Abstract
Fluoride is an important toxicological and environmental toxicant that is implicated in diverse cardiorenal system dysfunctions via the induction of oxidative stress. The present study aims at evaluating the cardioprotective and renoprotective effects of melatonin and vitamin E on fluoride toxicity on biomarkers of oxidative stress, clinical pathology, and their molecular mechanism of action. Apparently healthy male rats of the Wistar strain (n = 50; 160 ± 7.5 g), were randomly distributed into five groups of ten animals per group as follows: Control, sodium fluoride (NaF, 25 mg/kg), NaF and melatonin 20 mg/kg i.p.; NaF and vitamin E 50 mg/kg p/o, NaF plus melatonin and vitamin E administered orally. NaF and melatonin were administered for fifteen consecutive days, whereas vitamin E was administered every 72 h. Blood pressure parameters, oxidative stress biomarkers, electrocardiography, histopathology, and immunohistochemical staining were performed. From this study, NaF intoxication provoked reduction in renal and cardiac systemic antioxidants, alterations of haemodynamic and electrocardiographic parameters, heightened blood urea nitrogen (BUN), creatinine, angiotensin converting enzyme, angiotensin 2 type 1 receptor, kidney injury molecule 1, interleukin 1 beta in the renal tissues, cardiac troponin, and nuclear kappa beta. However, the administration of either melatonin or vitamin E, and its combination mitigated high blood pressure, normalized electrocardiographic changes, abrogated biomarkers of oxidative stress, improved renal function, and attenuated inflammation. The combination of melatonin and vitamin E effectively mitigated cardiovascular and renal toxicities associated with fluoride intoxication through the prevention of cardio-renal dysfunction, oxidative stress, and inflammatory processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sodium fluoride (NaF) is an inorganic compound of fluoride that is highly available orally, readily absorbed, and is estimated to be 2 to 5 times more available than other environmental and dietary forms of fluoride (Plumlee 2004). Fluoride is one of the most important toxicological and environmental hazards globally because of its ability to cause fluorosis in humans and livestock (Jha et al. 2013; Kashyap et al. 2021). Paradoxically, dietary fluoride is sine qua non to life (Zhang et al. 2013). In humans, fluorosis commonly manifests following excessive dietary consumption of fluoridated compounds, whereas poisoning of livestock with fluorides, more often than not, is caused by the ingestion of forages contaminated by industrial pollutants or volcanic emissions (Thompson 2018; Cowan and Blakley 2016). Furthermore, toxicity due to excessive fluoride exposure can occur from the use of fluoride containing household products such as dentifrices, special dyes, pesticides, ceramic polishing, and drinking of fluoride-enhanced water (Abdollahi and Momen-Heravi 2014). The wide application of fluoride as anticariogenic agent for the prevention of dental caries may further predispose humans to systemic toxicity (Azab et al. 2018). Hepato-renal, neuronal, and testicular toxicities associated with sodium fluoride intoxications have been documented (Caglayan et al. 2021; Ajibade et al. 2022; Varışlı et al. 2022; Abd-Allah and El-Rahman 2022; Das et al. 2023; Xu et al. 2023). Similarly, cardiovascular derangement has been positively correlated with sodium fluoride toxicities from our laboratory and elsewhere (Sharma et al. 2023; Labib et al. 2022; Zhang et al. 2022; Oyagbemi et al. 2017, 2018).
Fluoride reportedly causes diverse physiological alterations in mammalian systems via modulation of enzyme activity and antioxidant defenses, induction of apoptosis, release of cytochrome c from mitochondria, and heightened inflammatory processes (Guth et al. 2020). Alterations of proteins and inhibition of enzyme activities are other important mechanisms involved in the mediation of fluoride toxicities (Mendoza-Schulz et al. 2009). For instance, fluoride potently and specifically inhibits cytoplasmic pyrophosphatases, thereby inhibiting cellular protein functioning in vivo (Strunecka and Strunecky 2020). In addition to the inhibition of pyrophosphatases, fluoride exacerbates the generation of reactive oxygen species (ROS) while abating the levels and or activities of glutathione and other systemic antioxidants (Varol et al. 2013; Strunecká et al. 2019; Srivastava and Flora 2020).
The interactions among different antioxidant molecules can be such that the combined effect of two or more antioxidants is much greater than the sum of the effects of each antioxidant; additive (combined effect of two or more antioxidants is equal to the sum of the effects of each administered antioxidants); or antagonistic (the effects of the antioxidants cancel each other out) (Sonam and Guleria 2017). A logical advantage of the combined use of antioxidants is the significant reduction in the dose of each antioxidants required to produce a desired effect, thereby reducing toxicities associated with each of the exogenous antioxidants. Individually, melatonin and vitamin E are potent antioxidants that modulate the pathogenesis of hypertension and several other mammalian diseases (Borghi and Cicero 2017; Dominguez-Rodriguez et al. 2017; Ferlazzo et al. 2020; Ozkalayci et al. 2021). Vitamin E is a lipid-soluble antioxidant that inhibits the production of ROS and has been reported to protect biological membranes from lipid peroxidation (Hong et al. 2004). Furthermore, bioactive forms of vitamin E such as gamma-tocopherol have been reported to positively modulate cardiovascular system functioning (Rizvi et al. 2014).
