Abstract
Azadirachta indica A. Juss., Stemona curtisii Hook.F., and Mammea siamensis are popularly used as bioinsecticides by Thai farmers. To evaluate their safety on frog (Hoplobatrachus rugulosus) tadpoles, the ecologically and economically important species of Thailand, an acute static toxicity test was performed. The tadpoles (n = 10 per treatment group) were exposed to the bioinsecticides from A. indica (NSAI 5, 10, 15, 20, and 25 g/l) and the mixture of S. curtisii and M. siamensis (SCMS 0.8, 1.2, 1.6, 2.0, and 2.4 mg/l). It was found that the median lethal concentration (LC50) values at 96 h of the NSAI and the SCMS were 11.81 and 1.44 mg/l respectively. Hepatic failures were observed in tadpoles exposed to the NSAI and the SCMS. Histopathological changes included vacuolation, leukocyte infiltration, necrotic cell, and blood congestion. These lesions were more severe in the tadpoles treated with the NSAI or the SCMS at the high concentrations. Additionally, necrosis of bile duct epithelium, karyolysis, and sinusoidal dilation were apparently found in the tadpole exposed to 25 g/l of the NSAI, while the degeneration of bile duct was noted in the 1.6–2.4 mg/l of the SCMS-treated groups. However, the tadpoles exposed to 5 g/l of the NSAI showed only mild pathological changes in their livers. From a higher value of LC50 of the NSAI than the SCMS together with the mild histopathological changes in the tadpoles exposed to the low concentrations, thus the NSAI is less toxic to H. rugulosus tadpoles than the SCMS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The indiscriminate applications of industrial insecticides in agricultural areas around the world have created serious problems (Sparling et al. 2001; Aktar et al. 2009; Alavanja 2009). Agroinsecticides can contaminate into aquatic environments where they are the habitats of non-target organisms (Rohr et al. 2003; Relyea 2005; Hayes et al. 2010). Amphibians including frogs living in ponds or small water bodies within or around the contaminant fields can directly be exposed to pesticides due to their breeding and development occurrence in ponds. Earlier studies indicate that contaminations of insecticides in habitats of amphibian larvae have led to the toxicity and the alteration of normal development (Johansson et al. 2006; Shenoy et al. 2009; Bernabò et al. 2011). Toxicological investigations show that lethal effects of Maneb, a carbamate insecticide, were found in tadpoles of common toad (Bufo bufo) and green toad (Pseudepidalea viridis). This insecticide disrupted normal developments of the tadpoles. Histopathological changes such as visceral edema, liver necrosis, degenerations of somite, and deformations of tail and pronephric tubules were clearly noted in tadpoles exposed to Maneb (Gürkan and Hayretdağ 2015). Deltamethrin and cypermethrin, the pyrethroidal insecticides, at low concentrations caused acute toxicity on frog (Physalaemus gracilis) embryo and frog larvae. Moreover, the results demonstrated deltamethrin to be more toxic than cypermetrin and the frog larvae were more sensitive to these two insecticides than the embryo. Additionally, deltamethrin and cypermethrin induced neurological dysfunctions by producing spasmodic contractions in both developmental stages (Macagnan et al. 2017). Yu et al. (2013) found that three synthetic insecticides, parathion, alpha-cypermethrin, and endosulfan, inhibited normal growths and developments by significant decrease in total length, producing edema, and deforming gut and axial/tail of Xenopus laevis larvae when compared to normal larvae. Moreover, endosulfan at low concentrations caused acute mortality in the larvae of eight amphibians. Thus, these phenomena were the main causes of the mortality and the decline in frog populations. Since frogs play a key role in ecosystem by controlling insect pests and serve as food for predators, the drastic decline of frog populations as well as changes in normal growths are the ecologically global problems. In Thailand, frog (Hoplobatrachus rugulosus) is not only an important species in aquatic ecosystem, but it is a commercially important animal. Thus, the decline in frog populations is not only an ecological problem but it also adversely affects Thai farmers.
