Abstract
Nutrient, proximate and phytochemical analyses of Mucuna pruriens leaves were done. Acute and sub-acute toxicity studies on the methanol extract were done. Eighteen rats dosed with the extract at 10, 100, 1000, 1600, 2900 and 5000 mg/kg body weights were used for acute toxicity test. For the sub-acute toxicity study, 24 rats randomly allotted into four groups (A–D) of six animals each were used. Graded doses (100, 200, 400 mg/kg) of the extract were administered orally to groups A, B and C, respectively, for 28 days. Group D was the untreated control. Body weight changes, gross and histopathological examinations were carried out on the lungs, liver, brain, heart, spleen, testes and kidneys. The dried leaves contained 29.77% crude protein, vitamins A, C, E, B6, B9, B12, iron, copper and zinc. Anti-nutrients found were phenols, hydrogen cyanide, tannins, oxalate, saponin and anthocyanin. Alkaloids, terpenes and flavonoids were detected. The LD50 was >5000 mg/kg. Cytotoxic oedema of neurons, myocardial haemorrhages with hypertrophy of cells, perihepatic fibrinous exudation with haemorrhages and necrosis in liver, inflammation and hyperplasia of lungs, reduced spermatids and glomerulonephritis with hypercellularity of glomerulus were observed on histopathology. Leaves of M. pruriens, though nutritionally rich, contain potentially toxic principles and anti-nutrients which may have been responsible for widespread signs of toxicity in the brain, lungs, heart, testes, liver and kidneys of rats dosed at 400 mg/kg body weight of the extract for 28 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucuna pruriens L. (Fabaceae) is a wild tropical legume native to Africa and Asia (Duke 1981). It is commonly called ‘velvet bean’, ‘cowhage’ or ‘cowitch’. In the local dialect at Nsukka, South-Eastern Nigeria, it is called ‘Egbara’. The plant is notorious for the extreme itchiness it produces on contact (Andersen et al. 2015). The itch is caused by mucunain and serotonin (Reddy et al. 2008) contained in the hairs lining the seed pods. The seed is a good source of dietary protein (Pugalenthi et al. 2005). Its protein content is comparable to those of other pulses such as soybean, rice bean and lima bean (Gurumoorthi et al. 2003).
Seeds of M. pruriens have laxative, anthelmintic, alexipharmic and tonic effects (Taylor 2005). Its l-dopa content is also responsible for the efficacy of the seeds in the management of Parkinson’s disease (Manyam et al. 2004). The toxicity of the unprocessed bean may be why it has low susceptibility to insect pests (Duke 1981).
The dried crushed root is applied for relief of toothaches (Hishika et al. 1981). The leaves possess aphrodisiac and anti-microbial activities (Warrier et al. 1996) and are used in the management of ulcers, cephalgia and general debility. At Nsukka, South-Eastern Nigeria, leaves of M. pruriens are considered excellent blood boosters. The fresh leaves are washed and macerated in water to make a decoction which is drunk to boost blood supply. People suffering from debilitating disease conditions, acute blood loss and blood deficiency diseases consume leaves of M. pruriens (Obadoni and Ochuko 2001; Akindele and Busayo 2011).
Toxicity studies of medicinal plants are essential for the proper guidance of the populace, especially users of natural products (Agbaje et al. 2009). Some studies on the seed and whole plant have been done (Akindele and Busayo 2011; Chukwudi et al. 2011; Rafeeq et al. 2013). We studied the effects of daily administration of the methanol extract of M. pruriens for 28 days on some selected organs of rats as well as the nutrient, proximate and preliminary phytochemical analyses of the dried leaves obtained from South-Eastern, Nigeria.
Materials and methods
Plant collection and identification
Fresh plants of M. pruriens were collected during the dry season from the suburbs of Nsukka. They were identified by a botanist (Mr A. O. Ozioko of International Centre for Ethnomedicine and Drug Development, Nsukka). Herbarium samples were deposited (INTERCEDD/1569). The leaves were air dried and milled into fine powder then weighed and stored in an air-tight container at room temperature.
Nutritional, phytochemical and proximate composition of leaves
Plant material (100 g) was analysed for its nutritional, phytochemical and proximate composition as described by Nweze et al. (2016).
