Abstract
In this study, we investigated the phytochemical and toxicological impacts of the ethanol leaf extract of Prosopis africana in albino rats. Preliminary phytochemical analysis was done using established methods. Acute and 28-day sub-chronic toxicity evaluations of the extract were carried out in wistar rats. Five groups made up of 8 rats per group were used and treated with 500, 1000, 1500 and 2000 mg/kg b.wt. respectively; while the control group received 1 ml of 10% Tween-20 solution. Phytochemicals detected include tannins, saponins, flavonoids, alkaloids, anthocyanin, quinones, terpenoids and steroids. There were no signs of toxicity in the acute toxicity study neither was any death recorded. Weight loss and death were recorded after 18 days of treatment in groups treated with 1500 and 2000 mg/kg b.wt. No significant alteration of haematological parameters was observed, however increased WBC count was recorded. Liver and kidney function parameters were significantly (p < 0.05) reduced, while significant (p < 0.05) increase in triglycerides with lowered cholesterol was seen when compared with the control group. Histological evaluations of the liver, kidney, heart and testicular tissues revealed normal sections in majority of the animals; however, mild vascular congestions were observed at random doses (liver and heart at 1500 mg/kg b.wt.; kidney at 500 mg/kg b.wt.). Our study shows that ethanol leaf extract of P. africana would probably not cause significant toxic effects as indicated by haematological and biochemical parameters. However, there may be need to apply caution in its use at high doses given that death occurred at 1500 mg/kg b.wt. during the repeat-dose toxicity study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal plants are plants utilized in herbal medicine to manage ailments and diseases due to the presence of bioactive compounds reported to be responsible for eliciting such therapeutic effects [1]. Various parts of plants including the root, stem, bark, leaf, whole plant part etc. are used and since the history of man, medicinal plants have been used to maintain healthy state [2]. Medicinal plants have been reported to modulate a number of pathways implicated in disease onset and progression, as such, biological activities such as antidiabetic, antihypertensive, antioxidant, anticancer, anti-helminthic, analgesic properties, etc. have been of benefit to mankind. Prosopis africana (Guill. and Perr.). Taub is a continuously growing plant of the family; Fabaceae and it is indigenous to the African continent. The genus Prosopis comprises about 44 species, out of which 40 are indigenous to Northern and Southern Americas, three (3) are native to Asia and the remaining one (P. africana) from Africa [3]. Its common names include African mesquite as well as “Iron tree”. In Nigeria, the plant is known with various names such as “kiriya” in Hausa, “ubwo” in Igbo, “ayan” in Yoruba and “sanchi” in Nupe [4] and numerous medicinal uses even in other African countries [5]. Apart from their medicinal uses, Prosopis species have been used as food condiment, wood, charcoal, gum etc. [6]. In Nigeria, the seeds are important for the production of a food condiment called “ukpehe” (a high protein and fatty acid rich fermentation product) [7]. The roots are also used in making chewing sticks for prevention of tooth decay, while the hard wood is used to produce furniture, wooden farm implements, charcoal as well as mortar and pestle [8]. Ethnobotanical surveys indicate that in Nigeria, Ghana as well as Mali, the leaf part is employed in the treatment of migraines, hypertension, dysentery as well as rheumatism [9,10,11]. Rwang et al. [12] confirmed the presence of saponins, terpenes, tannins, steroids, anthraquinones and cardiac glycosides in the leaves of P. africana [12]. Biological activities reported for P. africana include analgesic, anti-inflammatory, as well as antibacterial [5, 9]. To the best of our understanding, there is a shortage of information on its safety hence the need to provide such useful information. This study therefore evaluates the phytochemical constituents as well as the impacts of the ethanol leaf extract of P. africana on haematological, biochemical and histopathological profiles in wistar rats, as indicators of toxicity.
Materials and methods
Study plant
Leaf parts of P. africana (PA) were harvested in November, 2018 from University of Ilorin, Ilorin, Kwara State through Forestry Research Institute of Nigeria (FRIN), Ibadan, Oyo State, Nigeria, and were identified and authenticated by Mr. K.A. Adeniji at FRIN and a sample was banked at the institution’s herbarium with voucher reference number FHI.112370. Leaves were lightly cleaned and air-dried for four weeks and thereafter, milled into powder with the aid of a laboratory blender.