In order to reduce these problems, bioinsecticide based from natural by-products have alternatively been used in many parts of the world (Shivanandappa and Rajashekar 2014; Siegwart et al. 2015). Several plant species have been used to manage insect pests in agricultural areas, farms, and gardens in Thailand. Azadirachta indica A. Juss. or neem, Mammea siamensis, and Stemona curtisii Hook.F. are well known as sadao, saraphi, and non tai yak, respectively, and they are popularly used by Thai farmers as insect control agents. In addition, these three plants have been reported to have repellent, deterrent, antifeedant, growth regulatory and killing properties (Kaltenegger et al. 2003; Mungkornasawakul et al. 2004; Siddiqui et al. 2004; Promsiri et al. 2006; Issakul et al. 2011). Besides the insecticidal activities of these plants, they have a wide range of biological efficacies such as contraceptive, antiseptic, antimicrobial, antipyretic, antiparasitic, and antitumor properties (Vasanth et al. 1990; Issakul et al. 2011; Agyare et al. 2014; Sharma et al. 2014; Rungrojsakul et al. 2016). All parts of A. indica have been reported to contain bioactive compounds such as triterpenoids, azadirachtin, quercetins, nimbolinin, gedunin, and β-sitosterol (Kumar et al. 1996; Ghimeray et al. 2009; Elteraifi and Hassanali 2011). Among these compounds, azadirachtin was the most effective substance against insect pests (Schmutterer 1990) and it was found in high amounts in the seed kernels (Mordue and Nisbet 2000; Siddiqui et al. 2004; Jadeja et al. 2011). The insecticidal compound in M. siamensis was found to be the coumarins and its derivatives presented in its seeds (Morris and Pagan 1953; Crombie et al. 1972; Mungkornasawakul et al. 2004). Several alkaloids such as stemocurtisine, pyridol[1,2-α]azepine, stemocurtisinol, and stemofoline were the phytosubstances in S. curtisii which possessed the insecticidal properties (Mungkornasawakul et al. 2004; Kongkiatpaiboon et al. 2013). Although natural by-products exhibit the potent activities against insects, the applications of these bioinsecticides with careless handling can adversely affect aquatic vertebrates. The toxic effects of insecticides based from several plants such as Milletia ferruginea (Hochst), Ipomoea aquatica, and Nicotiana tabacum on non-target vertebrate have been reported (Karunamoorthi et al. 2009; Oluwatoyin 2011; Millan et al. 2013). In this study, therefore, we used the tadpoles of H. rugulosus as an experimental model for examining the acute toxicity of bioinsecticides derived from A. indica, S. curtisii, and M. siamensis, which were traditionally used in Thailand. Moreover, we also investigated the histopathological alterations in livers of the H. rugulosus tadpoles after exposed to these bioinsecticides.
Materials and methods
Preparation of bioinsecticides
The fresh neem seed (Azadirachta indica A. Juss.) was purchased from the local market in Chiang Mai Province, Thailand. The mature seeds were washed and dried at 60 °C to obtain a constant weight. They were ground into coarse powder and extracted by soaking them in distilled water (1: 10 w/v) for 24 h (Cruz et al. 2004). The neem seed extract (NSAI) was obtained after removing the residues. The bioinsecticide (SCMS) consisted of the ethanolic extracts from the roots of Stemona curtisii Hook.F. and the seeds of Mammea siamensis. The SCMS was provided by the Natural Product Research Unit, Faculty of Science, Chiang Mai University. The NSAI was prepared freshly at the day of the experiments. Both bioinsecticides were diluted with distilled water to obtain various concentrations as required.
Experimental tadpoles
Eight-day-old frog tadpoles (Hoplobatrachus rugulosus) were purchased from a frog farm in Doi Sa Ket District, Chiang Mai Province, Thailand. They were kept in dechlorinated water with laboratory conditions at least 7 days for acclimatization. The frog tadpoles were fed with commercial pellet foods every 24 h. All procedures encompassing the animals were approved under permission number of Re. 005/07.
Evaluations of acute toxicity
The preliminary toxicity range-finding test was performed in frog tadpoles (Weber 1993) to investigate the ranges of suitable concentrations of the tested bioinsecticides. We found that the concentrations ranges of the NSAI and the SCMS which produced 0–100% mortality were 5–25 g/l and 0.8–2.4 mg/l respectively. Based on our results, the NSAI at the concentrations of 5–25 g/l and the SCMS at 0.8–2.4 mg/l were applied to frog tadpoles for determining the median lethal concentration (LC50).