A large number of drugs are available for the management of cardiovascular and renal dysfunctions but the use of these drugs is sometimes limited by severe side effects. Therefore, there is a need for the continuous search of safe and effective alternatives, or in the very least, biomolecules that may be used as supplements in the management of cardiac and renal diseases. Although melatonin and vitamin E have been well documented as modulators of cardiovascular and renal systems functioning, to our knowledge, no reports are currently available on the combined effects of the two antioxidants on fluoride-associated cardiovascular/ renal toxicities. Therefore, we sought to assess the mechanistic effects of melatonin and vitamin E alone and in combination on sodium fluoride (NaF)-associated derangements of the cardiorenal systems functioning. The prominent roles of oxidative stress in the pathogenesis of NaF-induced toxicities of the cardiovascular and renal systems made us to posit that melatonin-vitamin E will counteract NaF toxicities and provide new insights into the development of safe alternatives for the prevention and/or management of such diseases as hypertension and renal failure associated with fluoride toxicity in humans and animals.
Materials and methods
Experimental animals
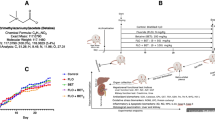
Apparently, healthy 8 weeks old male rats of the Wistar strain (n = 50; 160 ± 7.5 g) were randomly distributed without prejudice to size or weight into five groups of ten animals per group as follows: Control, sodium fluoride (NaF, 25 mg/kg), NaF and melatonin 20 mg/kg i.p.; NaF and vitamin E 50 mg/kg p/o, NaF plus melatonin and vitamin E administered orally. NaF and melatonin were administered for fifteen consecutive days, whereas vitamin E was administered every 72 h. The doses of NaF, melatonin, and vitamin E were used guided by earlier reports of Shanmugam et al. (2018), Ajibade et al. (2017), and Ajibade et al. (2021), respectively. The rats were kept at room temperature (27 °C) and 12-h light/dark cycle. Optimum feed from Ladokun Feeds Nigeria Ltd., Ibadan, Nigeria, and water were made available to the rats which were kept in very clean cages throughout the experimental period of fifteen consecutive days. Male rats were used for the study as estrous cycle of female might interfere with some clinical parameters. Ethical clearance was obtained from the University of Ibadan Animal Care and use research ethics committee (ACUREC) with approval code UIACUREC/ 19/124 assigned.
Chemicals
The chemicals used in this study were of analytical grade. They include melatonin (AK Scientific, CA, USA), sodium fluoride (NaF) (Molychem, Mumbai, India), trichloro aceticacid, anti-angiotensin converting enzyme1 polyclonal antibody (E-AB-16159: 1:500), angiotensin 2 type 1 receptor (ATR1) polyclonal antibody (E-AB-18016: 1:500), kidney injury molecule 1 (Kim-1) (ab78494: 100), interleukin 1 beta (IL-β) (E-AB-52153: 1:500), nuclear kappa beta (NF-κB) (E-AB-32232: 1:500), and cardiac troponin (TNNC1) polyclonal antibody (E-AB-18400: 1:50) (Elabscience Biotechnology Inc, Houston, TX, USA); vitamin E and O-dianisidine (Sigma Aldrich, St. Louis, Missouri, USA).
Plethysmography and electrocardiography
The primary haemodynamic parameters were recorded in conscious carefully restrained rats placed on a warm platform using a plethysmograph (Kent Scientific, USA). The plethysmograph employs the principle of indirect blood pressure measurement. The rats were trained for 30 min and allowed to acclimatize to the conditions of the blood pressure monitor before the haemodynamic parameters were recorded in the most quiescent state of the animals. An average of 30 most consistent readings was recorded for each rat. The electrocardiographic parameters were obtained in xylazine/ketamine anaesthetized rats using an electrocardiograph (EDAN VE-1010, China).
Preparation of tissues and serum for biochemical assays
The hearts and kidneys were carefully excised and immediately placed on ice to retard enzyme degradation. Thereafter, the tissues were homogenized in potassium phosphate buffer (0.1 M, pH 7.4) and centrifuged at 12,000 g for 15 min to obtain the post-mitochondria fraction (PMF) of the cardiac and renal tissues. The PMFs were persevered in a refrigerator in preparation for biochemical analyses.
Three milliliters of blood were collected from the retroorbital venous plexus in anaesthetized rats with the aid of capillary tubes, into nonheparinised haematological bottles. Thereafter, blood samples were kept at room temperature for 40 min to allow for clot formation and obtain the sera as the supernatant. Then, the separated sera were carefully removed with sterile Pasteur pipettes into clean sample bottles and preserves at −20 °C for biochemical assays.
Biochemical assays
In this study, the markers of antioxidant defense status such as superoxide dismutase, glutathione, and glutathione peroxidase were assayed in cardiac and renal tissues using the PMF as previously described (Misra and Fridovich 1972; Oyagbemi et al. 2019; Jollow et al. 1974; Buetler et al. 1963). Similarly, the markers of oxidative stress, including malondialdehyde (MDA), nitric oxide NO, and hydrogen peroxide H2O2, as well as protein concentration were assayed according to the methods established by Varshney and Kale (1990), Olaleye et al. (2007), Wolff (1994), and Gornal et al. (1949), respectively. The breakdown of protein metabolism (urea) and creatinine were assayed in the serum using the corresponding biochemical kits in accordance with the protocol provided by the manufacturer.
