Abstract
Sodium fluoride (NaF) is one of the neglected environmental toxicants that has continued to silently cause toxicity to both humans and animals. NaF is universally present in water, soil, and atmosphere. The persistent and alarming rate of increase in cardiovascular and renal diseases caused by chemicals such as NaF in mammalian tissues has led to the use of various drugs for the treatment of these diseases. The present study aimed at evaluating the renoprotective and antihypertensive effects of L-arginine against NaF-induced nephrotoxicity. Thirty male Wistar rats (150–180 g) were used in this study. The rats were randomly divided into five groups of six rats each as follows: Control, NaF (300 ppm), NaF + L-arginine (100 mg/kg), NaF + L-arginine (200 mg/kg), and NaF + lisinopril (10 mg/kg). Histopathological examination and immunohistochemistry of renal angiotensin-converting enzyme (ACE) and mineralocorticoid receptor (MCR) were performed. Markers of renal damage, oxidative stress, antioxidant defense system, and blood pressure parameters were determined. L-arginine and lisinopril significantly (P < 0.05) ameliorated the hypertensive effects of NaF. The systolic, diastolic, and mean arterial blood pressure of the treated groups were significantly (P < 0.05) reduced compared with the hypertensive group. This finding was concurrent with significantly increased serum bioavailability of nitric oxide in the hypertensive rats treated with L-arginine and lisinopril. Also, there was a significant reduction in the level of blood urea nitrogen and creatinine of hypertensive rats treated with L-arginine and lisinopril. There was a significant (P < 0.05) reduction in markers of oxidative stress such as malondialdehyde and protein carbonyl and concurrent increase in the levels of antioxidant enzymes in the kidney of hypertensive rats treated with L-arginine and lisinopril. The results of this study suggest that L-arginine and lisinopril normalized blood pressure, reduced oxidative stress, and the expression of renal ACE and mineralocorticoid receptor, and improved nitric oxide production. Thus, L-arginine holds promise as a potential therapy against hypertension and renal damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sodium fluoride (NaF) is one of the neglected environmental toxicants that has continued to cause toxicity to both humans and animals (Oyagbemi et al. 2021). NaF is universally present in water, soil, and the atmosphere (Oyagbemi et al. 2020). Human activity including massive global industrialization such as the industrial and pharmaceutical products and other sources has also contributed significantly to the presence of NaF in the environment (Irigoyen-Camacho et al. 2016; Choubisa and Choubisa 2016; Said et al. 2020). However, water-borne fluoride has been documented to represent the largest single component of NaF element’s daily intake (Catani et al. 2007; Molina-Frechero et al. 2012). Dental fluorosis has been observed to occur normally from excess fluoride ingestion during tooth formation (Aoba and Fejerskov 2002). However, other parts of the tooth such as the enamel and dentine can be affected by fluorosis resulting from fluoride exposure that occurs during childhood (Akpata 2001; DenBesten and Li 2011).
From our laboratory, we have documented organ and non-organ toxicities associated with NaF (Oyagbemi et al. 2021, 2017; Omóbòwálé et al. 2018). Cardiovascular and neurodegenerative dysfunctions such as hypertension and motor in-coordination have also been reported (Oyagbemi et al. 2021, 2020, 2018a, 2018b, 2018c, 2017; Omóbòwálé et al. 2018). Previous findings have reported generation of reactive oxygen species (ROS) and induction of oxidative stress as mechanism of action of fluoride-induced toxicity (Oyagbemi et al. 2017; Suzuki et al. 2015; Wu et al. 2015; Shuhua et al. 2012; Izquierdo-Vega et al. 2011).
L-arginine is one of the most metabolically versatile amino acids (Gad 2010). L-arginine is known to participate in the synthesis of nitric oxide and serves as a precursor for the synthesis of polyamines, proline, glutamate, creatine, agmatine, and urea (Viribay et al. 2020). Several human and experimental animal studies have indicated that exogenous L-arginine intake has multiple beneficial biological and pharmacological effects (Pahlavani et al. 2017; Dumont et al. 2001). Meta-analysis provides further evidence that oral L-arginine supplementation significantly lowers both systolic and diastolic blood pressure (Viribay et al. 2020; Dong et al. 2011). Nitric oxide (NO) is a well-known vasodilator produced by the vascular endothelium via the enzyme endothelial nitric oxide synthase (eNOS), the house-keeping enzyme. The inadequate production of NO has been linked to elevated blood pressure (BP) in both human and animal studies and might be due to substrate inaccessibility (Khalaf et al. 2019; Tsuboi et al. 2018). L-arginine administration has been demonstrated to improve endothelial function in various disease states (McRae 2016) and improved risk factors of cardiovascular diseases (CVD) as reported by Pahlavani et al. (2014). Interestingly, L-arginine supplementation was documented to have significant effect of lowering diastolic blood pressure and prolonging gestational age in pregnancy (Zhu et al. 2013). Another amino acid, L-citrulline has been reported to improve vascular function through increased L-arginine bioavailability and NO synthesis (Figueroa et al. 2017).
ACE inhibitors are medications used to treat and manage hypertension, which is a significant risk factor for coronary disease, heart failure, stroke, and a host of other cardiovascular conditions (Mall et al. 2021). Lisinopril is a non-sulfhydryl ACE inhibitor that lowers peripheral vascular resistance with a concomitant decrease in blood pressure (Mall et al. 2021). Lisinopril has now been shown to reduce mortality and cardiovascular morbidity in patients with myocardial infarction when administered as early treatment (Wihandono et al. 2021). Lisinopril produces a smooth, gradual BP reduction in hypertensive patients without affecting heart rate or cardiovascular reflexes (Wihandono et al. 2021). Lisinopril has been reported for its antioxidant (Scisciola et al. 2022), nephroprotective, and cardioprotective properties (Ruggenenti 2017; Brown et al. 2021; Wihandono et al. 2021; Østergaard et al. 2021).
The present study elucidated the molecular mechanism of anti-hypertensive action of L-arginine in a toxicant-induced hypertensive and nephrotoxic rat model.
Material and methods
Chemicals
Sodium fluoride, lisinopril, L-arginine, xylenol orange (XO), potassium hydroxide, reduced glutathione (GSH), oxidized glutathione (GSSG), thiobarbituric acid (TBA), trichloroacetic acid (TCA), sodium hydroxide, O-dianisidine, hydrogen peroxide (H2O2), and 1,2-dichloro-4-nitrobenzene were purchased from Sigma (St. Louis, MO, USA). Normal goat serum and Biotinylated 2-step plus Poly-HRP Anti Mouse/Rabbit IgG Detection System with DAB solution were purchased from Elabscience Biotechnology®, China), anti-ACE polyclonal antibody (E-AB-16159: 1:500 dilution) and anti-mineralocorticoid receptor polyclonal antibody (E-AB-70261: 1:50 Dilution). All other chemicals used for this study were of analytical grade.