Plant extraction and phytochemical screening
A procedure explained by Adebayo et al. [13] was followed, howbeit with some adjustments. Milled leaves of P. africana (2000 g) were steeped in 15L of 96% v/v ethanol for 72 h; thereafter filtration was done using cheese cloth and cotton wool. The extraction was done 3 times before the combined extract was concentrated using a rotary evaporator (50 °C) under reduced pressure. The percentage yield of 27.53% (550.6 g) was obtained. The presence of phytochemicals such as tannins, glycosides, alkaloids, quinones, flavonoids, saponins, terpenoids, etc., was determined in the ethanol extract using standard methods of phytochemical analysis as described by Harborne [14].
Experimental animal model
Male albino rats (Wistar strain) weighing between 163 and 172 g were purchased from the Physiology Department’s animal house, Lagos University Teaching Hospital Idi-araba, Lagos, Nigeria. They were kept at room temperature conditions (23–25 °C) and 12 h day and 12 h night circle in the Department of Biochemistry’s animal house, Covenant University, Ota, Ogun State, Nigeria. They were acclimatized for a period of 2 weeks before the experimental period and were granted access to water and feed (starter feed) ad libitum. Animals were handled according to the guidelines of National Institute of Health with ethical clearance from the Covenant Health Research Ethics Committee.
Acute toxicity
Evaluation of the lethal dose (LD50) was carried out as illustrated by Lorke [15]. The method entails two phases; in the first phase, rats were categorized into 3 consisting of 3 rats per group. Each rat was treated orally with the ethanol extract of P. africana (ELPA) at doses of 10, 100 and 1000 mg/kg b.wt. respectively. In the second stage, another set of rats (3 rats per group) were treated orally with 2000, 3000 and 5000 mg/kg b.wt. of ELPA. The rats were carefully observed for 48 h and a period of 1 week for signs of toxicity as well as mortality.
Experimental design
A total of 40 albino male rats were utilised for this study. They were categorized into five consisting of eight rats each. The crude ethanol extract was dispersed in Tween-20 and distilled water in a ratio of 1:4 prior to treatment and groups 1, 2, 3, 4 and 5 were utilized for the subchronic studies. Group 1 functioned as control and was given 0.5 ml of 10% Tween-20 solution, while Groups 2, 3, 4 and 5 were given 500, 1000, 1500 and 2000 mg/kg b.wt. of ELPA for 28 days through gatric intubation. Animals were weighed weekly during the treatment period and they were fasted overnight prior to the day of sacrifice. Animals were sacrificed by cervical dislocation and blood was collected through the orbital vein into ethylenediaminetetraacetic acid (EDTA) and plain tubes. Samples in plain tubes were centrifuged at 3000 × g for 10 min in order to obtain the serum that was used for biochemical analysis; while the whole blood obtained from the EDTA bottles was used for haematological analyses [13].
Biochemical assays
Biochemical assays such as alanine aminotransferase, alkaline phosphatase, glucose, aspartate aminotransferase, creatinine, urea, albumin, cholesterol, triglycerides, bilirubin, high density lipoprotein and total protein were analysed on the serum of experimental rats using an automated clinical chemistry analyser (Roche/Hitachi Codas systems, Model Cobas C311 India).
Haematological evaluations
Haematological parameters including red blood cell count, haemoglobin, packed cell volume and detailed white blood cell differentials were analysed on whole blood of experimental rats using an automated analyser (Mindray Haematology Analyser, BC 3200, China).
Histopathological evaluations
The rats’ organs (kidney, liver, heart and testis) were excised, cleaned and fixed for 72 h in formalin (40%) and thereafter sliced thinly and shrivelled using graded concentrations of alcohol. They were then treated with paraffin and cast into blocks, sectioned on a microtome to 5 μm, attached to a slide and allowed to dry. The sample slides were subsequently stained in haematoxylin–eosin dye and examined under a light microscope [16].