Extraction of plant materials
Extraction was done by cold maceration of the plant materials (212.5 g) in 2.5 L of HPLC grade absolute methanol (Sigma-Aldrich) for 72 h with intermittent shaking. The mixture was then filtered using Whatman’s filter paper no 1. The filtrate was allowed to evaporate at room temperature until dry. The extract was weighed and stored in a refrigerator. Percentage yield of the extract was calculated using the following formula:
Experimental animals
A total of 42 albino rats of mixed sexes were used for both the acute and sub-acute toxicity studies. Care of laboratory animals was as stated by Nweze et al. (2012). Briefly, the rats were housed in metal cages, and there was an acclimatization period of 1 week. Feeding using commercial feeds and provision of water were ad libitum. The guidelines contained in the Guide for the Care and Use of Laboratory Animals (DHHS, NIH Publication No. 85-23, 1985) were adhered to during the course of the experiment.
Acute toxicity test
Acute toxicity test was done as described by Nweze et al. (2016). Briefly, 18 rats allotted into six groups (1–6) of three animals each were dosed orally with 10, 100, 1000, 1600, 2900 and 5000 mg/kg body weight of the extract, respectively. The rats were observed for signs of toxicity or mortality for 24 h.
Sub-acute toxicity test
The procedure for sub-acute toxicity test adopted was according to the guidelines of the Organization for Economic Co-operation and Development (OECD 2008) and similar to that reported by other workers (Nweze et al. 2012). Twenty-four rats were randomly allocated into four groups (A–D) of six animals each. Varied doses (100, 200, 400 mg/kg body weight) of the extract were administered orally to groups A, B and C, respectively, for 28 days. Group D rats received equal volumes of distilled water. Body weights were recorded before dosing and weekly thereafter using an electronic weighing balance.
Necropsy
Necropsy procedures as described by Nweze et al. (2012) was carried out by day 29 of experiment after euthanasia of rats. Selected organs (brain, lungs, heart, liver, testes, spleen and kidneys) were examined both grossly and histopathologically.
Data analysis
Data are presented as mean ± standard error of mean. Data were analysed using one-way analysis of variance to determine significant differences between means. Variant means were considered significant at p < 0.05.
Results
The yield of extract was 5.7% while its colour was dark green. The results of the nutrient, phytochemical and proximate analyses of Iresine herbstii leaf are shown in Table 1. The dried leaves contained 29.77% crude protein, 6.40% ash, 16% moisture, 1.03% ether extract and 10.40% crude fibre. Vitamins A (1663.865 IU) C (9.075%) E (18.8 mg) B6 (0.284 mg) B9 (5 mg) B12 (0.0186 mg) iron (0.174%) copper (0.8 mg) and zinc (3200 mg) were present. Some anti-nutrients like phenol (8.409%), hydrogen cyanide (0.675%), tannin (0.124%), oxalate (0.168%), saponin (1%) and anthocyanin (0.5%) were found. Compounds like alkaloids (4%) terpenes (4%) and flavonoids (2.5%) were detected.
The result of the acute toxicity test is shown in Table 2. There was no mortality recorded in all the groups. The LD50 was calculated to be >5000 mg/kg.
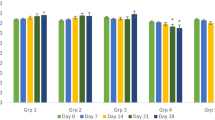
The result of mean group body weight changes is shown in Table 3. No significant differences were observed on days 0, 7, 14 and 28 in all the experimental groups. On day 21, mean group weight changes were significantly (p < 0.05) higher in groups A and C (100 and 400 mg/kg-treated) when compared with those of groups B and D (200 mg/kg-treated and untreated).
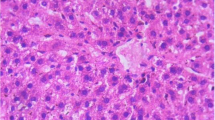
Results of the histopathological examinations are shown on Figs. 1, 2, 3, 4, 5, 6, and 7. Histopathological abnormalities of all organs examined, except the spleen, were found in the group fed with 400 mg/kg body weight of the extract. Lesions observed were cytotoxic oedema of degenerating brain neurons, myocardial haemorrhages, hypertrophy, degeneration and necrosis of myocytes, necrosis of epithelial cells of the bile duct, peri hepatic fibrinous exudation and haemorrhages, hyperplasia of the perivascular lymphoid tissue of the lungs with mononuclear cells infiltration of the interlobular septae, reduced spermatogenic activities in testes and glomerulonephritis of kidneys characterized by hypercellularity of the glomerulus and focal areas of haemorrhage.
Discussion
Results of the proximate and nutrient analyses show that leaves of M. pruriens are potentially nutritious. The crude protein content of the leaves (29%) is high and similar to that reported for the seed (27–31%) by Ngatchic et al. (2013). The leaves also contained some essential vitamins like B6, B9 and B12. Other beneficial vitamins found were A, C and E. Important trace elements such as iron, copper and zinc required for protein synthesis and maintenance of a healthy immune system were present. A lower concentration of iron (0.0186 mg) than that reported by Akindele and Busayo (2011) was found. The difference in concentration could be due to soil and climatic variations. Feeding of rats with the extract had no deleterious effects on the spleen (Fig. 7) which may suggest that erythropoiesis was not adversely affected.