Experimental animals and design
Thirty male Wistar rats (150–180 g) were used in this study; the rats were randomly divided into five groups of six rats per group as control, NaF (300 ppm), NaF + L-arginine (100 mg/kg), NaF + L-arginine (200 mg/kg), and NaF + lisinopril (10 mg/kg), respectively, orally for 8 days. The administration of drugs was given daily. The concentration of NaF (Oyagbemi et al. 2021) and the dosages of L-arginine (Adejare et al., 2020) and lisinopril (Oyagbemi et al. 2021) were chosen based on the previous literature. The rats were kept in wire mesh cages under controlled light cycle (12 h light/12 h dark) and fed with commercial rat chow ad libitum and liberally supplied with water. Body weight and kidney weight were also measured at the end of the experiment. The blood of the rats was taken on the 8th day and rats were euthanized on the 9th day. The rats were acclimatized for 2 weeks before the commencement of the experiment.
Ethical approval
The study was conducted following guidelines approved by the Animal Care and Use Research Ethics Committee (ACUREC) of the University of Ibadan with the approval number: UIACUREC/ 19/124.
Blood pressure measurement
The systolic (SBP), diastolic (DBP), and mean arterial (MAP) blood pressures were determined non-invasively in conscious animals by tail plethysmography using an automated blood pressure monitor (CODA S1, Kent Scientific Corporation, Connecticut, USA). The blood pressure parameters were obtained by an indirect method of blood pressure measurement as recently reported in our laboratory (Oyagbemi et al. 2019).
Serum preparation
Blood was collected from the retro-orbital venous plexus. The serum was obtained from whole blood collected into anticoagulant free sample bottles following a post-collection waiting period of 60 min. Thereafter, the serum was kept at a 4 °C temperature.
Determination of serum markers of renal damage
Serum creatinine and blood urea nitrogen (BUN) were determined following the manufacturer’s instructions in the purchased Randox® kits (Randox® Laboratories Ardmore, UK).
Preparation of renal post mitochondrial fractions (PMFs)
The kidneys were quickly excised, rinsed, weighed, and homogenized with homogenizing buffer (0.1 M phosphate buffer, pH 7.4) using a Teflon homogenizer. The homogenate was centrifuged at 10,000 g for 10 min at − 4 °C.
Biochemical assays
Estimation of renal oxidative stress
The malondialdehyde (MDA) content as an index of lipid peroxidation was quantified in the PMFs of renal tissues according to the method of Varshney and Kale (1990). The absorbance was measured against a blank of distilled water at 532 nm. Lipid peroxidation was calculated with a molar extinction coefficient of 1.56 × 105/M/cm. Protein carbonyl (PCO) contents in the renal tissues were measured using the method of Reznick and Packer (1994). The absorbance of the sample was measured at 370 nm. The carbonyl content was calculated based on the molar extinction coefficient of DNPH (2.2 104 cm1 M1) and expressed as nmoles/mg protein while vitamin C contents were measured as earlier described (Jacques-Silva et al. 2001).
Renal antioxidant status
The superoxide dismutase (SOD) assay was carried out by the method of Misra and Fridovich (1972), with slight modification (Oyagbemi et al. 2015). The increase in absorbance at 480 nm was monitored every 30 s for 150 s. The one unit of SOD activity was given as the amount of SOD necessary to cause 50% inhibition of the oxidation of adrenaline to adrenochrome. Reduced glutathione (GSH) was estimated by the method of Jollow et al. (1974). Glutathione peroxidase (GPx) activity was also measured according to Beutler et al. (1963). Glutathione S-transferase (GST) was estimated by the method of Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene as substrate. The protein and non-protein thiol contents were determined as described by Ellman (1959).
Estimation of serum nitric oxide concentration and total protein
The serum nitric oxide concentrations were measured spectrophotometrically at 548 nm as previously described (Olaleye et al. 2007). Protein concentration was determined by the Biuret method of Gornal et al. (1949), using bovine serum albumin (BSA) as standard.
Immunohistochemistry
Immunohistochemistry was done as described by Oyagbemi et al. (2019). Antibodies against renal ACE and mineralocorticoid receptor (MCR) were probed in the kidney using a 2-step plus Poly-HRP Anti Mouse/Rabbit IgG Detection System with DAB solution (Catalog number: E-IR-R217 from Elabscience Biotechnology®, China). The slides were subsequently dewaxed in xylene solution for 2 min and afterward, hydration was carried out in different concentrations of ethanol (100%, 90%, and 80%) for 2 min each. Antigen retrieval was performed and followed with endogenous peroxidase blocking. Goat serum (E-1R-R217A) was added to prevent nonspecific binding and the tissues were probed with primary antibodies viz-a-viz angiotensin converting enzyme polyclonal antibody (E-AB-16159: 1:500 dilution) and anti-mineralocorticoid receptor polyclonal antibody (E-AB-70261: 1:500 dilution). Thereafter, a secondary antibody labelled E-1R-R217B was added, and the slides were incubated in humidifying chamber at room temperature for 20 min. Finally, a few drops of the substrate diaminobenzidine (DAB) were added in the dark. The reaction was terminated with deionized water and slides were immersed in hematoxylin (Sigma-Aldrich, USA) for 3 s before rinsing with PBS. The slides were placed in 80%, 90%, and 100% of ethanol, and then xylene (100%) for 2 min each. Slides were removed, allowed to dry, and a DPX mountant was applied. Sections were observed with a light microscope (Leica LAS-EZ®) using Leica software application suite version 3.4 equipped with a digital camera.
Statistical analysis
All values are expressed as mean ± S.D. The test of significance between two groups was estimated by Student’s t test with P value less than 0.05. The one-way analysis of variance (ANOVA) with Turkey’s post-hoc test of Graph pad prism 5.0 was also carried out with p-values < 0.05 considered statistically significant (Fleiss et al. 2003).
Results
Sodium fluoride intoxication on body weight and kidney relative weight
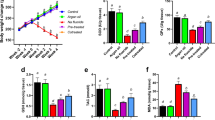
The results in Fig. 1 showed a significant (P < 0.05) reduction in relative body weight of rats intoxicated with NaF and those co-administered with either L-arginine or lisinopril. Similarly, there was a significant (P < 0.05) reduction in relative kidney weight of rats administered only NaF. However, L-arginine supplementation and lisinopril co-administration showed significant restorative effect on the relative kidney weight to near normal values (Fig. 1).