Data analysis
Variations between/within means of the experimental and control groups were evaluated using statistical package for social science (SPSS) for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA). Mean differences between various groups were done using analysis of variance (ANOVA) with significant differences assessed by DUNCAN multiple range test. Data were presented as mean ± SEM, with the level of significance taken as p < 0.05.
Results
Qualitative phytochemical evaluation of ELPA
Phytochemical evaluations as shown in Table 1 indicated that ELPA contains saponins, flavonoids, tannins, alkaloids, quinones, anthocyanin, steroids and terpenoids.
Acute toxicity study
No death was recorded in rats administered up to 5000 mg/kg b.wt. of ELPA within the short-term (48 h) and long-term (1 week) outcome of the single dose test as shown in Table 2. There were no observable changes in behaviour neither were toxicity signs seen. The LD50 value was thus estimated to be higher than 5000 mg/kg b.wt. via oral passage.
Repeated dose toxicity study (28 days)
Effect of ELPA on body weight and behaviour/toxicity signs of rats
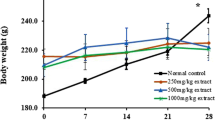
Consumption of food and water by the experimental animals in all treatment groups did not differ however, changes were seen in the body weights and behaviour of the rats. At the end of Week 2, there were no visible signs of toxicity or possible harm, however on Day 18, weight loss was observed in 50% of the rats in the group administered 2000 mg/kg b.wt. with 1 death recorded. On Day 22, 1 death each was recorded for the groups administered 1500 and 2000 mg/kg b.wt. Wet nose was also observed in rats that lost weight. At the end of week 4, rat groups administered 500 and 1000 mg/kg b.wt. were active and looked healthy however, rats treated with 1500 and 2000 mg/kg b.wt. were weak and clustered together with loss of weight. Figure 1 and Table 3 show the changes in body weight and organ/relative organ weights respectively of experimental rats for the treatment period. No significant (p > 0.05) changes were observed in the weight of all organs tested (liver, kidney, heart and testis) however, liver weight was significantly (p < 0.05) elevated in rats treated with 1500 mg/kg b.wt.
Changes in body weight of rats treated with ELPA. Values are presented as mean ± SEM with 5 replicates; values marked with (*) are significantly different at p < 0.05 as compared with Control (Grp 1). Grp 1, Grp 2, Grp 3, Grp 4 and Grp 5 represent groups of rats treated with 10% Tween-20 solution (0.5 ml), 500, 1000, 1500 and 2000 mg/kg b.wt. ELPA respectively
Effect of ELPA on haematological parameters of rats
Haematological evaluations show that there was no major alteration in the levels of haemoglobin (HGB), packed cell volume (PCV) and red blood count (RBC) compared with the control. There was however, significant increase (p < 0.05) in white blood count (WBC) and other white blood cells (MID) for all the treatment groups compared with the control. Also, significant increase (p < 0.05) was observed in rats treated with 2000 mg/kg b.wt. as compared to the control group. No significant (p > 0.05) change was observed for mean platelet volume (MPV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), red cell distribution width (RDW), mean corpuscular volume (MCV), platelet distribution width (PDW) and plateletcrit (PCT) as shown in Table 4.
Effect of ELPA on liver function of rats
There was significant decrease in the activities of the enzymes aspartate aminotransferase and alanine aminotransferase in all treatment groups, whereas significant reduction in the activity of alkaline phosphatase was observed for animals treated with 1000 and 1500 mg/kg b.wt. ELPA. Bilirubin concentration reduced significantly (p < 0.05) in rats treated with 1500 and 2000 mg/kg b.wt., whereas there was no major alteration in albumin concentration for all treatment groups compared with the control (Fig. 2).
Effect of ELPA on liver functions of rats after 28-day treatment. Values are presented as mean ± SEM with 5 replicates; values marked with (*) are significantly different at p < 0.05 as compared with Control, while values marked with similar superscript alphabet are not significantly different from each other at p < 0.05
Effect of ELPA on kidney function of rats
There was significant decline in the creatinine concentration in all the treatment groups compared with the control. Significant (p < 0.05) reduction in urea was observed in the groups treated with 1000 and 2000 mg/kg b.wt. Also, there was significant (p < 0.05) decrease in total protein concentrations in rats treated with 1000 and 1500 mg/kg b.wt. (Fig. 3).
