. The possible link between those two mutations remains unclear. Isometamidium kills protozoa by blocking the synthesis of nucleic acids. It intercalates between DNA base pairs and blocks the RNA and DNA polymerases. In sensitive strains, ISM is transported into and accumulated in the kinetoplast. It is assumed that changes in the efflux mechanism in the parasite result in a reduced accumulation of ISM inside the kinetoplast, thus creating resistance (Sutherland et al. 1991; Sutherland and Holmes 1993; Mulugeta et al. 1997).
In Mozambique, the use of chemotherapy for trypanosomosis control dates back to 1912 with the introduction of arsenic acid and later tartar emetic. Nevertheless, reports of failure of these compounds and, in some cases, the development of drug resistance were reported. In 1953, DA was introduced (Rafael 1959; Silva 1959). In the 1960s, ISM was introduced, and since then, it has been used, together with DA, as a chemoprophylactic and chemotherapeutic drug against trypanosomosis. Resistance to anti-trypanocidal drugs has been reported in at least 21 countries in Africa (Chitanga et al. 2011), including Mozambique, where it was experimentally confirmed for both ISM and DA (Jamal et al. 2005; Macucule 2014).
Despite the abovementioned explanations, the mechanism of resistance has not been fully elucidated for either drug. The lack of knowledge about the exact gene(s) that are responsible for the resistance profile in the livestock pathogenic species considered constrains the development of diagnostic molecular tools. In the absence of progress in this area, field tests (e.g., block treatment) can be considered as the alternative, as they have proven to be reliable, relatively easy, and fast to conduct. Furthermore, they do not require the isolation of trypanosomes (Eisler et al. 2001; McDermott et al. 2003). These advantages constitute the rationale of the approach used in the present study.
Drug resistance has been shown to be an important drawback to agricultural development in Africa in general, and in Mozambique in particular, where it has caused thousands of cattle deaths and a drastic reduction in the cattle population of Zambezia province. Despite this, local veterinary services have no structured program for trypanosomosis control and for regulating the use of trypanocidal drugs that is left to the initiative of the owner and/or animal health technicians. The present study provides an update on trypanocidal drug resistance in Nicoadala district and specifically evaluates the proportion of resistant strains in previously described hotspots. In addition to information on drug resistance, AAT epizootiology should be further investigated and the role of biological and mechanical transmission should be urgently revisited. Studies on vector species composition and distribution are in progress in order to shed light on the respective roles of tsetse flies and other biting flies in the transmission of trypanosomosis.
Material and methods
Study area
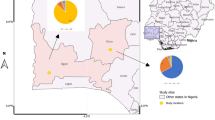
The study was conducted in five villages in Nicoadala district, Zambezia province (Fig. 1). The villages were chosen according to trypanosome prevalence obtained from a previous surveys conducted in Zambezia province between 2009 and 2013. The district has a history of high usage of trypanocides and has been previously identified as a drug resistance hot spot (Jamal et al. 2005).
Cross-sectional study and sampling framework
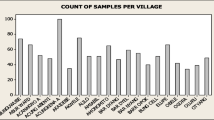
Blood samples for both parasitological and molecular analysis were collected in May–July 2014. Sample size was estimated according to Cannon and Roe (1982), where expected prevalence was 50% with a 95% confidence interval and a 5% desired absolute precision. In each of the villages, all animals were screened for trypanosomes using the buffy-coat technique (BCT) (Murray et al. 1977). Four milliliters of blood was collected using Vacutainer tubes containing di-sodium salt of ethylenediaminetetraacetic acid (EDTA) from the coccygeal vein of each animal. Two capillary tubes were then filled and sealed with crystaseal (Hawksley, Lancing, UK) and the blood was centrifuged at 1500 rpm for 5 min in a microhematocrit centrifuge (Hawksley®, Lancing, UK). One of the capillary tubes was used for packed cell volume (PCV) reading. The other was used for BCT and the preparation was examined for the presence of motile trypanosomes under a light microscope (Leica Mycrosystems, Wetzlar, Germany) at a magnification of × 400. In total, 467 heads of cattle from five villages were screened for trypanosomes.