Some anti-nutritional factors such as polyphenols, hydrogen cyanide, tannins, oxalates and saponins were found in leaves of M. pruriens. This result agrees with that of other workers (Awang et al. 1997). These factors contribute to an overall decrease in nutritional quality. Phenols hinder digestion by binding to enzymes. l-Dopa is the major phenolic constituent of M. pruriens (Misra and Wagner 2004). High creatinine levels, significant weight loss, decreased PCV, Hb and RBC counts observed in rats fed raw mucuna flour were associated with tannins, polyphenols and trypsin inhibitors contained in mucuna seed (Ngatchic et al. 2013). These anti-nutritional factors may have been responsible for all the other signs of toxicity observed in the brain, lungs, heart, testes, liver and kidneys of rats in this study.
Hydrogen cyanide causes both acute and chronic toxicity in man and animals. The concentration of it in plants considered dangerous is ≥200 ppm. The concentration of it found in M. pruriens leaves in this study (0. 675%) is, however, below the lethal level. This result agrees with that of other workers who found low levels (<50 μg/g) of cyanide in both mature and immature leaves of Mucuna curranii in the Philippines (Laurena et al. 1994).
Oxalates can bind with dietary calcium (Ca) or magnesium (Mg) to form insoluble Ca or Mg oxalate, which then may lead to low serum Ca or Mg levels as well as to renal failure because of precipitation of these salts in the kidneys (Rahman et al. 2013). Unlike hydrogen cyanide, non-ruminants have higher risks of oxalate poisoning (Braide and Anika 2007) because of the absence of rumen microflora that help in the breakdown of oxalic acid. The level of oxalate in M. pruriens (0.168%) is well below those of plants considered to be extreme oxalate accumulators that contain >5% oxalate by dry weight (Libert and Franceschi 1987).
Cytotoxic oedema observed in brain cells is usually due to transfer of fluid from the extracellular to intracellular spaces and, if not reversed, leads to cell death. Haemorrhages, hypertrophy, degeneration and necrosis seen in myocytes will cause heart problems in the long run. The liver showed signs of toxicity such as necrosis, fibrinous exudation and hyperplasia of lymphoid tissues. These lesions will undoubtedly precipitate impairment of liver functions as was found by Chukwudi et al. (2011) who stated that treatment with M. pruriens significantly reduced the levels of liver function enzymes in rats.
Infiltration of mononuclear cells into the lungs is a sign of inflammation (pneumonia). Depletion of spermatids in the testes will result in infertility if not reversed. Kidneys were characterized by focal areas of haemorrhage and hypercellularity which is indicative of glomerulonephritis. Glomerulonephritis, if not reversed, will ultimately lead to loss of kidney function and death. This report of glomerular hypercellularity agrees with the glomerular sclerosis reported by Ngatchic et al. (2013) after feeding rats with raw mucuna seed flour.
It is interesting to note that despite the fact that the acute toxicity result gave no indications of toxicity, toxicity was nonetheless present in organs examined. This underlines the importance of both sub-acute and chronic toxicity studies in validating the safety of natural products. Going by the results obtained in this study, prolonged consumption of raw M. pruriens leaf decoctions by the natives may be fraught with danger. It would be advisable to subject the leaf decoctions to some form of heat processing before consumption.
Leaves of M. pruriens, though nutritionally rich, contain potentially toxic principles and anti-nutrients which may have been responsible for widespread signs of toxicity observed in the brain, lungs, heart, testes, liver and kidneys of rats dosed at 400 mg/kg body weight of the extract for 28 days.