Effects of L-arginine and lisinopril on body weight and relative kidney weight. Superscript (a) indicates a significant increase in serum blood urea nitrogen and creatinine compared with control at P < 0.05. Superscript (b) indicates a significant difference in systolic blood pressure compared with the hypertensive group at P < 0.05. Abbreviations: HYPR, hypertensive; L-ARG, L-arginine; LISP, lisinopril
Hemodynamic parameters
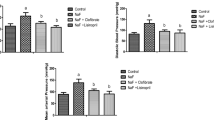
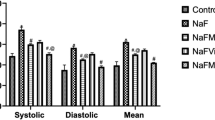
The blood pressure parameters measured in the present study indicated significant (P < 0.05) increases in the values of SBP, DBP, and MAP of rats intoxicated with NaF (Fig. 2). On the other hand, there was a dose-dependent reduction in the values of SBP, DBP, and MAP of rats intoxicated with NaF and treated with L-arginine, and lisinopril, respectively (Fig. 2). Lisinopril co-administration gave a better reduction of blood pressure parameters as recorded in Fig. 2.
Systolic, diastolic and mean arterial blood pressure of hypertensive rats treated with L-arginine and lisinopril. Superscript (a) indicates a significant increase in when compared with control at P < 0.05. Superscript (b) indicates significant difference when compared with the hypertensive group at P < 0.05. Abbreviations: HYPR, hypertensive; L-ARG, L-arginine; LISP, lisinopril
Renal antioxidant defense system
From Table 1, 200 mg/kg of L-arginine and lisinopril supplementation was found to significantly improve the activities of renal GPx, GSH, PSH, and NPSH content, respectively. Our results showed that NaF intoxication however significantly (P < 0.05) increased the activities of renal GST and SOD in comparison to the control (Table 1). It was interesting to observe that there was no appreciable improvement in the renal content of vitamin C except in the rats administered lisinopril (Table 1). It is worth noting that treatment with lisinopril gave better improvement renal antioxidant defense systems (Table 1).
Markers of renal damage and oxidative stress
We also observed that intoxication with NaF caused a significant (P < 0.05) increase in the values of serum BUN and creatinine when compared to the control and rats co-administered with L-arginine (100 mg/kg and 200 mg/kg) as indicated in Fig. 3. The nephron-protective effect of L-arginine and lisinopril was demonstrated as indicated by a significant (P < 0.05) reduction in the serum levels of BUN and creatinine in comparison to the NaF-intoxicated group (Fig. 3).
Effects of L-arginine and lisinopril on serum blood urea nitrogen (BUN) and creatinine. Superscript (a) indicates a significant increase in when compared with control at P < 0.05. Superscript (b) indicates significant difference when compared with the hypertensive group at P < 0.05. Abbreviations: HYPR, hypertensive; L-ARG, L-arginine; LISP, lisinopril
In Fig. 4, renal MDA which is the product of lipid peroxidation, in NaF-intoxicated rats, increased significantly as compared to the control group. There was a significant (P < 0.05) reduction in the MDA content of L-arginine and lisinopril co-administered rats when compared to the NaF alone–treated rats (Fig. 4). Our data also revealed an exaggerated increase in the content of PCO in NaF only–administered rats in comparison to the control (Fig. 4). The free radical scavenging action of L-arginine and lisinopril was demonstrated by a significant (P < 0.05) reduction in the content of renal PCO when compared to NaF only (Fig. 4). Also in Fig. 4, the administration of NaF caused a significant (P < 0.05) reduction in NO bioavailability relative to the control. Again, L-arginine supplementation caused a significant (P < 0.05) improvement in NO bioavailability similar to that of lisinopril (Fig. 4).
Effects of L-arginine and lisinopril on renal markers of oxidative stress and serum nitric oxide. Superscript (a) indicates a significant increase in when compared with control at P < 0.05. Superscript (b) indicates significant difference when compared with the hypertensive group at P < 0.05. Abbreviations: HYPR, hypertensive; L-ARG, L-arginine; LISP, lisinopril
Histopathology and immunohistochemistry
The histopathology of the kidney revealed mild tubular necrosis in rats intoxicated with NaF, while rats co-administered with L-arginine showed minute tubular necrosis, and no visible lesion was observed in lisinopril-treated group (Fig. 5). The renal immunohistochemistry of MCR revealed a higher expression of MCR in NaF-intoxicated rats relative to the control (Fig. 5). However, lower expression of MCR was observed in L-arginine and lisinopril–treated rats when compared to the NaF-alone rats (Fig. 5). It is important to note that lower expression of MCR was recorded in rats that received 100 mg/kg of L-arginine relative to rats that received 200 mg/kg of L-arginine and lisinopril (Fig. 5).
The immunohistochemistry of renal mineralocorticoid receptor (MCR). Group A (control), Group B (HYPR (NaF; 300 ppm), Group C (HYPR + L-ARG 100 mg/kg), Group D (HYPR + L-ARG 200 mg/kg), and Group E (HYPR + LISP 10 mg/kg). Slides stained with high definition Heamtoxylin (magnification × 400). Abbreviations: HYPR, hypertensive; L-ARG, L-arginine; LISP, lisinopril
In another experiment, our study revealed higher expression of ACE in renal tissues of rats intoxicated with NaF when compared to the control (Fig. 6). Interestingly, co-treatment with either L-arginine or lisinopril reduced the expression of ACE relative to the NaF-intoxicated rats (Fig. 6).
The immunohistochemistry of renal angiotensin converting enzyme (ACE). Group A (Control), Group B (HYPR (NaF; 300 ppm), Group C (HYPR + L-ARG 100 mg/kg), Group D (HYPR + L-ARG 200 mg/kg), and Group E (HYPR + LISP 10 mg/kg). Slides stained with high definition Hematoxylin (magnification × 400). Abbreviations: HYPR, hypertensive; L-ARG, L-arginine; LISP, Lisinopril
Discussion
This study showed that L-arginine and lisinopril ameliorated NaF-induced hypertension in male Wistar rats. This can be corroborated by a statistically significant reduction in high SBP, DBP, and MAP across the treated groups when compared with the hypertensive untreated rats. Our findings also confirmed earlier reports on the toxicity of NaF on cardiovascular system (Oyagbemi et al. 2021, 2018a, 2018b, 2018c, 2017; Omóbòwálé et al. 2018). Administration of NaF alone to rats led to a significant decrease in serum NO bioavailability in the hypertensive group. However, rats in the treated groups (L-arginine or lisinopril) had a noticeable increase in NO availability. The reduction in NO bioavailability has been reported to be involved in the pathogenesis of hypertensive conditions (Elmarakby and Sullivan 2021; Stamm et al. 2021; Travis et al. 2021) and other cardiac complications through generation of ROS (Oyagbemi et al. 2021, 2017). L-arginine is a precursor for the synthesis of NO (Almannai and El-Hattab 2021; Ma 2021; Yaremchuk et al. 2021), and the NO produced from vascular endothelium helps to maintain a continuous tone that is essential for the regulation of blood flow, blood pressure, platelet aggregation, and vasodilation (Umnyagina et al. 2021; Pautz et al. 2021). It was evident from our study that L-arginine or lisinopril significantly increased NO bioavailability and reversed high blood pressure precipitated by NaF intoxication.