To evaluate the LC50 of the NSAI and the SCMS, the static non-renewal test was used (Weber 1993). All experiments were done in three replicates. Ten acclimated tadpoles were randomly placed into each aerated aquarium containing various concentrations of the NSAI and the SCMS. Dechlorinated water was used in control groups. The numbers of dead tadpoles were recorded every 24 h, from 24 to 96 h. The LC50 was calculated and reported as LC50 at 96 h using the linear regression from the SPSS statistical software version 17 for Windows. Dead tadpoles were immediately removed from the aquaria and fixed in Bouin’s fixative for determining histopathological changes.
Histopathological examination
For histological observation, the livers were removed from the control and treated tadpoles. They were then processed following the routine paraffin-embedded section (Kurth et al. 2012). In brief, the liver samples were fixed in Bouin’s fixative for 24 h. They were dehydrated in increasing degree of alcoholic solutions. Xylene was used as clearing solution. The tissues were then embedded in paraplast. The 6-μm thickness was prepared. All sections were stained using hematoxylin and eosin (H & E). The stained slides were examined for histopathological alterations under a light microscope with a blind method.
Results
Acute toxicity of the NSAI and the SCMS
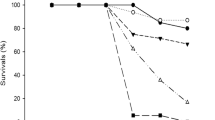
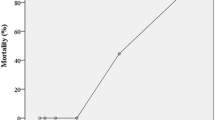
The numbers of dead tadpoles were observed and recorded every 24 h of exposure to the NSAI or the SCMS in different concentrations. Tables 1 and 2 showed that the numbers of dead tadpoles increased with concentrations of both bioinsecticides. One hundred percent of mortality was observed in group received with the NSAI at 25 g/l after 24 h of exposure and after 72 h at 15 g/l (Table 1). Interestingly, most of frog tadpoles could not survive after exposure to the SCMS at the concentrations of 2.0 (90% mortality) and 2.4 mg/l (100% mortality) for 24 h (Table 2). The SCMS is highly toxic to frog tadpoles more than the NSAI. The LC50 value at 96 h of the SCMS was 1.44 mg/l (equivalent to 1.44 ppm), while the NSAI was 11.81 g/l (equivalent to 11,810.00 ppm).
Histopathological changes in tadpole liver
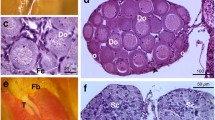
Histopathological examinations showed no alterations in liver tissues of frog tadpoles in the control group (Fig. 1). The severities of hepatic tissue damages after exposure to the NSAI and the SCMS were concentration-dependent. Histopathological alterations such as vacuolation, necrotic cell death and diffused necrosis of some hepatic area (Fig. 1), blood congestion in the sinusoids, and the leukocyte infiltration (Fig. 2) were clearly noted in the tadpole livers after exposure to the NSAI at the concentrations higher than 10 g/l or the SCMS at all concentrations. Additionally, necrosis of bile duct epithelium (Fig. 3), hepatocyte karyolysis (Fig. 1), and sinusoidal dilation (Figs. 1 and 2) were found in the 25 g/l NSAI-treated tadpole, whereas the degeneration of bile duct was found in the 1.6–2.4 mg/l SCMS-treated groups (Fig. 3). However, the tadpoles exposed to the NSAI at a concentration of 5 g/l had only mild pathological changes in the liver when compared to the control.