Drug resistance study test
The trypanosome-infected animals from each village were randomly divided into two groups with a minimum of nine animals assigned to each group, for drug sensitivity tests of DA (Inomazene®, INOUKO Generics, Paris, France) and ISM (Inomidium®, INOUKO Generics, Paris, France). The number of animals per group was estimated according to the formula from Cannon and Roe (1982) to detect the presence of at least one resistant isolate, using an infinite population size and an expected proportion of trypanosome-resistant isolates of 30%. The first group was treated with 3.5 mg/kg of body weight (b.w.) DA and the second one with 0.5 mg/kg b.w. ISM (2% solution), as described by Mungube et al. (2012a). The animals were monitored for relapses on days 14 and 28 post-treatment, using the BCT method. At day 28, the trypanocidal drugs were switched to expose the strains previously treated with DA to ISM and vice versa, and to verify if there was resistance to only one drug (single-) or to both drugs (multi-resistance). Animals were monitored for 14 more days. At the end of the experiment, all animals that remained positive were treated with DA at 7.0 mg/kg b.w. All positive animals were ear-tagged. Animal ID, owner’s name, location, GPS coordinates, age (calf, young, adult), sex, breed (zebu, taurine, crossbred), PCV, body condition (cachectic, lean, good), and infection type (trypanosome species) were recorded.
On monitoring days, for all animals, one vacutainer tube was filled with approximately 2 ml of blood per animal for DNA extraction and molecular tests (semi-nested 18S rRNA polymerase chain reaction (PCR)). Blood was kept at − 20 °C until analysis. Protocols and interpretation of the molecular analysis results followed the method described by Geysen et al. (2003).
DNA extraction and semi-nested 18S rRNA PCR
Genomic DNA was extracted from blood samples collected from the animals using a Qiagen® QIAamp DNA extraction kit (Qiagen, Hilden, Germany), following manufacturer instructions. For the molecular detection of trypanosomes, 18S semi-nested rRNA PCRs were run, targeting a fragment of the 18S ribosomal RNA gene, on an Eppendorf Mastercycler® gradient thermocycler (Eppendorf AG, Hamburg, Germany), under the following conditions: 10 s at 98 °C; 40 cycles of 98 °C for 1 s, 58 °C for 5 s, 72 °C for 15 s and a final step of 72 °C for 1 min. For this, two reactions were carried out in a final volume of 25 μl containing 1× Phusion Flash high-fidelity PCR Master Mix (Thermo Fisher Scientific, Sweden), 200 μM of each dNTP, 20 pmol of each primer, and 5 μl of eluted DNA for the first reaction and 2.5 μl of PCR product from the first reaction for the nested reaction. Water was added to obtain a final volume of 25 μl. The primers used were 18ST nF2 5′-CAA CGA TGA CAC CCA TGA ATT GGG GA-3′ and 18ST nR3 5′-TGC GCG ACC AAT AAT TGC AAT AC-3′ for the first reaction and 18ST nF2 and 18ST nR2 5′-GTG TCT TGT TCT CAC TGA CAT TGT AGT G-3′ for the nested reaction. For PCR product visualization, the samples were run in a 2% agarose gel where 2 μl of loading dye was mixed with 5 μl of nested PCR product and loaded onto the gel. A 1000-bp DNA ladder was also loaded (4 μl) for fragment size determination, and the gel was run for 45 min at 120 V. The gel was stained with GelRed (Biotium, Inc., Fremont, CA, USA) at 4 μl per 100 ml of gel directly added to the gel before polymerization.
Restriction fragment length polymorphism
All the nested products (positive samples in agarose gel) were digested with MspI and Eco571 (Acul) enzymes at 37 °C for 60 min, in a final volume of 15 μl containing 1× enzyme buffer, 0.5 μl MspI, 0.5 μl Eco571, 8.5 μl deionized water, and 4.0 μl of PCR product. The samples were then run in a 3% agarose gel at 80 V for 120 min, where 2 μl of loading dye was mixed with 4 μl of digested PCR product and loaded onto the gel together with a 100-bp DNA ladder. The gel was stained by adding GelRed (Biotium, Inc., Fremont, CA, USA) at 4 μl per 100 ml of gel before polymerization.