We observed from our study that NaF intoxication caused significant increase in blood urea nitrogen (BUN) and creatinine levels. The increase in BUN and creatinine has been associated with various degrees of renal injuries (Chen et al. 2021a, b; Ni et al. 2021; Nasiruddin et al. 2020). The observed nephrotoxicity by NaF might be due to free radical generation and increased protein catabolism with concomitant systemic oxidative damage. This finding might also suggest extensive glomerular and tubular epithelial cell damage observed in the histopathology are positively correlated with exaggerated levels of BUN and creatinine. Treatment with L-arginine or lisinopril significantly attenuated these deleterious effects by the reduction in BUN and creatinine levels across treated groups in comparison to the NaF-intoxicated group. This therefore indicates the nephroprotective effect of L-arginine or lisinopril against nephrotoxicity induced by NaF intoxication. Our study therefore is in support of nephropretective effect of L-arginine against nephrotoxicity and hepatorenal damage (Saka et al. 2021; Abdelhalim et al. 2018). The use of function foods and Cr-methionine has documented against oxidative stress in animals (Hoan et al., 2021; Bin-Jumah et al. 2020; Abdelnour et al. 2019).
The ability of L-arginine to mitigate oxidative stress in hypertensive rats was also demonstrated in the present study. Renal markers of oxidative stress including hydrogen peroxide (H2O2) generated, MDA, and PCO contents increased significantly in NaF-induced hypertensive rats compared with the control. The exaggerated production of H2O2 as classic example of ROS that has been reported during oxidative stress with ultimate damage to proteins, nucleic acids, cell membranes has been implicated in the development of some diseases (Yang et al. 2021; Yu et al. 2021; Zhang et al. 2021). The generated H2O2 can react with superoxide anion radical (O2∙) to initiate the Haber-Weis reaction, thereby producing hydroxyl radical (.OH). It was exciting to observe a significant reduction in H2O2 content in rats co-administered with L-arginine or lisinopril. The ability of L-arginine or lisinopril to reduce the renal content of H2O2 was an indication of free radical scavenging action of L-arginine.
Malondialdehyde (MDA) is one of the final products of peroxidation of polyunsaturated fatty acids (PUFA) in the cell (Wang et al. 2021; Torun et al. 2009; Gawel et al. 2004). The MDA is a toxic aldehyde that can initiate oxidative cellular damage in both target and non-target tissues (Morelli et al. 2021). In this study, NaF intoxication significantly increased the content of renal MDA. However, anti-oxidative action of L-arginine or lisinopril was demonstrated as shown in the reduction in aforementioned exaggerated production of renal MDA. Protein oxidation, and their level in tissues and plasma, has been reported as a relatively stable marker of oxidative damage (Dayanand et al. 2012). In fact, pathogenesis and pathophysiology of many disease conditions have been associated with increased protein carbonyl content (Akinrinde et al. 2021; Marques et al. 2021; Ommati et al. 2021; Rodríguez-Sánchez et al. 2021). From this study, L-arginine’s protection against NaF-induced renal protein carbonylation might be associated with the antioxidant activity of L-arginine or lisinopril which prevents protein oxidation. Protein carbonylation, one of the most harmful irreversible oxidative protein modifications, has been considered a major hallmark of oxidative stress–related disorders including aging and several age-related disorders (Fedorova et al. 2014). From this study, we can propose that L-arginine could be found applicable in the management of aging and several age-related disorders against protein oxidation and crosslinking.
Glutathione in its reduced form is an important intracellular antioxidant that protects against a variety of oxidant species (Masella et al. 2005). The protective mechanisms of glutathione against oxidative stress which can be through detoxification of enzymes such as glutathione peroxidase against oxidative stress, scavenging hydroxyl radicals, and singlet oxygen directly (Masella et al. 2005). Glutathione peroxidase (GPx) is a selenium-containing enzyme that catalyzes detoxification of lipid hydroperoxide and hydrogen peroxides to water and oxygen (O2). The reduction in the activity of GPx could lead to a concurrent increase in hydrogen peroxide with subsequent tissue damage (Farhat et al. 2018; Espinoza et al. 2008). Superoxide dismutase (SOD), on the other hand, catalyzes the dismutation of the superoxide anion radical to hydrogen peroxide (Pizzino et al. 2017).
Our data also showed a significant decrease in the activity of enzymatic and non-enzymatic antioxidants such as GPx, SOD, reduced GSH, and vitamin C in NaF-intoxicated hypertensive group, confirming the involvement of oxidative stress in the pathogenesis of hypertension. Treatment of the hypertensive rats with L-arginine at 100 mg/kg and 200 mg/kg brought about significant improvement in the antioxidant defense system. However, the increase in GSH level in the renal tissues of the hypertensive rats treated with L-arginine was not significant except in the group treated with 10 mg/kg lisinopril. The reduction in the levels of markers of oxidative stress and concurrent increase in antioxidant enzymes might suggest an ability of L-arginine to scavenge free radicals and mitigate oxidative stress associated with NaF toxicity.
The significant decrease in the activity of SOD and GPx in the hypertensive group may subsequently lead to an increase in superoxide anion radical and H2O2 levels, thereby potentiating oxidative stress as a major factor in the progression of hypertension. The accumulation of the superoxide anion radical was also a sequel to the observed decrease in the activity of SOD. Hence, increasing levels of the superoxide anion radical might enhance the uncoupling of eNOS with a resultant reduction in NO bioavailability. Furthermore, superoxide anion radical is also capable of reacting with NO to form peroxynitrite, which is a cytotoxic signaling molecule (Wu et al. 2020; Hu et al. 2019). Thus, the observable increase in the activity of GPx in the kidney tissues is suggestive of antioxidant and ameliorative roles of L-arginine against NaF toxicity.
The over-activation of MCR in animal models of chronic kidney disease (CKD) has been reported to play significant roles in the pathophysiology and pathogenesis of cardiorenal dysfunctions including inflammation and fibrosis in the kidneys and hearts and increased sodium retention and hypertension (Georgianos and Agarwal 2021). MCR antagonists have become novel therapeutic interventions to retard the progression of CKD with attendant improvement in cardiovascular morbidity and mortality (Droebner et al. 2021; Kovarik et al. 2021; Patrono and Volpe 2021). Our study revealed an over-activation of MCR by NaF intoxication as recorded with higher expression of renal MCR. The observed higher expression of MCR could be positively correlated with exaggerated high blood pressure obtained in rats administered only NaF. From our data, L-arginine or lisinopril co-administration with NaF caused a reduction in the expression of MCR. This might be indicative of renoprotective and anti-hypertensive action of L-arginine and lisinopril, respectively. The amino acid L-arginine could be found applicable for the management of toxicant-induced nephrotoxicity.