Histopathology
The preparation of the tissues for histopathological evaluation was carried out as described by Bancroft and Gamble (Drury et al. 1976), with the heart and kidney carefully dissected, fixed, processed, mounted on glass slides, and stained with haematoxylin and eosin.
Immunohistochemistry
The immune-localization of angiotensin converting enzyme (ACE), angiotensin 2 type 1 receptor (ATR1), kidney injury molecule 1 (Kim-1), interleukin 1 beta (IL-β), nuclear kappa beta (NF-κB), and cardiac troponin were carried out as described by Oyagbemi et al. (2019). Sections were observed with light microscope (Leica LAS-EZ®) using Leica software application suite version 3.4 equipped with a digital camera. Immunoreactivity was quantified using Image J (FIJI) software as described by Fuhrich et al. (2013).
Statistical analysis
The statistical analysis in this study was carried out using central tendency measures, mean ± standard deviation. Student’s t test and analysis of variance (ANOVA) were also carried out to compare the statistical difference between means of the experimental groups. Graph Pad Prism version 9.0 was used for the plotting of graphs. Values of probability less than 0.05 were considered statistically significant.
Results
Markers of oxidative stress
A significant increase (P < 0.05) was recorded in the markers of oxidative stress (hydrogen peroxide and malondialdehyde) in the cardiac and renal tissues of rats following sodium fluoride exposure. Rats administered with melatonin and vitamin E had lesser levels of oxidative stress markers relative to the sodium fluoride treated groups. The combination of melatonin and vitamin E caused a significantly lowered reduction (P < 0.05) of oxidative stress markers compared with the individual antioxidants. Moreover, protein carbonyl was elevated following fluoride exposure when compared with rats treated with melatonin and vitamin E.
The systemic antioxidants glutathione peroxidase (GPx), glutathione (GSH), glutathione S-transferase (GST), and superoxide dismutase (SOD) were significantly abated for sodium fluoride-treated group relative to rats administered antioxidants. Higher levels of the antioxidants GPx, GST, and SOD were seen in rats administered both melatonin and vitamin E compared with rats administered either of the antioxidants.
Blood pressure and electrocardiogram
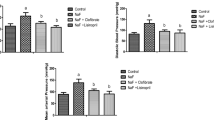
The arterial blood pressure (Fig. 1), flow rate (Fig. 2A), as well as heart rate (Fig. 2B) of rats administered sodium fluoride considerably exceeded those recorded for antioxidant-treated rats. Conversely, the electrocardiographic parameters PR interval, QRS complex, and QT interval were significantly shortened in rats administered sodium fluoride relative to the control and the antioxidants-treated rats (Fig. 2C).
Blood pressure parameters of rats exposed to sodium fluoride (NaF) and treated with melatonin (Mel) and vitamin E, alone and in combination. Asterisk (*) indicates significant increase (P < 0.05) compared with control; Number sign (#) indicates significant decrease (P < 0.05) compared with NaF; Commercial at (@) indicates significant difference (P < 0.05) compared with NaFMelVitE
Rates of blood flow, heart rate, and electrocardiographic parameters of rats exposed to sodium fluoride (NaF) and treated with melatonin (Mel) and vitamin E, alone and in combination. Asterisk (*) indicates significant increase (P < 0.05) compared with control; Number sign (#) indicates significant decrease (P < 0.05) compared with NaF; Commercial at (@) indicates significant difference (P < 0.05) compared with NaFMelVitE
Markers of renal function
The markers of renal function assayed in the serum, in this study (BUN and creatinine) (Fig. 3A, B, respectively), were significantly elevated in the sodium fluoride administered group compared with the control and antioxidant groups of rats.
Serum blood urea nitrogen (BUN) and creatinine of rats exposed to sodium fluoride (NaF) and treated with melatonin (Mel) and vitamin E, alone and in combination. Asterisk (*) indicates significant increase (P < 0.05) compared with control; Number sign (#) indicates significant decrease (P < 0.05) compared with NaF
Histopathology
Histopathological evaluation revealed necrosis of tubular epithelial cells and peritubular inflammation in the renal tissues, as well as multiple foci of myofiber degeneration in the cardiac tissues of rats administered sodium fluoride, but these lesions were not seen in the tissues of rats administered melatonin (Figs. 4 and 5).
Renal tissues of rats administered melatonin and vitamin E in sodium fluoride exposed rats. A Control shows no visible lesion. B Sodium fluoride shows necrosis of tubular epithelial cells and peritubular inflammation (black arrows). C Sodium fluoride and melatonin show mild atrophy of tubular cells. D Sodium fluoride and vitamin E shows mild peri-glomerular and tubular inflammation. E Sodium fluoride, melatonin, and vitamin E show no visible lesions. H & E. Mag × 400
Cardiac tissues of rats administered melatonin and vitamin E in sodium fluoride exposed rats. A (Control) shows no visible lesion. B (Sodium fluoride) shows multiple foci of myofibre degeneration (black arrows). C (Sodium fluoride and melatonin) shows no visible lesion. D (Sodium fluoride and vitamin E) shows visible lesion. E (Sodium fluoride, melatonin and vitamin E) shows no visible lesions. H & E. Mag × 400
Immunohistochemical analysis
Immunohistochemical analysis revealed greater expressions of ACE, ATR1, kidney injury molecule 1, and interleukin 1 beta in the renal tissues of rats administered sodium fluoride, compared with the rats treated with melatonin alone or a combination of melatonin and vitamin E (Figs. 6, 7, 8, and 9), whereas greater expressions of cardiac troponin and nuclear kappa beta (NF-κB) were seen in the cardiac tissues of rats exposed to sodium fluoride without antioxidant treatment, relative to those administered melatonin alone or combinations of melatonin and vitamin E (Figs. 10 and 11).