Results
Cross-sectional study: trypanosome prevalence
Out of the 467 animals screened using BCT, 107 were found to be positive for T. congolense infection in three of the five villages. No positive animals were diagnosed in both Zalala-Electrosul and Zalala-Madal villages. Only T. congolense infections were detected during the screening. Detailed description of the total prevalence per village can be found in Table 1. In the group of trypanosome-positive animals, the mean PCV (%) was 27%, significantly different from the negative sample group, i.e., 31% (P value < 0.0001, t test). The lowest PCV (12%) was found in one animal in Botao.
Treatment response to isometamidium chloride
On day 14 after treatment, three animals that had been treated with ISM were still positive in Botao, while in Mungama, four animals treated with ISM were positive. On day 28, the day on which the drug swap was carried out, four animals from the ISM group remained positive in Botao. In Mungama and Namitangurine however, no animals from the ISM group were found to be positive (Table 2).
Treatment response to diminazene aceturate
On day 14 after treatment with DA, the number of positive animals was six and three in Botao and Mungama, respectively. On day 28, four animals in Botao and four animals in Mungama were still positive after treatment with DA (Table 2). As was observed in the ISM group, no relapses were recorded in Namitangurine.
Multiple drug resistance
After swapping the drug, microscopic analysis on day 42 revealed six positive animals (9%) in Botao and two positive animals (9.5%) in Mungama, thus pointing to the presence of multiple drug resistance.
Molecular test
All the negative results from monitoring days 14, 28, and 42 were verified using semi-nested 18S PCR-RFLP. The 18S PCR allowed for the detection of positive cases where the BCT method detected negatives as shown in Table 2. A gel figure showing T. congolense-positive results detected by 18S PCR-RFLP can be found in Fig. 2.
Discussion
The Mozambican province of Zambezia is considered to be within an area infested by tsetse flies with medium to high trypanosome challenge, the “common fly belt” (RTTCP 2000).This is supported by the findings of the present study, where trypanosomes were detected in cattle in three out of five study villages. There may be several reasons for the absence of positive cases in Zalala-Madal and Zalala-Electrosul. One such reason could be the use of trypanocides, as it is known that most of the cattle in this area belong to the commercial sector and treatment with trypanocides is frequent (Specht 2008). However, the observed absence of positive cases could also be attributed to anthropogenic activities in the area (urbanization, clearing of vegetation for agriculture and new settlements), which may have altered the distribution of tsetse flies in the area or at least influenced their density. According to Malele et al. (2011), tsetse distribution can be affected by different factors such as changes in the land use and increases in human population and activities. These activities usually result in changes in the ecology of the areas previously occupied by tsetse flies, mainly through the elimination of breeding sites and hosts.
Specht (2008), working in Zambezia province in 2004, found a 15% prevalence of T. congolense, making it the dominant trypanosome species in the area. In the present study, only T. congolense infections were detected. Similar results were found by Jamal et al. (2005), when studying the susceptibility of T. congolense isolates in Zambezia province. In several studies conducted in sub-Saharan Africa, T. congolense has been found to be the most prevalent trypanosome species in cattle, especially in southern Africa (Sigaúque et al. 2000; Van Den Bossche 2001; Simukoko et al. 2007; Laohasinnarong et al. 2011; Mwandiringana et al. 2012; Simo et al. 2015), while in other areas, T. vivax is the dominant species (Ahmed et al. 2016; Cecchi et al. 2014).
One of the clinical evidences of trypanosomosis in cattle, especially in cases where it is caused by T. congolense infections, is low PCV (Mbewe et al. 2015; Marcotty et al. 2008). When comparing the mean PCV between trypanosome-negative and trypanosome-positive animals, a significant difference (P < 0.0001) was observed, with lower mean PCVs in trypanosome-positive animals.