Photomicrographs represent hepatic tissues of frog tadpoles in control and bioinsecticides-exposed groups. Liver tissue of the normal tadpole (a), the tadpoles exposed to the NSAI at 10, 15, 20, and 25 g/l (b–e), and the tadpoles exposed to the SCMS at 0.8, 1.2, and 1.6 mg/l (f–h). Vacuolation (arrowhead), necrotic cell death (dark arrow), karyolysis (star), and dilated sinusoid (askerisk). H & E, (× 40)
Photomicrographs represent hepatic tissues of frog tadpoles in control and bioinsecticides-exposed groups. Liver tissue of the normal tadpole (a), the tadpoles exposed to the NSAI at 5, 10, 15, 20, and 25 g/l (b–f), and the tadpoles exposed to the SCMS at 0.8, 1.2, and 1.6 mg/l (g–h). Blood congestion (white arrow), vacuolation (arrowhead), sinusoidal dilation (askerisk), and leukocyte infiltrations (yellow arrow). H & E, (× 20)
Photomicrographs of hepatic tissues of frog tadpoles show area of bile ducts. Liver tissue of the normal tadpole (a), the tadpoles exposed to the NSAI at 5, 10, 15, 20, and 25 g/l (b–f), and the tadpoles exposed to the SCMS at 1.2 and 1.6 mg/l (g–h). BD indicates bile duct. Necrotic cell of bile duct epithelium (dark arrow) and degeneration of bile duct (dark circle). H & E, (× 40)
Discussion
Although insecticides based from natural products have been used to replace synthetic insecticides, the careless applications of the bioinsecticides may produce various symptoms of toxicity on frogs (Karunamoorthi et al. 2009; Oluwatoyin 2011; Millan et al. 2013). We found the increases in numbers of dead tadpoles corresponded with concentrations of both bioinsecticides after 24 h of exposure. The mortality of the tadpoles may relate to the decrease of dissolved oxygen. Since oxygen has an essential role in decomposing the organic matters of the plant extracts, the reduction of dissolved oxygen occurs in high concentrations of the plant extracts (Milsom 1993). Furthermore, the accumulation of excessive plant extracts in the gills or the competitive reactions of the plant active compounds and the oxygen chemoreceptors at the gills of the tadpoles can disrupt gas exchanges (Aguinaga et al. 2014). Accordingly, these conditions initiated hypoventilation, altered physiological response, and produced mortality of the frog tadpoles.
The NSAI had the LC50 value at 96 h (11.81 g/l) more than the SCMS (1.44 mg/l), thus we suggested that the NSAI was less toxic to the tadpoles than the SCMS. This evidence may be due to the SCMS contained a higher level of phytoactive toxicant like alkaloids than the NSAI. Previous report indicated that the reaction of plant alkaloids with dopamine could alter the nervous and cardiac functions (Sakakibaru et al. 1999). Thus a variety of alkaloids presented in the SCMS (Mungkornasawakul et al. 2004; Kongkiatpaiboon et al. 2013) may produce the mortality through this mechanism. On the other hand, the NSAI mainly contained azadirachtin which was classified as terpenoid group (Kumar et al. 1996). However, both of the bioinsecticides were safer than the synthetic insecticides such as permethrin, esfenvalerate, Basudin, endosulfan, and deltamethrin which had the LC50 values at 96 h on frog tadpoles of 0.00025, 0.00073, 0.00086, 0.43, and 0.5 ppm respectively (Ferrari et al. 2004; Johansson et al. 2006; Bernabò et al. 2008; Ezemonye and Ilechie 2007). Because the above harmful insecticides have a broad spectrum against insects and they have properties different from the natural products, they are very high toxic to estuarine/marine invertebrates and other aquatic animals including fishes and amphibians (Das and Mukherjee 2003). Synthetic insecticides such as organophosphorus, organochlorine, and pyrethroidal compounds had neurotoxicity by altering cholinesterase enzymes in nervous tissues and neuromuscular junctions (Eddleston et al. 2008) as well as altering voltage-dependent sodium channels (Wolansky and Harrill 2008) and inhibiting ATPase and the influx of calcium ions (Jayaraj et al. 2016). On the other hand, the by-products of plant materials are the ideal insecticides because they are eco-friendly to the environment. Furthermore, the natural products are easily photodegraded and they leave their residues in environments less than commercial insecticides. Thus, the NSAI and the SCMS have less of adverse effects on frog tadpoles as compared to some synthetic insecticides. From the results of acute toxicity, we suggested that the NSAI and the SCMS appear to be safe for H. rugulosus tadpoles and the environment.