Recently, science has taken the advantage of selectively inhibiting ACE as a therapeutic target for preventing CKD and better management of hypertension (Puspita et al. 2021; Bas 2021; Alves-Lopes et al. 2021; Chen et al. 2021a, b). In this study, we also investigated renal immunolocalization of ACE following NaF intoxication. The immunohistochemistry revealed a higher expression of renal ACE in rats administered with NaF relative to the control and rats co-administered with either L-arginine or lisinopril. The increased expression of ACE was similar to that of MCR as stated above, meaning that NaF nephrotoxicity might be through over-activation of MCR and ACE signaling pathways. The over-activation of these pathways could actually be responsible for the nephrotoxicity and hypertension. The ability of L-arginine to block the activities of MCR and ACE could be maximized as a novel therapeutic agent in the management and treatment of kidney damage and associated hypertension.
Conclusion
The results of this study showed that 200 mg/kg of L-arginine normalized high blood pressure, reduced oxidative stress, improved renal antioxidant defense system, offered protection against renal damage and nephrotoxicity, and improved nitric oxide bioavailability thereby serving as a precursor to nitric oxide production. Thus, L-arginine could serve as a potential alternative therapy against toxicant-induced oxidative stress, nephrotoxicity, and hypertension via increase in the supply of endogenous nitric oxide.
Data availability
Data will be made available on request.
References
Abdelhalim MAK, Qaid HA, Al-Mohy Y, Al-Ayed MS (2018) Effects of quercetin and arginine on the nephrotoxicity and lipid peroxidation induced by gold nanoparticles in vivo. Int J Nanomed 13:7765–7770. https://doi.org/10.2147/IJN.S183281
Abdelnour SA, Abd El-Hack ME, Swelum AA, Saadeldin IM, Noreldin AE, Khafaga AF et al (2019) The usefulness of retinoic acid supplementation during in vitro oocyte maturation for the in vitro embryo production of livestock: a review. Animals (basel) 9(8):561
Adejare A, Oloyo A, Anigbogu C, Jaja S (2020) L-arginine supplementation increased only endothelium-dependent relaxation in Sprague-Dawley rats fed a high-salt diet by enhancing abdominal aorta endothelial nitric oxide synthase gene expression. Clin Med Insights Cardiol. https://doi.org/10.1177/1179546820902843
Akinrinde AS, Oduwole O, Akinrinmade FJ, Bolaji-Alabi FB (2021) Nephroprotective effect of methanol extract of Moringa oleifera leaves on acute kidney injury induced by ischemia-reperfusion in rats. Afr Health Sci 20(3):1382–1396. https://doi.org/10.4314/ahs.v20i3.44
Akpata ES (2001) Occurrence and management of dental fluorosis. Int Dent J 51(5):325–333
Almannai M, El-Hattab AW (2021) Nitric oxide deficiency in mitochondrial disorders: the utility of arginine and citrulline. Front Mol Neurosci 14:682780. https://doi.org/10.3389/fnmol.2021.682780
Alves-Lopes R, Montezano AC, Neves KB, Harvey A, Rios FJ, Skiba DS et al (2021) Selective inhibition of the C-domain of ACE (angiotensin-converting enzyme) combined with inhibition of NEP (neprilysin): a potential new therapy for hypertension. Hypertension 78(3):604–616. https://doi.org/10.1161/HYPERTENSIONAHA.121.17041
Aoba T, Fejerskov O (2002) Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med 13(2):155–170. https://doi.org/10.1177/154411130201300206
Bas Z (2021) Inhibition effect of nicotinamide (vitamin B (3)) and reduced glutathione (GSH) peptide on angiotensin-converting enzyme activity purified from sheep kidney. Int J Biol Macromol 189:65–71. https://doi.org/10.1016/j.ijbiomac.2021.08.109
Bin-Jumah M, Abd El-Hack ME, Abdelnour SA, Hendy YA, Ghanem HA, Alsafy SA et al (2020) Potential use of chromium to combat thermal stress in animals: a review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.135996
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Brown AK, Nichols A, Coley CA, Ekperikpe US, McPherson KC, Shields CA et al (2021) Treatment with lisinopril prevents the early progression of glomerular injury in obese dahl salt-sensitive rats independent of lowering arterial pressure. Front Physiol 12:765305. https://doi.org/10.3389/fphys.2021.765305
Catani DB, Hugo FN, Cypriano S, de Sousa MKR, Cury JA (2007) Relationship between fluoride levels in the public water supply and dental fluorosis. Rev Saude Publica 41(5):732–739. https://doi.org/10.1590/s0034-89102007000500007
Chen R, Suchard MA, Krumholz HM, Schuemie MJ, Shea S, Duke J et al (2021a) Comparative first-line effectiveness and safety of ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers: a multinational cohort study. Hypertension 78(3):591–603. https://doi.org/10.1161/HYPERTENSIONAHA.120.16667
Chen S, Zhou M, Ying X, Zhou C (2021b) Ellagic acid protects rats from chronic renal failure via MiR-182/FOXO3a axis. Mol Immunol 138:150–160. https://doi.org/10.1016/j.molimm.2021.08.007
Choubisa SL, Choubisa D (2016) Status of industrial fluoride pollution and its diverse adverse health effects in man and domestic animals in India. Environ Sci Pollut Res Int 23(8):7244–7254. https://doi.org/10.1007/s11356-016-6319-8
Dayanand CD, Vegi PK, Kutty AVM (2012) Protein carbonyl content as a stable oxidative stress marker in type ii diabetes. Int J Biol Med Res 3(4):2362–2365
DenBesten P, Li W (2011) Chronic fluoride toxicity: dental fluorosis. Monogr Oral Sci 22:81–96. https://doi.org/10.1159/000327028
Dong JY, Qin LLQ, Zhang Z, Zhao Y, Wang J, Arigoni F et al (2011) Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Review Am Heart J 162(6):959–965. https://doi.org/10.1016/j.ahj.2011.09.012
Droebner K, Pavkovic M, Grundmann M, Hartmann E, Goea L, Nordlohne J et al (2021) Direct blood pressure-independent anti-fibrotic effects by the selective nonsteroidal mineralocorticoid receptor antagonist finerenone in progressive models of kidney fibrosis. Am J Nephrol 52(7):588–601. https://doi.org/10.1159/000518254
Dumont Y, D’Amours M, Lebel M, Larivière M (2001) Supplementation with a low dose of L-arginine reduces blood pressure and endothelin-1 production in hypertensive uraemic rats. Nephrol Dial Transplant 16(4):746–754. https://doi.org/10.1093/ndt/16.4.746
Ellman GL (1959) Tissue Sulfhydryl Groups Arc Biochem Biophy 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Elmarakby AA, Sullivan JC (2021) Sex differences in hypertension: lessons from spontaneously hypertensive rats (SHR). Clin Sci (lond) 135(15):1791–1804. https://doi.org/10.1042/CS20201017