Drug resistance (DR) to trypanocides is a dynamic process, and it is spreading and increasing in Africa. The detection of DR and identification of resistant Trypanosoma sp. populations remain a challenge. In the present study, a block treatment test was applied to assess single and multiple drug resistance to ISM and DA (Mungube et al. 2012a). PCR and BCT, which are both methods that are typically employed to estimate the prevalence of trypanosome infection, were used to detect ISM and DA treatment failure in the current study. Although BCT is a commonly used method for field assessment of trypanosomosis and for individual animal treatment monitoring, it is limited by the levels of parasitemia in the infected animals (Paris et al. 1982). The visualization of parasites in body fluids is currently the most widely used method for the diagnosis of animal trypanosomosis in endemic regions (Cecchi et al. 2014). However, according to Van den Bossche (2001), parasitemia can fluctuate below the levels of detection by microscopy. Nevertheless, with the advent of PCR, this limitation was surpassed, as it allows for the detection of trypanosomes in samples with very low parasitemia (Clausen et al. 1998; Desquenes and Davila 2002). Nonetheless, due to its high cost, PCR is not the technique of choice to support decisions on treatment options for individually diagnosed animals (Cox et al. 2010). In the present study, using BCT, trypanosome-positive animals were diagnosed, treated, and monitored for relapses in the field. However, PCR was shown to be a more sensitive technique for the assessment of relapses as it allowed for the detection of positives in cases where BCT indicated negatives during the monitoring phase on days 14, 28, and 42 post-treatment.
Using the BCT method to check for trypanosomes 28 days post-treatment, the treatment failure rate for ISM was 6 and 0%, while the one for DA, it was 6 and 19%, in Botao and Mungama, respectively. These results revealed the difficulty that exists for farmers to be successful in the treatment of trypanosomosis. After the drug swap, conducted to detect multi-resistant strains, a total of eight isolates that were resistant to both drugs were identified on day 42. The present results confirmed those obtained by Jamal et al. (2005), which indicated that resistance to both ISM and DA is present in Zambezia province. Multi-resistant cases have also been detected, e.g., in Burkina Faso (Clausen et al. 1992), Zambia (Sinyangwe et al. 2004), Ethiopia (Miruk et al. 2008), Mali (Mungube et al. 2012a), and Togo (Tchamdja et al. 2017). In the present study, contrary to what was reported by Geerts et al. (2001), Mungube et al. (2012a), and Tchamdja et al. (2017), treatment failure was higher for DA than that for ISM. Multiple drug resistance can be a serious problem to cattle keepers, especially in this specific case, where the tested drugs are considered to be the most reliable ones presently available on the market.
Most of the cattle in Zambezia province belong to commercial farmers, and this is usually associated with intensive use of trypanocides. Although it cannot be assumed that the results from the present study are a reflection of this, it is a hypothesis that cannot be disregarded.
The results from the present study confirmed the existence of single- and multi-drug resistance in Nicoadala district, Zambezia province. Furthermore, based on the previous reports of resistance in the area, it is worth noting that the current results demonstrated that the geographical location of trypanocides-resistant hot spots has remained unchanged for at least the past 12 years. This information is fundamental when considering the control of trypanosomosis in the area. A better understanding of the drug resistance in the area will allow to define evidence-based, adapted measures for the progressive control of AAT (Diall et al. 2017).
The use of best bet strategies (Mungube et al. 2012b; Tchamdja et al. 2017) for the control of the disease in the area could be a valuable approach as it can be seen that the sanative pair of drugs can, at any moment, no longer be an option. The use of strategies involving rational drug use, which consists in the treatment of sick animals only and after proper diagnosis and using the correct dose should be urgently implemented by the local veterinary services. Moreover, general improvement of animal health conditions by deworming and reduction of animal disease risk, and vector control as described by Clausen et al. (2010) can also be of great impact. The development of molecular tools to allow for a faster assessment of the status of drug resistance is also advisable and should be encouraged.