Aquatic vertebrates living in ponds within and around agricultural areas may be exposed to contaminants directly in their habitats, causing mass mortality, and accumulating and affecting non-target vertebrates. The toxic effects of insecticides on freshwater fishes and frogs have been well documented (Bridges 2000; Chindah et al. 2004; Johansson et al. 2006; Brühl et al. 2013). The tadpoles are aquatic amphibian closed with the environment and they have many chances to encounter acquired environment contaminants. The tadpoles live in both stagnant water and stream water. They consume algae and small insects while adult frogs feed insects and small aquatic animals. These prey organisms may contain undesired materials from the surroundings and unquestionably transferred them to the frog. Moreover, the larvae and adult frogs respire through their skins, gills, and lungs, making them directly in contact with the environmental contaminants and accumulate them in their body/organs (Wake 1991; Blaustein and Wake 1995; Netting 2000).
The liver functions of animals are to get some wastes rid of blood and also to degrade many poisons and detoxicate drugs (Thapa and Walia 2007). It has been well accepted that alterations of the hepatic functions can be used as a biological marker that indicates the accumulation of toxins in the environment. Histopathological changes of exposed tadpoles seen in this study were similar to previous study (Wattanasirmkit et al. 2003) who reported the effect of A. indica var siamensis Valelton seed extract on H. rugulosus, demonstrating karyolysis of hepatocytes, white blood cell infiltration, increased fat droplet, and hydropic swelling of some hepatic cells. Clinically, inflammation of hepatocyte, leukocyte, and macrophages infiltrations and spreading of sinusoidal endothelial cells were the similar symptoms of cirrhosis in rat (Kasai et al. 1990). Moreover, Akah et al. (1992) reported that the high levels of aminotransferase enzymes, and inflammation of bile duct and necrotic cells were observed in rabbit liver after fed with neem’s leaf extract at a dose of 2.3 mg/kg. As seen in our study, the NSAI and the SCMS at the higher concentrations could cause severe damages to liver tissues of frog tadpoles although they have much lower toxicity than the synthetic chemicals including pyrethroid, organophosphorus, and organochlorine insecticides as mentioned above. We also agree to prior research (Schmuttere 1990), who suggested that safety utilization of the NSAI and the SCMS extracts should be used at low concentrations and far from water resources. It could decrease toxic effects on frog’s and fish’s larvae and small fish, and be safe for the environment.
As described above, both the NSAI and the SCMS are less toxic as compared to some synthetic insecticides, therefore they are safer to apply bioinsecticides provided from raw plant materials because they have little or no adverse effects on frog tadpoles. Although some pesticides can produce mortality at high concentrations, long-term exposure to low concentrations may generate negative effects on growth rates, developments, behaviors, reproduction, and biochemical parameters. From our results, low concentrations of the NSAI and the SCMS did not cause lethal toxicities, but sublethal responses to these two bioinsecticides should also be considered. Therefore, the further investigations on long-term treatments of the NSAI or the SCMS on H. rugulosus are required. In addition, the study of their toxicities on other aquatic vertebrates should be strongly recommended to support the safe uses of bioinsecticides derived from A. indica, S. curtisii, and M. siamensis.
Conclusion
In summary, we concluded that although the NSAI and the SCMS at high concentrations caused acute toxicity and produced mild histopathological alterations in livers of H. rugulosus tadpoles, they were less toxic than synthetic insecticides. However, the direct application in agricultural fields near the habitats of frog tadpoles should be considerably attentive.