Espinoza SE, Guo H, Fedarko N, DeZern A, Fried LP, Xue QL et al (2008) Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci 63(5):505–509. https://doi.org/10.1093/gerona/63.5.505
Farhat Z, Browne RW, Bonner MR, Tian L, Deng F, Swanson M et al (2018) How do glutathione antioxidant enzymes and total antioxidant status respond to air pollution exposure? Environ Int 112:287–293. https://doi.org/10.1016/j.envint.2017.12.033
Fedorova M, Bollineni RC, Hoffmann R (2014) Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 33(2):79–97. https://doi.org/10.1002/mas.21381
Figueroa A, Wong A, Jaime SJ, Gonzales JU (2017) Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Review Curr Opin Clin Nutr Metab Care 20(1):92–98. https://doi.org/10.1097/MCO.0000000000000340
Fleiss JL, Levin B, Paik MC (2003) Statistical methods for rates and proportions, 3rd edn. John Wiley & Sons, New York, pp 598–626
Gad MZ (2010) Review anti-aging effects of L-arginine. J Adv Res 3(1):169–177
Gaweł S, Wardas M, Niedworok E, Wardas P (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 57(9–10):453–455
Georgianos PI, Agarwal R (2021) Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney Int Rep 6(9):2281–2291. https://doi.org/10.1016/j.ekir.2021.05.027
Gornal AG, Bardawill JC, David MM (1949) Determination of serum proteins by means of biuret reaction. J Biol Chem 177(2):751–766
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Bio Chem 249(22):7130–7139
Hoan DN, Dung TH, Hoan PD, Thang TV (2021) Effect of supplementation of green tea extract on blood corticosterone concentration and growth performance in heat-stressed broiler. Livestock Res Rural Develop 33(1):2021
Hu Y, Lv T, Ma Y, Xu J, Zhang Y, Hou Y et al (2019) Nanoscale coordination polymers for synergistic NO and chemodynamic therapy of liver cancer. Nano Lett 19(4):2731–2738. https://doi.org/10.1021/acs.nanolett.9b01093
Irigoyen-Camacho ME, García Pérez A, Mejía González A, Huizar Alvarez R (2016) Nutritional status and dental fluorosis among schoolchildren in communities with different drinking water fluoride concentrations in a central region in Mexico. Sci Total Environ 541:512–519. https://doi.org/10.1016/j.scitotenv.2015.09.085
Izquierdo-Vega JA, Sánchez-Gutiérrez M, Del Razo LM (2011) NADPH oxidase participates in the oxidative damage caused by fluoride in rat spermatozoa. Protective role of α-tocopherol. J Appl Toxicol 31(6):579–588. https://doi.org/10.1002/jat.1600
Jacques-Silva MC, Nogueira CW, Broch LC, Flores EMM, Rocha JBT (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol 88(3):119–125. https://doi.org/10.1034/j.1600-0773.2001.d01-92.x
Jollow DJ, Mitchell JR, Zampaglione (1974) N Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol 11:151–169
Khalaf D, Krüger M, Wehland M, Infanger M, Grimm D (2019) The effects of Oral L-arginine and l-citrulline supplementation on blood pressure. Review Nutrients 11(7):1679. https://doi.org/10.3390/nu11071679
Kovarik JJ, Kaltenecker CC, Domenig O, Antlanger M, Poglitsch M, Kopecky C et al (2021) Effect of mineralocorticoid receptor antagonism and ace inhibition on angiotensin profiles in diabetic kidney disease: an exploratory study. Diabetes Ther 12(9):2485–2498. https://doi.org/10.1007/s13300-021-01118-7
Ma SX (2021) Low electrical resistance properties of acupoints: roles of noergic signaling molecules and neuropeptides in skin electrical conductance. Chin J Integr Med 27(8):563–569. https://doi.org/10.1007/s11655-021-3318-5
Mall S, Srivastava R, Sharma N, Patel CN, Rolta R, Sourirajan A et al (2021) Antihypertensive activity of phytocompounds from selected medicinal plants via inhibition of angiotensin-converting enzyme (ACE) protein: an in-silico approach. Nat Prod Res 1-4.https://doi.org/10.1080/14786419.2021.1990917
Marques LS, Zborowski VA, Heck SO, Fulco BCW, Nogueira CW (2021) 4,4’-dichloro-diphenyl diselenide modulated oxidative stress that differently affected peripheral tissues in streptozotocin-exposed mice. Can J Physiol Pharmacol 99(9):943–951. https://doi.org/10.1139/cjpp-2020-0652
Masella R, Benedetto RD, Varì R, Filesi C, Giovannini C (2005) Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16(10):577–586. https://doi.org/10.1016/j.jnutbio.2005.05.013
McRae MP (2016) Therapeutic benefits of L-arginine: an umbrella review of meta-analyses. Review J Chiropr Med 15(3):184–189. https://doi.org/10.1016/j.jcm.2016.06.002
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Molina-Frechero N, Pierdant-Rodríguez AI, Oropeza-Oropeza AR (2012) Fluorosis and dental caries: an assessment of risk factors in Mexican children. Bologna-Molina Rev Invest Clin 64(1):67–73
Morelli NR, Maes M, Bonifacio KL, Vargas HO, Nunes SOV, Barbosa DS (2021) Increased nitro-oxidative toxicity in association with metabolic syndrome, atherogenicity and insulin resistance in patients with affective disorders. J Affect Disord 294:410–419. https://doi.org/10.1016/j.jad.2021.07.057
Nasiruddin RM, Karim N, Changlek S, Atiar Rahman M, Tangpong J, Hajjar D et al (2020) Thunbergia laurifolia leaf extract partially recovers lead-induced renotoxicity through modulating the cell signaling pathways. Saudi J Biol Sci 27(12):3700–3710. https://doi.org/10.1016/j.sjbs.2020.08.016
Ni W, Zhang Y, Yin Z (2021) The protective mechanism of Klotho gene-modified bone marrow mesenchymal stem cells on acute kidney injury induced by rhabdomyolysis. Regen Ther 18:255–267. https://doi.org/10.1016/j.reth.2021.07.003
Olaleye SB, Adaramoye OA, Erigbali PP, Adeniyi OS (2007) Lead exposure increases oxidative stress in the gastric mucosa of HCl/ethanol-exposed rats. World J Gastroenterol 13(38):5121–5126. https://doi.org/10.3748/wjg.v13.i38.5121
Ommati MM, Farshad O, Azarpira N, Ghazanfari E, Niknahad H, Heidari R (2021) Silymarin mitigates bile duct obstruction-induced cholemic nephropathy. Naunyn Schmiedebergs Arch Pharmacol 394(6):1301–1314. https://doi.org/10.1007/s00210-020-02040-8
Omóbòwálé TO, Oyagbemi AA, Alaba BA, Ola-Davies OE, Adejumobi OA, Asenuga ER et al (2018) Ameliorative effect of Azadirachta indica on sodium fluoride-induced hypertension through improvement of antioxidant defence system and upregulation of extracellular signal regulated kinase 1/2 signaling. J Basic Clin Physiol Pharmacol 29(2):155–164. https://doi.org/10.1515/jbcpp-2017-0029
Østergaard MV, Secher T, Christensen M, Salinas CG, Roostalu U, Skytte JL et al (2021) Therapeutic effects of lisinopril and empagliflozin in a mouse model of hypertension-accelerated diabetic kidney disease. Am J Physiol Renal Physiol 321(2):F149–F161. https://doi.org/10.1152/ajprenal.00154.2021