References
Ahmed SK, Rahman AH, Hassan MA, Salih EM, Paone M, Cecchi G (2016) An atlas of tsetse and bovine trypanosomosis in Sudan. Parasit Vectors 9(1):194. https://doi.org/10.1186/s13071-016-1485-6
Baker N, de Koning HP, Mäser P, Horn D (2013) Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol 29(3):110–118. https://doi.org/10.1016/j.pt.2012.12.005
Barret MP, Burchmore RJ, Stich AS, Lazzari JO, Frasch AC, Cazulo JJ, Krishna S (2003) The trypanosomiases. Lancet 362(9394):1469–1480. https://doi.org/10.1016/S0140-6736(03)14694-6
Büscher P, Cecchi G, Jamonneau V, Priotto G (2017) Human African trypanosomiasis. Lancet 390(10110):2397–2409. https://doi.org/10.1016/S0140-6736(17)31510-6
Cannon RM, Roe RT (1982) Livestock disease surveys: a field manual for veterinarians. Australian Government Publishing Service, Canberra
Cecchi G, Paone M, Feldmann U, Vreysen MJ, Diall O, Mattioli RC (2014) Assembling a geospatial database of tsetse-transmitted animal trypanosomosis for Africa. Parasit Vectors 7(1):39. https://doi.org/10.1186/1756-3305-7-39
Chitanga S, Marcotty T, Namangala R, Van den Bossche P, Van Den Abbeele J, Delespaux V (2011) High prevalence of drug resistance in animal trypanosomes without a history of drug exposure. PLoS Negl Trop Dis 5(12):e1454. https://doi.org/10.1371/journal.pntd.0001454
Clausen PH, Sidibe I, Kabore I, Bauer B (1992) Development of multiple drug resistance of Trypanosoma congolense in Zebu cattle under high natural tsetse fly challenge in the pastoral zone of Samorogouan, Burkina Faso. Acta Trop 51:229–236
Clausen PH, Wiemann A, Patzelt R, Kakaire D, Poetzsch C, Peregrine A, Mehlitz D (1998) Use of a PCR assay for the specific and sensitive detection of Trypanosoma spp. in naturally infected dairy cattle in peri-urban Kampala, Uganda. Ann N Y Acad Sci 849(1):21–32. https://doi.org/10.1111/j.1749-6632.1998.tb11029.x
Clausen PH, Bauer B, Zessin KH, Diall O, Bocoum Z, Sidibe I, Affognon H, Waibel H, Grace D, Randolph T (2010) Preventing and containing trypanocide resistance in the cotton zone of West Africa. Transbound Emerg Dis 57:28–32
Cox AP, Tosas O, Tilley A, Picozzi K, Coleman P, Hide G, Welburn SC (2010) Constraints to estimating the prevalence of trypanosome infections in East African zebu cattle. Parasit Vectors 3(1):82. https://doi.org/10.1186/1756-3305-3-82
Dávila AM, Majiwa PA, Grisard EC, Aksoy S, Melville SE (2003) Comparative genomics to uncover the secrets of tsetse and livestock-infective trypanosomes. Trends Parasitol 19(10):436–439. https://doi.org/10.1016/S1471-4922(03)00196-X
De Koning HP, Anderson LF, Stewart M, Burchmore RJ, Wallace LJ, Barrett MP (2004) The trypanocides diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in African trypanosomes. Antimicrob Agents Chemother 48(5):1515–1519. https://doi.org/10.1128/AAC.48.5.1515-1519.2004
Delespaux V, De Koning HP (2007) Drugs and drug resistance in African trypanosomiasis. Drug Resist Update 10:30–50
Delespaux V, Chitanga S, Geysen D, Goethals A, Van den Bossche P, Geerts S (2006) SSCP analysis of the P2 purine transporter TcoAT1 gene of Trypanosoma congolense leads to a simple PCR-RFLP test allowing the rapid identification of diminazene resistant stocks. Acta Trop 100(1-2):96–102. https://doi.org/10.1016/j.actatropica.2006.10.001
Delespaux V, Dinka H, Masumu J, Van den Bossche P, Geerts S (2008) Five fold increase in Trypanosoma congolense isolates resistant to diminazene aceturate over a seven-year period in Eastern Zambia. Drug Resist Update 11:205–209