Effect of ELPA on lipid profile of rats
There was significant (p < 0.05) decrease in plasma concentration of cholesterol in all treatment groups; however, there was with significant (p < 0.05) decrease in high density lipoprotein cholesterol (HDL-cholesterol) concentration observed in the group treated with 500 mg/kg b.wt. However, significant (p < 0.05) rise in triglyceride concentration was seen in treatment groups 2 and 3 (500 and 1000 mg/kg b.wt.) compared with the control group. Low density lipoprotein cholesterol was not significantly affected (Fig. 4).
Effect of ELPA on lipid profile of rats after 28-day treatment. Values are presented as mean ± SEM with 5 replicates; values marked with (*) are significantly different at p < 0.05 as compared with Control while values marked with similar superscript alphabet are not significantly different from each other at p < 0.05
Effect of ELPA on histology of rat organs (liver, heart, kidney and testis)
The histological sections of the liver showing structure, portal vein, central vein while sections of heart showed fascicles of the heart muscle cells. No abnormalities were seen in both organs except for the group treated with 1500 mg/kg b.wt. in which mild vascular congestion was seen (Figs. 5, 6). The histological section of the kidney tissue showed normocellular glomerular tufts with vascular congestion observed in treatment Grp 2 (Fig. 7). Evaluation of the testes showed no abnormalities in all the treatment groups (Fig. 8).
Effect of ELPA on the histology of rat livers after 28-day treatment. a Grp 1 (control), b Grp 2 (500 mg/kg b.wt.), c Grp 3 (1000 mg/kg b.wt.), d Grp 4 (1500 mg/kg b.wt.) and e Grp 5 (2000 mg/kg b.wt.). NR and MVC represent normal histology and mild vascular congestion respectively. H and E × 100 of representative group organs
Effect of ELPA on the histology of rat hearts after 28-day treatment. a Grp 1 (control), b Grp 2 (500 mg/kg b.wt.), c Grp 3 (1000 mg/kg b.wt.), d Grp 4 (1500 mg/kg b.wt.) and e Grp 5 (2000 mg/kg b.wt.). NR and VC represent normal histology and vascular congestion respectively. H and E × 100 of representative group organs
Effect of ELPA on histology of rat kidneys after 28-day treatment. a Grp 1 (control), b Grp 2 (500 mg/kg b.wt.), c Grp 3 (1000 mg/kg b.wt.), d Grp 4 (1500 mg/kg b.wt.) and e Grp 5 (2000 mg/kg b.wt.). NR and VC represent normal histology and vascular congestion respectively. H and E × 100 of representative group organs
Discussion
Medicinal plants and products derived from them are of paramount importance in health management as they play a crucial role as alternative therapies for conventional drugs. Many persons living in developing nations including Nigeria, depend on medicinal plants (traditional medicine) for the management of various diseases and ailments. However, there is a major drawback to their use—potential toxicity. It has been reported that there are more than 5000 plants being used as medicines in Africa, with very few of them being studied [13]. This could be as a result of general belief that these plants are safe for consumption having been used for ages. However, this may not be the case as some studies have recorded some level of toxicity in organs of experimental animals treated with medicinal plant extracts perceived to be safe. Toxicity studies help to ascertain if there are possible lethal effects that could arise from consumption of such plants and also gives a guide on the dose to be consumed. Research work on P. africana is scanty, thus this study was conducted in order to evaluate the acute and subchronic toxicity of ethanol extract (ELPA) of the plant.
Preliminary phytochemical analyses of ELPA revealed that tannins, alkaloids, saponins, flavonoids, anthocyanins, terpenoids, quinones and steroids were present. This is similar to the reports of Atawodi and Ogunbusola on the methanol extracts of P. africana [17]. There are biochemical, haematological as well as histological indices for assessing the toxicity of plant extracts in animal models and the parameters evaluated in this study are important indicators of toxicity evaluation.