The immunohistochemistry of renal angiotensin converting enzyme (ACE). Group A (control), group B (sodium fluoride; 600 ppm), group C (sodium fluoride; 600 ppm + melatonin 50 mg/kg), group D (sodium fluoride; 600 ppm + vitamin E 50 mg/kg), group E (sodium fluoride; 600 ppm + melatonin + vitamin E). The arrows in slides B, C, and E indicate the expression of ACE which is highest in slide B. The slides are stained only with haematoxylin. Magnification at × 100
The immunohistochemistry of renal angiotensin 2 type I receptor (ATR1). Group A (control), group B (sodium fluoride; 600 ppm), group C (sodium fluoride; 600 ppm + melatonin 50 mg/kg), group D (sodium fluoride; 600 ppm + vitamin E 50 mg/kg), group E (sodium fluoride; 600 ppm + melatonin + vitamin E). The arrows in slides B, C, D, and E indicate the levels of expression of ATR 1 which is highest in slide B. The slides are stained only with haematoxylin. Magnification at × 100
The immunohistochemistry of renal kidney injury molecule 1 (Kim-1). Group A (control), group B (sodium fluoride; 600 ppm), group C (sodium fluoride; 600 ppm + melatonin 50 mg/kg), group D (sodium fluoride; 600 ppm + vitamin E 50 mg/kg), group E (sodium fluoride; 600 ppm + melatonin + vitamin E). The arrows in slides B, C, D, and E indicate the expression of Kim-1, which is highest in slide B. The slides are stained only with haematoxylin. Magnification at × 100
The immunohistochemistry of interleukin 1 beta (IL-β). Group A (control); B (sodium fluoride); C (sodium fluoride and melatonin); D (sodium fluoride and vitamin E); E (sodium fluoride, melatonin, and vitamin E). The arrows in slides B, C and D indicate the levels of expression of IL-β which is highest in slide B. The slides are stained only with haematoxylin. Magnification × 100
The immunohistochemistry of cardiac troponin. Group A (control), group B (sodium fluoride; 600 ppm), group C (sodium fluoride; 600 ppm + melatonin 50 mg/kg), group D (sodium fluoride; 600 ppm + vitamin E 50 mg/kg), group E (sodium fluoride; 600 ppm + melatonin + vitamin E). The arrows in slides A, B, C, D, and E indicate the levels of expression of cardiac troponin which is highest in slide B. The slides are stained only with haematoxylin. Magnification at × 100
The immunohistochemistry of cardiac nuclear kappa beta (Nf-kβ). Group A (control), group B (sodium fluoride; 600 ppm), group C (sodium fluoride; 600 ppm + melatonin 50 mg/kg), group D (sodium fluoride; 600 ppm + vitamin E 50 mg/kg), group E (sodium fluoride; 600 ppm + melatonin + vitamin E). The arrows in slides A, B, C, D, and E indicate the levels of expression of Nf-kβ which is highest in slide B. The slides are stained only with haematoxylin. Magnification at × 100
Discussion
The heart and kidney are two of the most susceptible soft tissues to fluoride toxicity, with significant alterations of normal cardiovascular and renal systems functioning reported in human and animal models (Jha et al. 2011). In this study, the exposure to sodium fluoride caused severe dysregulation of the cardiovascular and renal systems, manifested as elevated primary haemodynamic parameters and kidney functional markers, respectively. Fluoride is a pro-inflammatory factor that has been reported to mediate significant elevation of blood pressure parameters via the induction of oxidative stress and inflammation in cardiac and renal tissues (Gutowska et al. 2010; Flora et al. 2012). An association between increased fluoride in ground water and increased prevalence of hypertension in adult males has been reported (Amini et al. 2011). However, the administration of the two antioxidants, melatonin, and vitamin E, in this study prevented the development of hypertension and maintained normal levels of serum blood urea nitrogen (BUN) and creatinine in rats. Remarkably, this observation is more pronounced in rats administered both melatonin and vitamin E.