Desquenes M, Davila AM (2002) Applications of PCR-based tools for detection and identification of animal trypanosomes: a review and perspectives. Vet Parasitol 109(3-4):213–231. https://doi.org/10.1016/S0304-4017(02)00270-4
Diall O, Cecchi G, Wanda G, Argilés-Herrero R, Vreysen MJ, Cattoli G, Viljoen GJ, Mattioli R, Bouyer J (2017) Developing a progressive control pathway for African animal trypanosomosis. Trends Parasitol 33(7):499–509. https://doi.org/10.1016/j.pt.2017.02.005
Eisler M, Brandt J, Bauer B, Clausen P, Delespaux V, Holmes P, Ilemobade A, Machila N, Mbwambo H, McDermott J, Mehlitz D, Murilla G, Ndung’u J, Peregrine A, Sidibe I, Sinyangwe L, Geerts S (2001) Standardized tests in mice and cattle for detection of drug resistance in tsetse transmitted trypanosomes of African domestic cattle. Vet Parasitol 97(3):171–182. https://doi.org/10.1016/S0304-4017(01)00415-0
Franco JR et al (2017) Monitoring the elimination of human African trypanosomiasis: update to 2014. PLoS Negl Trop Dis 11(5):e00005585
Geerts S, Holmes PH (1998) Drug management and parasite resistance in bovine trypanosomiasis in Africa. Drug management and parasite resistance in bovine trypanosomiasis in Africa. PAAT Technical and Scientific Series. FAO
Geerts S, Holmes PH, Eisler MC, Diall O (2001) African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol 17(1):25–28. https://doi.org/10.1016/S1471-4922(00)01827-4
Geysen D, Delespaux V, Geerts S (2003) PCR–RFLP using Ssu-rDNA amplification as an easy method for species-specific diagnosis of Trypanosoma species in cattle. Vet Parasitol 110:171–180
Jamal SAJ, Sigauque I, Macuamule C, Neves L, Penzhorn BL, Marcotty T, Van den Bossche P (2005) The susceptibility of Trypanosoma congolense isolated in Zambezia Province, Mozambique, to isometamidium chloride, diminazene aceturate and homidium chloride. Onderstepoort J Vet Res 72(4):333–338
Laohasinnarong D, Thekisoe OM, Malele I, Namangala B, Ishii A, Goto Y, Kawazu SI, Sugimoto C, Inoue N (2011) Prevalence of Trypanosoma sp. in cattle from Tanzania estimated by conventional PCR and loop-mediated isothermal amplification (LAMP). Parasitol Res 109(6):1735–1739. https://doi.org/10.1007/s00436-011-2513-2
Macucule PA (2014) Diagnosis and mapping of diminazene aceturate resistance in Trypanosoma congolense, Broden 1904, strains circulating in cattle in Matutuíne district, Mozambique. Dissertation, University of Pretoria
Malele II, Magwisha HB, Nyingilili HS, Mamiro KA, Rukambile EJ, Daffa JW, Lyaruu EA, Kapange LA, Kasilagila G, Lwitiko N, Msami HM, Kimbita EN (2011) Multiple Trypanosoma infections are common amongst Glossina species in the new farming areas of Rufiji district, Tanzania. Parasit Vectors 4(1):217. https://doi.org/10.1186/1756-3305-4-217
Marcotty T, Simukoko H, Berkvens D, Vercruysse J, Praet N, Van den Bossche P (2008) Evaluating the use of packed cell volume as an indicator of trypanosomal infections in cattle in eastern Zambia. Prev Vet Med 87(3-4):288–300. https://doi.org/10.1016/j.prevetmed.2008.05.002
Matovu E, Stewart ML, Geiser F, Brun R, Mäser P, Wallace LJ, Burchmore RJ, Enyaru JC, Barret MP, Kaminsky R, Seebeck T, De Koning HP (2003) Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell 2(5):1003–1008. https://doi.org/10.1128/EC.2.5.1003-1008.2003