References
Aguinaga JY, Claudiano GS, Marcusso PF, Ikefuti C, Ortega GG, Eto SF, de Cruz C, Moraes JRE, Moraes FR, Fernandes JBK (2014) Acute toxicity and determination of the active constituents of aqueous extract of Uncaria tomentosa bark in Hyphessobrycon eques. J Toxicol 2014:ID 412437
Agyare C, Spiegler V, Sarkodie H, Asase A, Liebau E, Hensel A (2014) An ethnopharmacological survey and in vitro confirmation of the ethnopharmacological use of medicinal plants as anthelmintic remedies in the Ashanti region, in the central part of Ghana. J Ethnopharmacol 158:255–263
Akah PA, Offiah VN, Onuogu E (1992) Hepatotoxic effect of Azadirachta indica leaf extracts in rabbits. Fitoterapia 63:311–319
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12
Alavanja MCR (2009) Pesticides use and exposure extensive worldwide. Rev Environ Health 24:303–309
Bernabò I, Brunelli E, Berg C, Bonacci A, Tripepi S (2008) Endosulfan acute toxicity in Bufo bufo gills: ultrastructural changes and nitric oxide synthase localization. Aquat Toxicol 86:447–456
Bernabò I, Sperone E, Tripepi S, Brunelli E (2011) Toxicity of chlorpyrifos to larval Rana dalmatina: acute and chronic effects on survival, development, growth and gill apparatus. Arch Environ Contam Toxicol 61:704–718
Blaustein AR, Wake DB (1995) The puzzle of declining amphibian populations. Sci Am 272:52–57
Bridges CM (2000) Long-term effects of pesticide exposure at various life stages of the Southern leopard frog (Rana sphenocephala). Arch Environ Contam Toxicol 39:91–96
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Sci Rep 3:1135
Chindah AC, Sikoki FD, Vincent-Akpu I (2004) Toxicity of an organophosphate pesticides (chlorpyrifos) on a common Niger Delta Wetland fish-Tilapia guineensis (Blecker 1862). J Appl Sci Environ Mgt 8:11–17
Crombie L, Games D, Haskins N, Reed G (1972) Extractives of Mammea americana L. Part V. The insecticidal compounds. J Chem Soc Perkin Trans 0:2255–2260
Cruz C, Machado-Neto JG, Menezes ML (2004) Toxicidade aguda do inseticida Paration metílico e do biopesticida azadiractina de folhas de neem (Azadirachta indica) para alevino e juvenile de pacu (Piaractus mesopotamicus). Pesticidas: R Ecotoxicol e Meio Ambiente 14:92–102
Das BK, Mukherjee SC (2003) Toxicity of cypermethrin in Labeo rohita fingerlings: biochemical, enzymatic and haematological consequences. Comp Biochem Physiol C: Toxicol Pharmacol 134:109–121
Eddleston M, Buckley N, Eyer P, Dawson AH (2008) Management of acute organophosphorus pesticide poisoning. Lancet 371:597–607
Elteraifi IE, Hassanali A (2011) Oil and Azadirachtin contents of neem (Azadirachta indica A. Juss) seed kernels collected from trees growing in different habitats in Sudan. Int J Biol Chem Sci 5:1063–1072
Ezemonye LIN, Ilechie I (2007) Acute and chronic effects of organophosphate pesticides (Basudin) to amphibian tadpoles (Ptychadena bibroni). Afr J Biotechnol 6:1554–1558
Ferrari A, Anguiano OL, Soleño J, Venturino A, de D’ Angelo AMP (2004) Different susceptibility of two aquatic vertebrates (Oncorhynchus mykiss and Bufo arenarum) to azinphos methyl and carbaryl. Comp Biochem Physiol C 139:239–243
Ghimeray AK, Jin CW, Ghimire BK, Cho DH (2009) Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta indica A. Juss grown in foothills of Nepal. Afr J Biotechnol 8:3084–3091
Gürkan M, Hayretdağ S (2015) Acute toxicity of maneb in the tadpoles of common and green toad. Arh Hig Rada Toksikol 66:189–195
Hayes TB, Falso P, Gallipeau S, Stice M (2010) The cause of global amphibian declines: a developmental endocrinologist’s perspective. J Exp Biol 15:921–933
Issakul K, Jatisatienr A, Pawelzik E, Jatisatienr C (2011) Potential of Mammea siamensis as a botanical insecticide: its efficiency on diamondback moth and side effects on non-target organisms. J Med Plant Res 5:2149–2156