Oyagbemi AA, Adebiyi OE, Adigun KO, Ogunpolu BS, Falayi OO, Hassan FO et al (2020) Clofibrate, a PPAR-alpha agonist, abrogates sodium fluoride-induced neuroinflammation, oxidative stress, and motor incoordination via modulation of GFAP/Iba-1/anti-calbindin signaling pathways. Environ Toxicol 35(2):242–253. https://doi.org/10.1002/tox.22861
Oyagbemi AA, Adejumobi OA, Jarikre TA, Ajani OS, Asenuga ER, Gbadamosi IT et al (2021) Clofibrate, a peroxisome proliferator-activated receptor-alpha (PPARalpha) agonist, and its molecular mechanisms of action against sodium fluoride-induced toxicity. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02722-1
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243. https://doi.org/10.1002/tox.21994
Oyagbemi AA, Omobowale TO, Asenuga ER, Adejumobi AO, Ajibade TO, Ige TM et al (2017) Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol 32(4):1089–1101. https://doi.org/10.1002/tox.22306
Oyagbemi AA, Omobowale TO, Awoyomi OV, Ajibade TO, Falayi OO, Ogunpolu BS et al (2019) Cobalt chloride toxicity elicited hypertension and cardiac complication via induction of oxidative stress and upregulation of COX-2/Bax signaling pathway. Hum Exp Toxicol 38(5):519–532. https://doi.org/10.1177/0960327118812158
Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA et al (2018a) Luteolin-mediated Kim-1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. BioFactors 44(6):518–531. https://doi.org/10.1002/biof.1449
Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA et al (2018b) Ameliorative effect of Rutin on sodium fluoride-induced hypertension through modulation of Kim-1/NF-κB/Nrf2 signaling pathway in rats. Environ Toxicol 33(12):1284–1297. https://doi.org/10.1002/tox.22636
Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA et al (2018c) Quercetin attenuates hypertension induced by sodium fluoride via reduction in oxidative stress and modulation of HSP 70/ERK/PPARγ signaling pathways. BioFactors 44(5):465–479. https://doi.org/10.1002/biof.1445
Pahlavani N, Entezari MH, Nasiri M, Miri A, Rezaie M, Bagheri-Bidakhavidi M et al (2017) The effect of L-arginine supplementation on randomized controlled trial. Eur J Clin Nutr 71(4):544–548. https://doi.org/10.1038/ejcn.2016.266
Pahlavani N, Jafari M, Sadeghi O, Rezaei M, Rasad H, Rahdar HA et al (2014) L-arginine supplementation and risk factors of cardiovascular diseases in healthy men: a double-blind randomized clinical trial. F1000Res 3:306. https://doi.org/10.12688/f1000research.5877.2. eCollection 2014.
Patrono C, Volpe M (2021) Aldosterone receptor antagonism in patients with diabetes and chronic kidney disease: new promises and old problems. Eur Heart J 42(1):14–15. https://doi.org/10.1093/eurheartj/ehaa992
Pautz A, Li H, Kleinert H (2021) Regulation of NOS expression in vascular diseases. Front Biosci (Landmark Ed) 26(5):85–101. https://doi.org/10.52586/4926.
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V et al (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017:8416763. https://doi.org/10.1155/2017/8416763
Puspita FM, Yunir E, Agustina PS, Sauriasari R (2021) Effect of angiotensin receptor blocker and angiotensin converting enzyme inhibitor on kidney function and blood potassium level in Indonesian type 2 diabetes mellitus with hypertension: a three-month cohort study. Diabetes Metab Syndr Obes 14:3841–3849. https://doi.org/10.2147/DMSO.S310091
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363. https://doi.org/10.1016/s0076-6879(94)33041-7
Rodríguez-Sánchez E, Navarro-García JA, Aceves-Ripoll J, González-Lafuente L, Corbacho-Alonso N, Baldan-Martín M (2021) Analysis of global oxidative status using multimarker scores reveals a specific association between renal dysfunction and diuretic therapy in older adults. J Gerontol A Biol Sci Med Sci 76(7):1198–1205. https://doi.org/10.1093/gerona/glab012
Ruggenenti P (2017) Dual renin-angiotensin system blockade for nephroprotection. Nephrol Ther Suppl 1:S43–S45. https://doi.org/10.1016/j.nephro.2017.02.006
Said AB, Telmoudi C, Louati K, Telmoudi F, Amira D, Hsairi M (2020) Evaluation of the reliability of human teeth matrix used as a biomarker for fluoride environmental pollution. Ann Pharm Fr 78(1):21–33. https://doi.org/10.1016/j.pharma.2019.10.006
Saka WA, Akhigbe RE, Abidoye AO, Dare OS, Adekunle AO (2021) Suppression of uric acid generation and blockade of glutathione dysregulation by L-arginine ameliorates dichlorvos-induced oxidative hepatorenal damage in rats. Biomed Pharmacother 138:111443. https://doi.org/10.1016/j.biopha.2021.111443
Scisciola L, Fontanella RA, Surina Garofalo G, Rizzo MR, Paolisso G (2022) Potential role of lisinopril in reducing atherosclerotic risk: evidence of an antioxidant effect in human cardiomyocytes cell line. Front Pharmacol 13:868365. https://doi.org/10.3389/fphar.2022.868365
Shuhua X, Ziyou L, Ling Y, Fei W, Sun G (2012) A role of fluoride on free radical generation and oxidative stress in BV-2 microglia cells. Mediators Inflamm 2012:102954. https://doi.org/10.1155/2012/102954
Stamm P, Oelze M, Steven S, Kröller-Schön S, Kvandova M, Kalinovic S (2021) Direct comparison of inorganic nitrite and nitrate on vascular dysfunction and oxidative damage in experimental arterial hypertension. Nitric Oxide 113–114:57–69. https://doi.org/10.1016/j.niox.2021.06.001
Suzuki M, Bandoski C, Bartlett JD (2015) Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic Biol Med 89:369–378. https://doi.org/10.1016/j.freeradbiomed.2015.08.015
Torun AN, Kulaksizoglu S, Kulaksizoglu M, Pamuk BO, Isbilen E, Tutuncu NB (2009) Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin Endocrinol (oxf) 70(3):469–474. https://doi.org/10.1111/j.1365-2265.2008.03348.x
Travis OK, Tardo GA, Giachelli C, Siddiq S, Nguyen HT, Crosby MT et al (2021) Tumor necrosis factor-alpha blockade improves uterine artery resistance, maternal blood pressure, and fetal growth in placental ischemic rats. Pregnancy Hypertens 25:39–47. https://doi.org/10.1016/j.preghy.2021.05.002
Tsuboi T, Maeda M, Hayashi T (2018) Administration of L-arginine plus L-citrulline or L-citrulline alone successfully retarded endothelial senescence. PLoS ONE 13(2):e0192252. https://doi.org/10.1371/journal.pone.0192252
Umnyagina IA, Blinova TV, Strakhova LA, Ivanova YV, Troshin VV, Kolesov SA et al (2021) Endothelin-1 and nitrogen oxide metabolites in risk diagnostics of arterial hypertension in persons of young and middle ages occupied in harmful working conditions. Klin Lab Diagn 66(9):525–532. https://doi.org/10.51620/0869-2084-2021-66-9-525-532.