Mbewe NJ, Namangala B, Sitali L, Vorster I, Michelo C (2015) Prevalence of pathogenic trypanosomes in anaemic cattle from trypanosomosis challenged areas of Itezhi-tezhi district in central Zambia. Parasit Vectors 8(1):638. https://doi.org/10.1186/s13071-015-1260-0
McDermott J, Woitag T, Sidibé I, Bauer B, Diarra B, Oue’draogo D, Kamuanga M, Peregrine A, Eisler M, Zessin KH, Mehlitz D, Clausen PH (2003) Field studies of drug-resistance cattle trypanosomes in Ke’ne’dougou Province, Burkina Faso. Acta Trop 86(1):93–103. https://doi.org/10.1016/S0001-706X(03)00019-6
Miruk A, Hagos A, Yacob HT, Asnake F, Basu AK (2008) Prevalence of bovine trypanosomosis and trypanocidal drug sensitivity studies on Trypanosoma congolense in Wolyta and Dawero zones of southern Ethiopia. Vet Parasitol 152(1-2):141–147. https://doi.org/10.1016/j.vetpar.2007.12.007
Mulugeta W, Wilkes J, Mulatu W, Majiwa PA, Masake R, Peregrine AS (1997) Long-term occurrence of Trypanosoma congolense resistant to diminazene, isometamidium and homidium in cattle at Ghibe, Ethiopia. Acta Trop 64(3-4):205–217. https://doi.org/10.1016/S0001-706X(96)00645-6
Munday J, López K, Eze A, Delespaux V, Van Den Abbeele J, Rowan T, Barret M, Morrison L, De Koning H (2013) Functional expression of TcoAT1 reveals it to be a P1-type nucleoside transporter with no capacity for diminazene uptake. Int J Parasitol Drugs Drug Resist 3:69–76. https://doi.org/10.1016/j.ijpddr.2013.01.004
Mungube E, Vitouley H, Diall O, Cudjoe E, Boucoum Z, Diarra B, Sanogo Y, Randolph T, Bauer B, Zessin K, Clausen PH (2012a) Detection of multiple drug-resistant Trypanosoma congolense populations in village cattle of south-east Mali. Parasit Vectors 5(1):155. https://doi.org/10.1186/1756-3305-5-155
Mungube E, Diall O, Baumann M, Sanogo Y, Randolph T, Bauer B, Hoppenheit A, Hinney B, Maiga B, Zessin K, Clausen PH (2012b) Best-bet integrated strategies for containing drug-resistant trypanosomes in cattle. Parasit Vectors 5(1):164. https://doi.org/10.1186/1756-3305-5-164
Murray M, Murray PK, McIntyre WI (1977) An improved parasitological tecnique for diagnosis of African trypanosomiasis. Trans R Soc Trop Med Hyg 71(4):325–326. https://doi.org/10.1016/0035-9203(77)90110-9
Mwandiringana E, Gori E, Nyengerai T, Chidzwondo F (2012) Polymerase chain reaction (PCR) detection of mixed trypanosome infection and blood meal origin in field-captured tsetse flies from Zambia. Afr J Biotechnol 11:14490–14497
Paris J, Murray M, McOdimba F (1982) A comparative evaluation of the parasitological techniques currently available for the diagnosis of African trypanosomiasis in cattle. Acta Trop 39(4):307–316
Rafael AAG (1959) Ensaios com o Pro-salt de Antricide R.F. e Berenil. Separata do Boletim No 117
RTTCP (2000) An update of the distribution of bovine trypanosomosis in Mozambique (1986–1998)
Shah-Fischer M, Say RR (1989) Manual of tropical veterinary parasitology. U.K. The Technical Centre for Agriculture and Rural Cooperation
Shaw APM, Cecchi G, Wint GRW, Mattioli RC, Robinson TP (2014) Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Prev Vet Med 113(2):197–210. https://doi.org/10.1016/j.prevetmed.2013.10.024
Sigauque I, Van den Bossche P, Moiana M, Jamal S, Neves L (2000) The distribution of tsetse (Diptera: Glossinidae) and bovine trypanosomosis in the Matutuine District, Maputo Province, Mozambique. Onderstepoort J Vet Res 67(3):167–172
Silva JM (1959) Aspectos das trypanossomiases animais em Moçambique – Revisão da sua terapêutica. Separata do Boletim No115