Jadeja GC, Maheshwari RC, Naik SN (2011) Extraction of natural insecticide azadirachtin from neem (Azadirachta indica A. Juss) seed kernels using pressurized hot solvent. J Supercirt Fluids 56:253–258
Jayaraj R, Megha P, Sreedev P (2016) Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol 9:90–100
Johansson M, Piha H, Kylin H, Merila J (2006) Toxicity of six pesticides to common frog (Rana temporaria) tadpoles. Environ Toxicol Chem 25:3164–3170
Kaltenegger E, Brem B, Mereiter K, Kalchhauser H, Kahlig H, Hofer O, Vajrodaya S, Greger H (2003) Insecticidal pyrido (1,2-a)azepine alkaloids and related derivatives from Stemona species. Phytochemistry 63:803–816
Karunamoorthi K, Bishaw D, Mulat T (2009) Toxic effects of traditional Ethiopian fish poisoning plant Milletia ferruginea (Hochst) seed extract on aquatic macroinvertebrates. Eur Rev Med Pharmacol Sci 13:179–185
Kasai N, Osanai T, Miyoshi I, Kamimura E, Yoshida MC, Dempo K (1990) Clinico-pathological studies of LEC rats with hereditary hepatitis and hepatoma in the acute phase of hepatitis. Lab Anim Sci 40:502–505
Kongkiatpaiboon S, Keeratinijakal V, Gritsanapan W (2013) Simultaneous quantification of stemocurtisine, stemocurtisinol and stemofoline in Stemona curtisii (Stemonaceae) by TLC-densitometric method. J Chromatogr Sci 51:430–435
Kumar CSSR, Srinivas M, Yakkundi S (1996) Limonoids from the seeds of Azadirachta indica. Phytochemistry 43:451–455
Kurth T, Weiche S, Vorkel D, Kretschmar S, Menge A (2012) Histology of plastic embedded amphibian embryos and larvae. Genesis 50:235–250
Macagnan N, Rutkoski CF, Kolcenti C, Vanzetto GV, Macagnan LP, Sturza PF, Hartmann PA, Hartmann MT (2017) Toxicity of cypermethrin and deltamethrin insecticides on embryos and larvae of Physalaemus gracilis (Anura: Leptodactylidae). Environ Sci Pollut Res 24:20699–20704
Millan DC, Shiogiri NS, Souza NES, Silva HR, Fernandes MN (2013) Ecotoxicity and hematological effects of a natural insecticide based on tobacco (Nicotiana tabacum) extract on Nile tilapia (Oreochromis niloticus). Acta Sci Biol Sci 35:157–162
Milsom WK (1993) Afferent inputs regulating ventilation in vertebrates. In: Bicudo JEPW (ed) The vertebrate gas transport cascade. Adaptations and mode of life. CRC Press, USA, pp 94–105
Mordue AJ, Nisbet AJ (2000) Azadirachtin from the neem tree Azadirachta indica: its action against insects. An Soc Entomol Brasil 29:615–632
Morris M, Pagan C (1953) The isolation of the toxic principle of mammey. J Am Chem Soc 75:1489
Mungkornasawakul P, Pyne SG, Jatisatienr A, Supyen D, Jatisatienr C, Lie W, Ung AT, Skelton BW, White AH (2004) Phytochemical and larvicidal studies on Stemona curtisii: structure of a new pyrido[1,2-a]azepine Stemona alkaloid. J Nat Prod 67:675–677
Netting J (2000) Pesicides implicated in declining frog number. Nature 408:760
Oluwatoyin AS (2011) Histopathology of Nile tilapia (Oreochromis niloticus) juveniles exposed to aqueous and ethanolic extracts of Ipomoea aquatica leaf. Int J Fish Aquac 3:244–257
Promsiri S, Naksathit A, Kruatrachue M, Thavara U (2006) Evalutions of larvicidal activity of medicinal plant extracts to Aedes aegypti (Diptera: Culicidae) and other effects on a non target fish. Insect Sci 13:179–188
Relyea RA (2005) The lethal impact of roundup on aquatic and terrestrial amphibians. Ecol Appl 15:1118–1124
Rohr JR, Elskus AA, Shepherd BS, Crowley PH, McCarthy TM, Niedzwiecki JH, Sager T, Sih A, Palmer BD (2003) Lethal and sublethal effects of atrazine, carbaryl, endosulfan, and octylphenol on the streamside salamander (Ambystoma barbouri). Environ Toxicol Chem 22:2385–2392