In this study, no death was recorded in rats used for the acute toxicity evaluations up to 5000 mg/kg b.wt. of ELPA. In another study, lethal dose (LD50) of the methanol extract of the stem bark of the same plant was > 5000 mg/kg b.wt. [9]. The outcome of this study suggests that the ethanol leaf extract of P. africana may not exert toxic effects when administered via gastric intubation in the short term. This outcome however may not hold for the repeated dose toxicity study. In the repeat dose study of ELPA, death occurred and weight loss was observed in the third and fourth weeks in the groups administered 1500 and 2000 mg/kg b.wt. This could mean that at higher doses, ELPA may exert toxic effects. No substantial changes were observed in the weight of all organs tested (liver, kidney, heart and testis); however, there was significant increase in the liver weight of rats treated with 1500 mg/kg b.wt. The liver is the major organ dedicated to xenobiotic metabolism and as such, is faced with possible harm from toxic materials. The observed increase in liver weight of rats treated with 1500 mg/kg b.wt. could be random adaptive response of the animals to the extract, given that at the higher dose of 2000 mg/kg b.wt., there was no significant increase in liver weight.
The blood is of paramount importance in evaluating the effect of substances or xenobiotics on animals. Haematological parameters such as packed cell volume, red blood cell count, white blood cell count, packed cell volume, etc., are used to evaluate the degree of harmful effects or beneficial functions of plant extracts [13]. Results from the haematological evaluations revealed that there was no major alteration in the levels of haemoglobin, packed cell volume and red blood count compared with the control. This suggests that there was no difference in the rate of production and degradation of erythrocytes. This result is comparable to an earlier study by Robertson et al., in which there was no major change in these parameters [18]. The significant increase observed in the white blood count and platelets levels could indicate anti-inflammatory response of the animals to the extract. Increased mobilization of leucocytes may be correlated to the extent of assault experienced by the animal [19]. This observed effect of the extract at 2000 mg/kg b.wt. could infer potential toxicity of ELPA at higher doses given that death occurred at this dose. The works of Robertson et al., on the toxicity of Prosopis cineraria showed no significant alterations in these parameters up to 1000 mg/kg b.wt. [18].
Significant reduction was observed in the liver function parameters across all treatment groups (AST, ALP and ALT), with no significant change in total protein concentration. Elevated liver function parameters is an indication of hepatic injury [20]. Under normal physiological conditions, tissue specific enzymes such as ALT, AST, ALP are localized in the respective tissues (in this case-liver), however, during tissue damage, these enzymes leak into the blood, thus elevating their concentrations in blood and suggesting tissue damage. This result is corroborated by the observed significant reduction in plasma bilirubin concentration in rats treated with higher doses of ELPA (1500 and 2000 mg/kg b.wt.). The lower serum activities of the liver function parameters observed in this study as compared to the control could suggest that ELPA may possess hepatoprotective abilities or “non-adverse” effects. This is comparable to the reports of Adebayo et al. in which extracts of Chrysophyllum albidum conferred hepatoprotection [13]. The observed reduction in liver function parameters in the rats when compared with the control however, is not consistent with the earlier observed toxic potentials of the extract, thus it could be asserted that the induction of liver xenobiotic metabolism by the extract may not produce adverse effects but adaptive responses. Creatinine is a major catabolite of muscle breakdown and its excessive presence in the blood is an indication of impaired kidney function. In this study, there was significant decrease in the creatinine concentration across the groups in comparison with the control. The extract may not induce nephrotoxicity. Significant reduction in urea concentration was observed in the groups administered 1000 and 2000 mg/kg b.wt., whereas albumin concentration was not significantly affected. Urea is the major nitrogenous product of protein metabolism and it is usually excreted in the urine. Elevated concentrations of urea in the blood suggests that the kidney is not functioning adequately or possible blockage of the urinary tract. Higher doses of the extract in our study reduced blood urea concentration significantly which could suggest enhanced kidney function. Lack of significant alteration of albumin concentration in this study corroborates the beneficial effects observed. Albumin is the main protein produced by the liver and it helps to maintain the integrity of the vasculature, thus preventing fluid from leaking into other tissues.