Simo G, Fongho P, Farikou O, Ndjeuto-Tchouli PIN, Tchouomene-Labou J, Njiokou F, Asonganyi T (2015) Trypanosome infection rates in tsetse flies in the “silent” sleeping sickness focus of Bafia in the centre region in Cameroon. Parasit Vectors 8(1):528. https://doi.org/10.1186/s13071-015-1156-z
Simukoko H, Marcotty T, Phiri I, Geysen D, Vercruysse J, Van den Bossche P (2007) The comparative role of cattle, goats and pigs in the epidemiology of livestock trypanosomiasis on the plateau of eastern Zambia. Vet Parasitol 147(3-4):231–238. https://doi.org/10.1016/j.vetpar.2007.04.005
Sinyangwe L, Delespaux V, Brandt J, Geerts S, Mubanga J, Machila N, Holmes PH, Eisler MC (2004) Trypanocidal drug resistance in eastern province of Zambia. Vet Parasitol 119(2-3):125–135. https://doi.org/10.1016/j.vetpar.2003.11.007
Specht EJK (2008) Prevalence of bovine trypanosomosis in Central Mozambique from 2002 to 2005. Onderstepoort J Vet Res 75(1):73–81
Sutherland IA, Holmes PH (1993) Alterations in drug transport in resistant Trypanosoma congolense. Acta Trop 54(3-4):271–278. https://doi.org/10.1016/0001-706X(93)90099-W
Sutherland IA, Peregrine AS, Lonsdale-Eccles JD, Holmes PH (1991) Reduced accumulation of isometamidium by drug-resistant Trypanosoma congolense. Parasitology 103(02):245–251. https://doi.org/10.1017/S0031182000059527
Sutherland IA, Mounsey A, Holmes PH (1992) Transport of isometamidium (Samorin) by drug-resistant and drug-sensitive Trypanosoma congolense. Parasitology 104(03):461–467. https://doi.org/10.1017/S0031182000063721
Tchamdja E, Kulo AE, Vitouley HS, Batawui K, Bankolé AA, Adomefa K, Cecchi G, Hoppenheit A, Clausen PH, De Deken R, Van Den Abbeele J, Marcotty T, Delespaux V (2017) Cattle breeding, trypanosomosis prevalence and drug resistance in Northern Togo. Vet Parasitol 236:86–92. https://doi.org/10.1016/j.vetpar.2017.02.008
Van den Bossche P (2001) Some general aspects of the distribution and epidemiology of bovine trypanosomosis in southern Africa. Int J Parasitol 31(5-6):592–598. https://doi.org/10.1016/S0020-7519(01)00146-1
Acknowledgements
We would like to acknowledge the European Union for financing the study through the EU funded TRYRAC project (TRYRAC/DCI-FOOD/2011/279-754), the Biotechnology Center - Eduardo Mondlane University and the University of Pretoria for all the laboratory assistance.
FAO assistance to this study was provided in the framework of the Programme Against African Trypanosomosis (PAAT), and supported by the Government of Italy (Project ‘Improving food security in sub-Saharan Africa by supporting the progressive reduction of tsetse-transmitted trypanosomosis in the framework of the NEPAD’, codes GTFS/RAF/474/ITA and GCP/RAF/502/ITA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed and all procedures performed in studies involving animals were in accordance with the ethical standards of Biotechnology Centre—Eduardo Mondlane University and the practice at which the study was conducted. Permit number CBUEM/COMETH_0014/2014 issued on 17 March 2014.
Rights and permissions
About this article
Cite this article
Mulandane, F.C., Fafetine, J., Van Den Abbeele, J. et al. Resistance to trypanocidal drugs in cattle populations of Zambezia Province, Mozambique. Parasitol Res 117, 429–436 (2018). https://doi.org/10.1007/s00436-017-5718-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5718-1