Rungrojsakul M, Katekunlaphan T, Saiai A, Ampasavate C, Okonogi S, Sweeney CA, Anuchapreeda S (2016) Down-regulatory mechanism of mammea E/BB from Mammea siamensis seed extract on Wilms’ tumor 1 expression in K562 cells. BMC Complement Altern Med 16:130
Sakakibaru I, Terabayashi S, Jubo M, Hiquchi M, Komatsu Y, Okada M, Taki K, Kamei J (1999) Effect on locomotion of indole alkaloids from the hooks of Uncaria plants. Phytomed 6:163–168
Schmutterer H (1990) Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu Rev Entomol 35:271–297
Sharma J, Gairola S, Sharma YP, Gaur RD (2014) Ethnomedicinal plants used to treat skin diseases by Tharu community of district Udham Singh Nagar, Uttarakhand, India. J Ethnopharmacol 158:140–206
Shenoy K, Cunningham BT, Renfroe JW, Crowley PH (2009) Growth and survival of northern leopard frog (Rana pipiens) tadpoles exposed to two common pesticides. Environ Toxicol Chem 28:1467–1474
Shivanandappa T, Rajashekar Y (2014) Mode of action of plant-derived natural insecticides. In: Singh D (ed) Advances in plant biopesticides. Springer, New Delhi
Siddiqui BS, Rasheed M, Ilyas F, Gulzar T, Tariq RM, Nagvi SN (2004) Analysis of insecticidal Azadirachta indica A. Juss. fractions. Z Naturforsch C 59:104–112
Siegwart M, Graillot B, Lopez CB, Besse S, Bardin M, Nicot PC, Lopez-Ferber M (2015) Resistance to bio-insecticides or how to enhance their sustainability: a review. Front Plant Sci 6:381
Sparling DW, Fellers GM, McConnell LL (2001) Pesticides and amphibian population declines in California, USA. Environ Toxicol Chem 20:1591–1595
Thapa BR, Walia A (2007) Liver function tests and their interpretation. Indian J Pediatr 74:663–671
Vasanth S, Gopal RH, Rao RH, Rao RB (1990) Plant antimalarial agents. Ind. J Sci Res 49:68–77
Wake DB (1991) Declining amphibian population. Sci 253:860
Wattanasirmkit K, Singh-asa P, Fagtongpan P (2003) Subchronic effect of Thai neem Azadirachta indica var. siamensis seed extract on liver and blood of Tiger frog Hoplobatrachus rugulosus Weighman. Proceeding of the 8th Biological Science Graduate Congress, National University of Singapore, p 53
Weber CI (1993) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organism. US Environmental Protection Agency Office of Water, Washington, DC
Wolansky MJ, Harrill JA (2008) Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol 30:55–78
Yu S, Wages MR, Cai Q, Maul JD, Cobb GP (2013) Lethal and sublethal effects of three insecticides on two developmental stages of Xenopus laevis and comparison with other amphibians. Environ Toxicol Chem 32:2056–2064
Acknowledgements
We would like to thank the Natural Product Research Unit, Department of Biology, Faculty of Science, Chiang Mai University, for providing the bioinsecticide.
Funding
This research was supported by Center of Excellence in Bioresources for Agriculture, Industry and Medicine, Faculty of Science, Chiang Mai University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was funded by Center of Excellence in Bioresources for Agriculture, Industry and Medicine, Faculty of Science, Chiang Mai University.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures encompassing the animals were conducted with strict adherence to guidelines and procedures reviewed and approved by the Institutional Animal Care and Use Committee of the Biology Department, Faculty of Science, Chiang Mai University, permission number Re. 005/07.
Rights and permissions
About this article
Cite this article
Saenphet, K., Saenphet, S., Intamong, J. et al. Acute toxicity and histopathological changes in livers of frog tadpoles (Hoplobatrachus rugulosus) exposed to bioinsecticides derived from Azadirachta indica A. Juss., Stemona curtisii Hook.F., and Mammea siamensis. Comp Clin Pathol 27, 939–946 (2018). https://doi.org/10.1007/s00580-018-2685-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2685-6