Melatonin is an endogenous neurohormone involved in the control of a number of physiological processes, including circadian rhythms, mood regulation, anxiety, sleep, appetite, immune responses, maintenance of oxidant/antioxidant balance, and cardiovascular functioning (Comai and Gobbi 2014). Melatonin, due to its direct radical scavenging activity, exerts potent antioxidant effects on tissues/organs and antiapoptotic effects on cells (Tamura et al. 2012; Onaolapo et al. 2017). In this study, melatonin alone and in combination with vitamin E significantly decreased the heart rate, as well as the systolic, diastolic, and mean arterial blood pressure, while increasing the duration of the electrocardiographic parameters PR and QT intervals, QRS complex and ST segment, compared with the sodium fluoride administered group. This observation suggests a potent ability of melatonin to mitigate fluoride-induced toxicological effects probably by preventing the development of oxidative stress in the cardiac and renal tissues. In an earlier study, melatonin was reported to inhibit oxidative stress in the brain of rats chronically exposed to NaF with significant decreases reported in the levels of TBARS and ROS while increasing the activities of antioxidant enzymes and GSH content (Jain et al. 2015). In a similar experiment, melatonin, vitamin C, and vitamin E reportedly caused a significant reduction in serum MDA levels of rats exposed to the toxic effects of lead acetate (Aziz et al. 2012). From our results, melatonin, and vitamin E, alone and in combination significantly reduced the biomarkers of oxidative stress—MDA, H2O2, and PCO, whereas increased levels were recorded for the systemic antioxidants—GPx, GSH, GST, and SOD—relative to rats exposed to the toxicity of NaF without antioxidant treatment. Melatonin together with its metabolites such as cyclic 3-hydroxymelatonin have been reported to scavenge and neutralize a wide array of oxidizing agents and free radicals including singlet oxygen, superoxide anion radical, hydrogen peroxide, nitric oxide, hypochlorous acid, hydroxyl radical, and peroxynitrite anion (Reiter et al. 2014). In earlier reports, melatonin reportedly exerts indirect antioxidant effects via the potentiation of several enzymatic antioxidants and heightening the efficiency of the mitochondrial electron transport chain (Loren et al. 2017). In addition to its potent antioxidant properties, melatonin exerts highly efficacious anti-inflammatory effects, thereby preventing tissue damage by blocking transcriptional factors of pro-inflammatory cytokines (Guerra and Devesa 2021).
Also, in this study, immunohistochemical evaluation revealed increased expressions of angiotensin 2 type 1 receptor (ATR1), kidney injury molecule 1 (Kim-1), and Interleukin 1 beta (IL-β) in the kidney tissues, whereas expressions of cardiac troponin and nuclear kappa beta were elevated in the cardiac tissues of rats exposed to toxic levels of NaF without antioxidant treatment. In previous studies, fluoride exposure was linked with chronic tubular interstitial nephritis and apoptosis in kidney tissues (Malin et al. 2019), while myocardial necrosis with increased levels of myocardial troponin I, creatine kinase, lactate dehydrogenase, and aspartate transaminase have been reported following experimental fluoride exposure (Panneerselvam et al. 2015). Furthermore, fluoride-induced cell apoptosis in the heart and kidney has been reported to be associated with altered expression of B cell lymphoma/leukemia 2 (Bcl-2), increased cytochrome c, caspase 3p20, and terminal deoxynucleotidyl transferase dUTP nick end labeled positive cells (Agalakova and Gusev 2012). Therefore, the observed attenuation of cardiac troponin and nuclear kappa beta expression, particularly in rats administered both melatonin and vitamin E suggests a mediation of cardioprotective effect via the prevention of inflammatory processes and tissue damage. Melatonin reportedly has a cardioprotective role in a variety of cardiovascular processes via antioxidant, anti‐inflammatory, antihypertensive, antithrombotic, and antilipemic effects (Misaka et al. 2019). Interestingly, lowered expressions of cardiac troponin and nuclear factor kappa B (NF-κB) were recorded in the melatonin than the vitamin E administered group; an observation that corroborates an earlier report of Wahab et al. (2000) that melatonin convers a better cardioprotective effect than vitamin E against doxycycline-induced cardiotoxicity. The attenuation of the expression of the markers of tissue damage (cardiac troponin) and inflammation (NF-κB) in the antioxidants treated groups, in this study, is a pointer to the positive modulatory role of melatonin and vitamin E on fluoride-induced cardiovascular dysfunctions. This assertion is further strengthened by the absence of histopathologic lesions in the cardiac tissues of rats administered melatonin and vitamin E. In the kidney tissues, the immunohistochemical expressions of angiotensin converting enzyme (ACE) and kidney injury molecule 1 (Kim-1) were higher in the vitamin E administered group than the melatonin administered group, but the greatest attenuation of immunohistochemical expressions of the two proteins were maximal with the combination of vitamin E and melatonin. In earlier reports, melatonin has been reported to attenuate cisplatin-induced acute kidney injury by suppressing apoptosis and necroptosis in rats (Kim et al. 2019) a report corroborated, in this study, by the absence of histopathological lesions in the heart and kidney tissues of rats administered melatonin alone or in combination with vitamin E.
In conclusion, melatonin and vitamin E effectively mitigate cardiovascular and renal toxicities associated with fluoride exposure through the prevention of oxidative and inflammatory processes as probable mechanisms of action. A major limitation of the study was funding due to the global COVID-19 pandemic that impacted negatively global financial burden. We hope to explore genetic alterations associated with sodium fluoride toxicity during the present post COVID-19 era.
Data Availability
Data will be made available on request.