This study also recorded significant decrease in concentration of total cholesterol across all treatment groups. Elevated plasma cholesterol levels have been implicated in cardiovascular diseases [21]. Thus, the observed reduction in cholesterol by P. africana extract, may be beneficial to the management of cardiovascular diseases. Also, significant reduction in HDL-cholesterol concentration was observed in rats treated with 500 mg/kg b.wt. however, at higher doses, there was no significant change in HDL-cholesterol concentration when compared with the control group. HDL-cholesterol has been reported to prevent cardiovascular diseases through enhanced mobilisation of triglycerides and cholesterol to the liver for catabolism as well as elimination [22]. Elevated triglyceride concentration was also observed at lower doses (500 and 1000 mg/kg b.wt.), whereas there was no significant change in triglycerides concentration at higher doses of 1500 and 2000 mg/kg b.wt., when compared with the control group. Higher triglyceride levels have been ascribed to elevated quantities of small LDL-cholesterol atherogenic particles [22]. LDL-cholesterol levels in this study was not significantly altered when compared with the control group. This is different from the results obtained by Yakubu et al. in which ethanol leaf extracts of Ricinodendron heudelotii induced elevated blood cholesterol as well as high density lipoprotein cholesterol concentrations [21]. Our result suggests that P. africana may confer cardiovascular benefits when used at the right dosage. This is similar to the reports of Omodamiro and Nwankwo, who reported reduced LDL-cholesterol in rats treated with extracts of Voacanga africana [23]. The observed modulatory properties of P. africana on lipid profile in this study, may be attributed to its phytochemical composition. Phytochemicals such as flavonoids have been reported to improve cardiovascular health due to their antioxidant and anti-inflammatory properties [24].
The histological sections of the liver showed structure, portal vein, central vein while sections of heart showed fascicles of the heart muscle cells. No abnormalities were seen in both organs except for the group treated with 1500 mg/kg b.wt. in which mild vascular congestion was seen. Histopathological assessments could readily define hypertrophy histologically, however, the interpretations could vary from increased organ weight to increased cells or enzyme induction [25]. The histological section of the kidney tissues showed normocellular glomerular tufts with vascular congestion observed in the group administered 500 mg/kg b.wt. Results from the liver and kidney functions parameters did not indicate any significant toxic effects of the extract. There may be need for further studies on the effect of P. africana extract on hepatotoxicity as well as nephrotoxicity. Evaluation of the testes showed no abnormalities in all treatment groups.
The ethanol leaf extract of P. africana may not exert toxic effects when administered gastric intubation in the short term. The extract may not cause major toxic effects as indicated by haematological and biochemical parameters from our study. There is the possibility of the extract conferring hepatoprotection (this assertion could be verified in a further study). The extract also exerted hypolipidemic properties and as such may be a promising lead for the management of cardiovascular diseases. However, there may be need to apply caution in its use at higher doses given that death occurred at 1500 mg/kg b.wt. during the repeated-dose toxicity study.
References
Singh R (2015) Medicinal plants: a review. J Plant Sci 3:50–55
Azmir J, Zaidul IS, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MH, Ghafoor K, Norulaini NA, Omar AK (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117:426–436
Henciya S, Seturaman P, James AR, Tsai YH, Nikam R, Wu YC, Dahms HU, Chang FR (2017) Biopharmaceutical potentials of Prosopis spp (Mimosaceae, Leguminosa). J Food Drug Anal 25:187–196
Dosumu OO, Oluwaniyi OO, Awolola GV, Oyedeji OO (2012) Nutritional composition and antimicrobial properties of three Nigerian condiments. Niger Food J 30:43–52