Varshney R, Kale RK (1990) Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol 58(5):733–743. https://doi.org/10.1080/09553009014552121
Viribay A, Burgos J, Fernández-Landa J, Seco-Calvo J, Mielgo-Ayuso J (2020) Effects of arginine supplementation on athletic performance based on energy metabolism: a systematic review and meta-analysis. Meta-Analysis Nutrients 12(5):1300. https://doi.org/10.3390/nu12051300
Wang L, Pan F, Luo T (2021) Sinapic acid attenuates rheumatoid arthritis through reducing inflammation and oxidative stress by downregulating IkappaB kinase. J Interferon Cytokine Res 41(9):347–354. https://doi.org/10.1089/jir.2021.0044
Wihandono A, Azhar Y, Abdurahman M, Hidayat S (2021) The role of lisinopril and bisoprolol to prevent anthracycline induced cardiotoxicity in locally advanced breast cancer patients. Asian Pac J Cancer Prev 22(9):2847–2853. https://doi.org/10.31557/APJCP.2021.22.9.2847.
Wu J, Cheng M, Liu Q, Yang J, Wu S, Lu X et al (2015) Protective role of tert-butylhydroquinone against sodium fluoride-induced oxidative stress and apoptosis in PC12 cells. Cell Mol Neurobiol 35(7):1017–1025. https://doi.org/10.1007/s10571-015-0196-4
Wu W, Zhang C, Rees TW, Liao X, Yan X, Chen Y et al (2020) Lysosome-targeting iridium(III) probe with near-infrared emission for the visualization of NO/O(2)(-) Crosstalk via in vivo peroxynitrite imaging. Anal Chem 92(8):6003–6009. https://doi.org/10.1021/acs.analchem.0c00259
Yang L, Dong L, Zhang L, Bai J, Chen F, Luo Y (2021) Acrylamide induces abnormal mtDNA expression by causing mitochondrial ROS accumulation, biogenesis, and dynamics disorders. J Agric Food Chem 69(27):7765–7776. https://doi.org/10.1021/acs.jafc.1c02569
Yaremchuk O, Lisnychuk N, Nebesna Z, Kramar S, Kulitska M, Shanaida M et al (2021) Morphological changes in the liver of mice with antiphospholipid syndrome and administration of nitric oxide synthesis modulators. Georgian Med News 315:177–180
Yu F, Wei J, Cui X, Yu C, Ni W, Bungert J et al (2021) Post-translational modification of RNA m6A demethylase ALKBH5 regulates ROS-induced DNA damage response. Nucleic Acids Res 49(10):5779–5797. https://doi.org/10.1093/nar/gkab415
Zhang L, Fang Y, Zhao X, Zheng Y, Ma Y, Li S et al (2021) miR-204 silencing reduces mitochondrial autophagy and ROS production in a murine AD model via the TRPML1-activated STAT3 pathway. Mol Ther Nucleic Acids 24:822–831. https://doi.org/10.1016/j.omtn.2021.02.010
Zhu Q, Yue X, Tian QY, Saren G, Wu MH, Zhang Y et al (2013) Effect of L-arginine supplementation on blood pressure in pregnant women: a meta-analysis of placebo-controlled trials. Meta-Analysis Hypertens Pregnancy 32(1):32–41. https://doi.org/10.3109/10641955.2012.697952
Acknowledgements
The authors deeply thank Prof. O. O. Oguntibeju of Cape Peninsula University of Technology (CPUT), Cape Town, South Africa, for providing some of the antibodies used for this study.
Author information
Authors and Affiliations
Contributions
The authors, Ademola Adetokunbo Oyagabemi, Olusola Adedayo Awodele, and Temidayo Olutayo Omobowale, designed the experiment. Histopathology was carried out by Monsuru Oladunjoye Tijani. The blood pressure was performed by Temitayo Olabisi Ajibade, Olumuyiwa Abiola Adejumobi, and Temidayo Olutayo Omobowale. Olusola Adedayo Awodele and Ademola Adetokunbo Oyagabemi performed the immunohistochemistry and biochemical assays Moses Olusola Adetona, Aduragbenro Deborah A. Adedapo, Temidayo Olutayo Omobowale, Abimbola Obemisola Aro, Olufunke Eunice Ola-Davies, Adebowale Benard Saba, Adeolu Alex Adedapo, Sanah Malomile Nkadimeng, Lyndy Joy McGaw, Prudence Ngalula Kayoka-Kabongo, Oluwafemi Omoniyi Oguntibeju, and Momoh Audu Yakubu supervised, proofread, and approved the submission.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted following guidelines approved by the Animal Care and Use Research Ethics Committee (ACUREC) of the University of Ibadan (Approval number: UIACUREC/19/124).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajibade, T.O., Awodele, O.A., Tijani, M.O. et al. L-arginine and lisinopril supplementation protects against sodium fluoride–induced nephrotoxicity and hypertension by suppressing mineralocorticoid receptor and angiotensin-converting enzyme 3 activity. Environ Sci Pollut Res 30, 23263–23275 (2023). https://doi.org/10.1007/s11356-022-23784-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23784-1