References
Abd-Allah ER, El-Rahman HAA (2022) Ameliorative effects of nano Moringa on fluoride-induced testicular damage via down regulation of the StAR gene and altered steroid hormones. Reprod Biol 23(1):100724. https://doi.org/10.1016/j.repbio.2022.100724
Abdollahi M, Momen-Heravi F (2014) Encyclopedia of Toxicology, 3rd Edition, 606–610. Academic Press, Amsterdam
Agalakova NI, Gusev GP (2012) Fluoride induces oxidative stress and ATP depletion in the rat erythrocytes in vitro. Environ Toxicol Pharmacol 34(2):334–337. https://doi.org/10.1134/S0022093019020029
Ajibade T, Bolaji-Alabi F, Oyagbemi A, Ajileye I, Omobowale T (2021) Supplementation of antihypertensive drug regimen with vitamin E ameliorates alterations of primary haemodynamic parameters and total antioxidant capacity in ovariectomised rats. J Basic Clin Physiol Pharmacol. https://doi.org/10.1515/jbcpp-2020-0097
Ajibade TO, Awodele OA, Tijani MO, Adejumobi OA, Adetona MO, Oyagbemi AA, Adedapo AD, Omobowale TO, Aro AO, Ola-Davies OE, Saba AB, Adedapo AA, Nkadimeng SM, McGaw LJ, Kayoka-Kabongo PN, Oguntibeju OO, Yakubu MA (2022) L-arginine and lisinopril supplementation protects against sodium fluoride-induced nephrotoxicity and hypertension by suppressing mineralocorticoid receptor and angiotensin-converting enzyme 3 activity. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-022-23784-1
Ajibade TO, Oyagbemi AA, Durotoye LA, Omóbòwálé TO, Asenuga ER, Olayemi FO (2017) Modulatory effects of melatonin and vitamin C on oxidative stress-mediated haemolytic anaemia and associated cardiovascular dysfunctions in rats. J Integr Comp Med 14(1). https://doi.org/10.1515/jcim-2015-0082
Amini H, Mahmood T, Mohamad A, Majid M, Yaser M, Masoud Y (2011) Drinking water fluoride and blood pressure? An environmental study. Bio Trace Elem Res 144:157–163. https://doi.org/10.1007/s12011-011-9054-5
Azab A, Albasha M, Jbireal J, Adwas A (2018) Sodium fluoride induces hepato-renal oxidative stress and pathophysiological changes in experimental animals. Open J Apoptosis 7:1–23. https://doi.org/10.4236/ojapo.2018.71001
Aziz FM, Maulood IM, Chawsheen MAH (2012) Effects of melatonin, vitamin C and E alone or in combination on lead-induced injury in liver and kidney organs of rats. IOSR J Pharm 22:13–18. https://doi.org/10.9790/3013-25201318
Borghi C, Cicero AF (2017) Nutraceuticals with clinically detectable blood pressure lowering effect: a review of available randomized clinical trials and their meta-analyses. Brit J Clin Pharmacol 83:163–171. https://doi.org/10.1111/bcp.12902
Buetler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Caglayan C, Kandemir FM, Darendelioğlu E, Küçükler S, Ayna A (2021) Hesperidin protects liver and kidney against sodium fluoride-induced toxicity through anti-apoptotic and anti-autophagic mechanisms. Life Sci 281:119730. https://doi.org/10.1016/j.lfs.2021.119730
Comai S, Gobbi G (2014) Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psych Neurosci 39(1):6–21
Cowan V, Blakley B (2016) Characterizing 1341 cases of veterinary toxicoses confirmed in western Canada: a 16-year retrospective study. Can Vet J 57:53–58
Das S, Dey A, Maji S, Sahoo A, Barman A, Santra S, Mondal S, Mondal KC, Chattopadhyay S (2023) Attenuation of fluoride-induced hepatorenal oxidative stress by ferulic acid in vivo: an approach with in-silico analysis and interaction informatics of ferulic acid. J Trace Elem Med Biol 77:127133. https://doi.org/10.1016/j.jtemb.2023.127133
Dominguez-Rodriguez A, Abreu-Gonzalez P, de la Torre-Hernandez JM (2017) Usefulness of early treatment with melatonin to reduce infarct size in patients with ST-elevation myocardial infarction receiving percutaneous coronary intervention. Ame J Cardiol 120:522–526. https://doi.org/10.1016/j.ijcard.2014.04.044
Drury RA, Wallington EA, Cancerson R (1976) Carlton’s histopathological techniques, 4th edn. Oxford University Press, Oxford
Ferlazzo N, Andolina G, Cannata A, Costanzo MG, Rizzo V (2020) Is melatonin the cornucopia of the 21st century? Antioxidants 9:1088. https://doi.org/10.3390/antiox9111088
Flora SJ, Mittal M, Pachauri V, Dwivedi N (2012) A possible mechanism for combined arsenic and fluoride induced cellular and DNA damage in mice. Metallomics 4(1):78–90. https://doi.org/10.1039/c1mt00118c
Fuhrich DG, Lessey BA, Savaris RF (2013) Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytopathol Histpathol 35(4):210–216
Gornal AG, Bardawill JC, David MM (1949) Determination of serum proteins by means of Biuret reaction. J Bio Chem 177:751–766
Guerra J, Devesa J (2021) Melatonin exerts anti-inflammatory, antioxidant, and neuromodulatory effects that could potentially be useful in the treatment of vertigo. Inter J Otolaryngol 6641055. https://doi.org/10.1155/2021/6641055
Guth S, Hüser S, Roth A, Degen G, Diel P, Edlund K (2020) Toxicity of fluoride: critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch Toxicol 94:1375–1415. https://doi.org/10.1007/s00204-020-02725-2