Ezike AC, Akah PA, Okoli CO, Udegbunam S, Okwume N, Okeke C, Iloani O (2010) Medicinal plants used in wound care: a study of Prosopis africana (Fabaceae) stem bark. Indian J Pharm Sci 72:334–339
Mwangi E, Swallow B (2008) Prosopis juliflora invasion and rural livelihoods in the Lake Baringo area of Kenya. Conservat Soc 6:130–140
Sanni AI, Lie E, Lindberg AM (1993) Fatty acid composition of Prosopis africana and its fermented product okpehe. Chem Mikrobiol Technol Lebensm 15:89–90
Agboola DA (2004) Prosopis africana (Mimosaceae): stem, roots, and seeds in the economy of the savanna areas of Nigeria. Econ Bot 58:S34–S42
Ayanwuyi LO, Yaro AH, Abodunde OM (2010) Analgesic and anti-inflammatory effects of the methanol stem bark extract of Prosopis africana. Pharm Biol 1 48:296–299
Raji NO, Adebisi IM, Bello SO (2013) Ethnobotanical survey of antihypertensive agents in Sokoto, Northwest Nigeria. Int J Innov Res Dev 2:1820–1835
Bekoe EO, Kretchy IA, Sarkodie JA, Okraku A, Sasu C, Adjei D, Twumasi M (2017) Ethnomedicinal survey of plants used for the management of hypertension sold in the makola market, Accra, Ghana. Eur J Med Plants 19:1–9
Rwang PG, Fabiyi JP, Suleiman M, Mercy KP (2016) Evaluation and phytochemical analysis of Prosopis africana and Erythrina senegalensis used against immature stages of Schistosoma haematobium. Eur J Med Plants 7:1–9
Adebayo AH, Zeng GZ, Fan JT, Ji CJ, He WJ, Xu JJ, Zhang YM, Akindahunsi AA, Kela R, Tan NH (2010) Biochemical, haematological and histopathological studies of extract of Ageratum conyzoides L. in Sprague Dawley rats. J Med Plants Res 4:2264–2272
Harborne JB (1984) Methods of plant analysis. In: Harbone JB (ed) Phytochemical methods 1984. Springer, Dordrecht, pp 1–36
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54:275–287
Aliyu R, Adebayo AH, Gatsing D, Garba IH (2007) The effects of ethanolic leaf extract of Commiphora africana (Burseraceae) on rat liver and kidney functions. J Pharmacol Toxicol 2:373–379
Atawodi SE, Ogunbusola F (2009) Evaluation of anti-trypanosomal properties of four extracts of leaves, stem and root barks of Prosopis africana in laboratory animals. Biokemistri 21:101–108
Robertson S, Narayanan N, Ravinargis NR (2012) Toxicity evaluation on hydroalcoholic extract of leaf and stem bark of Prosopis cineraria. Int J Pharm Pharm Sci 4:113–118
Celik I, Suzek H (2008) The hematological effects of methyl parathion in rats. J Hazard Mat 153:1117–1121
Adebayo AH, Aliyu R, Gatsing D, Garba HI (2006) The effects of ethanolic leaf extract of Commiphora africana (Burseraceae) on lipid profile in rats. Int J Pharmacol 2:618–622
Yakubu OF, Adebayo AH, Famakinwa TO, Adegbite OS, Ishola TA, Imonikhe LO, Adeyemi AO, Awotoye OA, Iweala EEJ (2018) Antimicrobial and toxicological studies of Ricinodendron heudelotii (Baill.). Asia J Pharm Clin Res 11:299–305
Harnafi H, Caid HS, el Houda Bouanani N, Aziz M, Amrani S (2008) Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem 108:205–212
Omodamiro OD, Nwankwo CI (2013) The effect of Voacanga africana leaves extract on serum lipid profile and haematological parameters on albino wistar rats. Eur J Exp Biol 3:140–148
Leng E, Xiao Y, Mo Z, Li Y, Zhang Y, Deng X, Zhou M, Zhou C, He Z, He J, Xiao L (2018) Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complement Altern Med 18:122–132
Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A et al (2012) Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes-conclusions from the 3rd International ESTP Expert Workshop. Toxicol Pathol 40:971–994
Acknowledgements
The authors do acknowledge the support of the technical staff at the Department of Biochemistry, Covenant University, Ota for their technical assistance. The effort of the Covenant University Centre for Research, Innovation and Discovery is quite appreciated for paying the cost of publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Obode, O.C., Adebayo, A.H. & Li, C. Phytochemical and toxicological evaluations of Prosopis africana (GUILL. and PERR.) extract on albino wistar rats. Toxicol Res. 37, 183–195 (2021). https://doi.org/10.1007/s43188-020-00052-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-020-00052-3