References
Agbaje EO, Adeneye AA, Daramola AO (2009) Biochemical and toxicological studies of aqueous extract of Syzigium aromaticum (L.) Merr. & Perry Myrtaceae in rodents. Afr J Trad CAM 6(3):241–254
Akindele AJ, Busayo FI (2011) Effects of the hydroethanolic extract of Mucuna pruriens (L.) DC (Fabaceae) on haematological profile in normal and haloperidol treated rats. Nig Q J Hosp Med 21(2):93–98
Andersen HH, Elberling JP, Arendt-Nielsen L (2015) Human surrogate models of histaminergic and non-histaminergic itch. Acta Derm Venereol. doi:10.2340/00015555-2146
Awang D, Buckles D, Arnason JT (1997) Chapeco, Catarina, Brazil, Santa Catarina, Brazil: paper presented at the International Workshop on Green Manure—Cover Crop Systems for Smallholders in Tropical and Subtropical Regions 6–12 Apr, Rural Extension and Agricultural Research Institute of Santa Catarina; The phytochemistry, toxicology and processing potential of the covercrop velvetbean (cow(h)age, cowitch) (Mucuna Adans. spp, Fabaceae)
Braide VB, Anika SM (2007) Environmental toxicology. Snaap press Ltd., Enugu, pp 31–60
Chukwudi NH, Simeon O, Chinyere AJ (2011) Analysis of some biochemical and haematological parameters for Mucuna pruriens (DC) seed powder in male rats. Pak J Pharm Sci 24(4):523–526
Duke JA (1981) Handbook of legumes of world economic importance. Plenum Press, New York
Gurumoorthi P, Pugalenthi M, Janardhanan K (2003) Nutritional potential of five accessions of a South Indian tribal pulse Mucuna pruriens var. utilis; II investigation on total free phenolics, tannins, trypsin and chymotrypsin inhibitors, phytohaemagglutinins, and in vitro protein digestibility. Trop Subtrop Agroecosys 1:153–158
Hishika R, Shastry S, Shinde S, Guptal SS (1981) Preliminary phytochemical and anti-inflammatory activity of seeds of Mucuna pruriens. Indian J pharmacol 13(1):97–98
Laurena AC, Revilleza MJR, Mendoza EMT (1994) Polyphenols, phytate, cyanogenic glycosides and trypsin inhibitor activity of several Philippine indigenous food legumes. J Food Comp Analysis 7(3):194–202
Libert B, Franceschi VR (1987) Oxalate in plants. J Agric Food Chem 35:926–938
Manyam BV, Dhanasekaran M, Hare TA (2004) Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res 18:706–712
Misra L, Wagner H (2004) Alkaloidal constituents of Mucuna pruriens seeds. Phytochemistry 65:2565–2567
Ngatchic JT, Sokeng SD, Njintang NY, Maoundombaye T, Oben J, Mbofung CM (2013) Evaluation of some selected blood parameters and histopathology of liver and kidney of rats fed protein-substituted mucuna flour and derived protein rich product. Food Chem Toxicol 57:46–53
Nweze NE, Anene BM, Asuzu IU, Ezema WS (2012) Subacute toxicity study of the methanolic seed extract of Buchholzia coriacea (Capparaceae) in rats. Comp Clin Pathol 21:967–974
Nweze NE, Nwachukwu KA, Adieme IC (2016) The effect of Iresine herbstii Hook on some haematological parameters of experimentally induced anaemic rats. Comp Clin Pathol 25:797–803
Obadoni BO, Ochuko PO (2001) Phytochemical studies and comparative efficacy of the crude extract of some haemostatic plants in Edo and Delta states of Nigeria. Global J Pure and Applied Science 8:203–208
OECD (2008) The Organization for Economic Co-operation and development (OECD) guideline for testing of chemical: 407 repeated dose 28-day oral toxicity study in rodents. OECD, Paris, pp 1–13
Pugalenthi M, Vadivel V, Siddhuraju P (2005) Alternative food/feed perspectives of an under-utilized legume Mucuna pruriens var. utilis—a review. Linn J Plant Foods Human Nutr 60:201–218
Rafeeq AK, Muhammad A, Bushra S, Mansoor A (2013) Acute and subchronic toxicity of Mucuna pruriens, Cinnamomum zeylanicum, Myristica fragrans and their effects on haematological parameters. Aust J Basic Appl Sci 7(8):641–647
Rahman MM, Abdullah RB, Wan Khadijah WE (2013) A review of oxalate poisoning in domestic animals: tolerance and performance aspects. J Anim Physiol Anim Nutr (Berl) 97:605–6144
Reddy VB, Luga AO, Shimada SG, LaMotte RH, Lerner EA (2008) Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 28:4331–4335
Taylor L (2005) The healing power of rainforest herbs: a guide to understanding and using herbal medicinal. Square One Publishers, Garden City Park, New York
Warrier PK, Nambiar VPK, Mankutty C (1996) Indian medicinal plants. Orient Longman, Chennai
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No fund was received from any external body for the conduct of this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Nweze, N.E., Ezema, C., Ezema, A.S. et al. Toxicity and nutritional studies on Mucuna pruriens leaves from Nsukka, South-Eastern Nigeria. Comp Clin Pathol 26, 569–574 (2017). https://doi.org/10.1007/s00580-017-2412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2412-8