Gutowska I, Baranowska-Bosiacka I, Baśkiewicz M, Milo B, Siennicka A, Marchlewicz M (2010) Fluoride as a pro-inflammatory factor and inhibitor of ATP bioavailability in differentiated human THP1 monocytic cells. Toxicol Lett 196(2):74–79. https://doi.org/10.1016/j.toxlet.2010.03.1167
Hong JH, Kim MJ, Park MR, Kwag OG, Lee IS, Byun BH, Lee SC, Lee KB, Rhee SJ (2004) Effects of vitamin E on oxidative stress and membrane fluidity in brain of sterptozotocin-induced diabetes rats. Clin Chim Acta 340:107–115. https://doi.org/10.1016/j.cccn.2003.10.003
Jain A, Metha VK, Chittora R, Mahdi A, Bhatnagar M (2015) Melatonin ameliorates fluoride induced neurotoxicity in young rats. Asian J Pharmaceut Clin Res 8:164–167
Jha S, Mishra V, Sharma D, Damodaran T (2011) Fluoride in the environment and its metabolism in humans. Rev Environ Contam Toxicol 211:121–142. https://doi.org/10.1007/978-1-4419-8011-3_4
Jha SK, Singh RK, Damodaran T, Mishra VK, Sharma DK, Rai D (2013) Fluoride in groundwater: toxicological exposure and remedies. J Toxicol Environ Health Part B 16(1):52–66. https://doi.org/10.1080/10937404.2013.769420
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol 11(3):151–69. https://doi.org/10.1159/000136485
Kashyap HJ, Ravi S, Madhu GM (2021) Fluoride sources, toxicity, and fluorosis management techniques – a brief review. J Hazard Mater Lett 100033. https://doi.org/10.1016/j.hazl.2021.100033
Kim JW, Jo J, Kim JY, Choe M, Leem J, Park JH (2019) Melatonin attenuates cisplatin-induced acute kidney injury through dual suppression of apoptosis and necroptosis. Bio 8(3):64. https://doi.org/10.3390/biology8030064
Labib H, Badr AM, Abdelgwad M, Abd El-Galil TI (2022) Keap1/Nrf2 pathway in sodium fluoride-induced cardiac toxicity and the prophylactic role of vitamin C versus platelet-rich plasma. Folia Morphol (warsz) 81(3):663–678. https://doi.org/10.5603/FM.a2021.0053
Loren P, Sánchez R, Arias ME, Felmer R, Risopatrón J, Cheuquemán C (2017) Melatonin scavenger properties against oxidative and nitrosative stress: impact on gamete handling and in vitro embryo production in humans and other mammals. Inter J Mol Sci 18(6):1–17. https://doi.org/10.3390/ijms18061119
Malin AJ, Lesseur C, Busgang SA, Curtin P, Wright RO, Sanders AP (2019) Fluoride exposure and kidney and liver function among adolescents in the United States: NHANES, 2013–2016. Environ Int 132:105012. https://doi.org/10.1007/s12403-021-00448-y
Mendoza-Schulz A, Solano-Agama C, Arreola-Mendoza L, Reyes-Márquez B, Barbier O, Del Razo LM, Mendoza-Garrido ME (2009) The effects of fluoride on cell migration, cell proliferation, and cell metabolism in GH4C1 pituitary tumour cells. Toxicol Lett 190(2):179–186. https://doi.org/10.1016/j.toxlet.2009.07.014
Misaka T, Yoshihisa A, Yokokawa T, Sato T, Oikawa M, Kobayashi A (2019) Plasma levels of melatonin in dilated cardiomyopathy. J Pineal Res 66(4): e12564. https://doi.org/10.1111/jpi.12564
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Bio Chem 217:3170–3175
Olaleye SB, Adaramoye OA, Erigbali PP, Adeniyi OS (2007) Lead exposure increases oxidative stress in the gastric mucosa of HCl/ethanol-exposed rats. World J Gastroenterol 13(38):5121–5126. https://doi.org/10.3748/wjg.v13.i38.5121
Onaolapo AY, Aina OA, Onaolapo OJ (2017) Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed Pharmacother 92:373–383. https://doi.org/10.1177/11790695198273
Oyagbemi AA, Omobowale TO, Asenuga ER, Adejumobi AO, Ajibade TO, Ige TM, Ogunpolu BS, Adedapo AA, Yakubu MA (2017) Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol 32(4):1089–1101. https://doi.org/10.1002/tox.22306
Oyagbemi AA, Omobowale TO, Awoyomi OV, Ajibade TO, Falayi OO, Ogunpolu BS (2019) Cobalt chloride toxicity elicited hypertension and cardiac complication via induction of oxidative stress and upregulation of COX-2/Bax signaling pathway. Hum Exp Toxicol 38(5):519–532. https://doi.org/10.1177/0960327118812158
Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA, Afolabi JM, Ogunpolu BS, Falayi OO, Saba AB, Adedapo AA, Yakubu MA (2018) Luteolin-mediated Kim-1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. BioFactors 44(6):518–531. https://doi.org/10.1002/biof.1449
Ozkalayci F, Kocabas U, Altun BU (2021) Relationship between melatonin and cardiovascular disease. Cureus 13:e12935. https://doi.org/10.7759/cureus.12935
Panneerselvam L, Govindarajan V, Ameeramja J, Nair HR, Perumal E (2015) Single oral acute fluoride exposure causes changes in cardiac expression of oxidant and antioxidant enzymes, apoptotic and necrotic markers in male rats. Biochimie 119:27–35. https://doi.org/10.1016/j.biochi.2015.10.002
Plumlee KH (2004) Metals and minerals. Clinical Veterinary Toxicology. Mosby, St. Louis, pp 193–230
Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA (2014) Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Repro 20(2):293–307
Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F (2014) The role of vitamin E in human health and some diseases. Sul Qab Univ Med J 14(2):e157–e165
Shanmugam T, Abdulla S, Yakulasamy V (2018) A mechanism underlying the neurotoxicity induced by sodium fluoride and its reversal by epigallocatechin gallate in the rat hippocampus: involvement of NrF2/Keap-1 signaling pathway. JoBAZ 79:17. https://doi.org/10.1186/s41936-018-0020-z
Sharma P, Verma PK, Sood S, Singh M, Verma D (2023) Impact of chronic sodium fluoride toxicity on antioxidant capacity, biochemical parameters, and histomorphology in cardiac, hepatic, and renal tissues of Wistar rats. Biol Trace Elem Res 201(1):229–241. https://doi.org/10.1007/s12011-022-03113-w
Sonam KS, Guleria S (2017) Synergistic antioxidant activity of natural products. Ann Pharmacol Pharmaceut 2(8):1086
Srivastava S, Flora SJS (2020) Fluoride in drinking water and skeletal fluorosis: a review of the global impact. Curr Environ Health Rep 7:140–146. https://doi.org/10.1007/s40572-020-00270-9
Strunecká A, Strunecký O, Guan Z (2019) The resemblance of fluorosis pathology to that of autism spectrum disorder: a mini-review. Fluoride 52:105–115
Strunecka A, Strunecky O (2020) Mechanisms of fluoride toxicity: from enzymes to underlying integrative networks. Appl Sci 10:1–24. https://doi.org/10.3390/app10207100
Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L (2012) The role of melatonin as an antioxidant in the follicle. J Ovar Res 26:5. https://doi.org/10.1186/1757-2215-5-5
Thompson LJ (2018) Veterinary toxicology. Basic and Clinical Principles, 3rd edn. Elsevier, Amsterdam, pp 429–431
Varışlı B, Darendelioğlu E, Caglayan C, Kandemir FM, Ayna A, Genç A, Kandemir Ö (2022) Hesperidin attenuates oxidative stress, inflammation, apoptosis, and cardiac dysfunction in sodium fluoride-induced cardiotoxicity in rats. Cardiovasc Toxicol 22(8):727–735. https://doi.org/10.1007/s12012-022-09751-9
Varol E, Icli A, Aksoy F, Bas HA, Sutcu R, Ersoy IH (2013) Evaluation of total oxidative status and total antioxidant capacity in patients with endemic fluorosis. Toxicol Ind Health 29:175–180. https://doi.org/10.1177/030089160008600210
Varshney R, Kale RK (1990) Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Inter J Rad Bio 58:733–743. https://doi.org/10.1177/030089160008600210
Wahab MH, Akoul ES, Abdel-Aziz AA (2000) Modulatory effects of melatonin and vitamin E on doxorubicin-induced cardiotoxicity in Ehrlich ascites carcinoma-bearing mice. Tumori 86(2):157–162. https://doi.org/10.1177/030089160008600210
Wolff SF (1994) Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydrogen peroxides. Meth Enzymol 233:182–189. https://doi.org/10.1016/S0076-6879(94)33021-2
Xu W, Hu Z, Zhang J, Tang Y, Xing H, Xu P, Ma Y, Niu Q (2023) Cross-talk between autophagy and ferroptosis contributes to the liver injury induced by fluoride via the mtROS-dependent pathway. Ecotoxicol Environ Saf 250:114490. https://doi.org/10.1016/j.ecoenv.2022.114490
Zhang S, Jiang C, Liu H, Guan Z, Zeng Q, Zhang C, Lei R, Xia T, Gao H, Yang L (2013) Fluoride-elicited developmental testicular toxicity in rats: roles of endoplasmic reticulum stress and inflammatory response. Toxicol Appl Pharmacol 271:206–215. https://doi.org/10.1016/j.taap.2013.04.033
Zhang J, Tang Y, Xu W, Hu Z, Xu S, Niu Q (2022) Fluoride-induced cortical toxicity in rats: the role of excessive endoplasmic reticulum stress and its mediated defective autophagy. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03463-5
Author information
Authors and Affiliations
Contributions
The authors Ademola Adetokunbo Oyagabemi, Temitayo Olabisi Ajibade, and Temidayo Olutayo Omobowale designed the experiment. The blood pressure was performed by Temitayo Olabisi Ajibade and Temidayo Olutayo Omobowale. Oluwaseun Olanrewaju Esan, Omolola Victoria Awoyomi, and Ademola Adetokunbo Oyagabemi performed the immunohistochemistry and biochemical assays. Moses Olusola Adetona, Temidayo Olutayo Omobowale, Olufunke Eunice Ola-Davies, Adebowale Benard Saba, Adeolu Alex Adedapo, Sanah Malomile Nkadimeng, Lyndy Joy McGaw, Prudence Ngalula Kayoka-Kabongo, Momoh Audu Yakubu, Evaristus Nwulia, and Oluwafemi Omoniyi Oguntibeju supervised, proof-read, and approved the submission.
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was conducted following guidelines approved by the Animal Care and Use Research Ethics Committee (ACUREC) of the University of Ibadan (Approval number: UIACUREC/ 19/124).
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oyagbemi, A.A., Ajibade, T.O., Esan, O.O. et al. Cardioprotective and renoprotective effects of melatonin and vitamin E on fluoride-induced hypertension and renal dysfunction in rats. Comp Clin Pathol 33, 33–45 (2024). https://doi.org/10.1007/s00580-023-03519-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03519-5