Abstract
The methanol extract of Buchholzia coriacea seed has shown some anti-trypanosomal activity against Trypanosoma brucei brucei. The acute toxicity test of this extract showed no signs of toxicity. This present study was designed to assess its sub-acute toxicity in rats. Rats of both sexes were dosed daily with 125, 250 and 500 mg/kg body weight of the extract by oral gavage for 28 days. Dosing with the extract showed no significant effect on body weights. The temperatures of extract-treated rats were significantly (p < 0.05) higher during the first and last weeks of the experiment. There were also significant reductions (p < 0.05) of red and white blood cell counts, packed cell volumes and haemoglobin concentrations in the extract-treated animals. Blood biochemistry revealed no significant changes. On necropsy, pale mucous membranes, bile stains on spleen, congestion of the lungs and caudal lobes of the liver were evident in the extract-treated rats. There were significant (p < 0.05) reductions in relative liver weights of the extract-treated male rats. Twenty-eight days dosing of rats with the methanol extract of B. coriacea seed caused signs of toxicity at the tested doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The seeds of Buchholzia coriacea Engler are called elephant colas or wonderful colas. They are folklorically used in the treatment of feverish conditions (Nweze et al. 2009). They are chopped up and soaked overnight in the local gin. The infusion is drunk for the cure of such ailments as malaria. The already bottled preparations are available from the herbalists and those traders who hawk herbal remedies. The oil from the seeds is also reputed to have powerful analgesic properties. The methanol extract of B. coriacea has been shown to have anti-trypanosomal activity in mice experimentally infected with Trypanosoma brucei brucei (Nweze et al. 2009). At the dose of 1,000 mg/kg i.p. for three consecutive days, the methanol extract was able to clear the parasites from peripheral blood circulation.
The antimicrobial properties of the fresh seeds and seed extracts of B. coriacea were investigated by Ezekiel and Onyeoziri (2009). The fresh seed, as well as its hexane and methanol extracts, showed antimicrobial activities against some food-borne bacteria like Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Trichoderma viride and Aspergillus niger. The zones of inhibitions ranged from 0 to 62 mm depending on the susceptibility of the tested organism and the plant preparation used, but the fresh seeds were found to be the most active.
B. coriacea leaves have been shown to have anthelmintic effects on Fasciola hepatica (Ajaiyeoba et al. 2001). The ethanolic extract of B. coriacea seed caused larval deaths of the infective stage larvae of Haemonchus contortus and Heligmosomoides polygrus at various concentrations in vitro (Nweze and Asuzu 2006). Fractions prepared from the methanol extract of B. coriacea stem back exhibited a high concentration-dependent antibacterial and antifungal activity comparable to standard antibiotics such as Ampicillin and Tioconazole (Ajaiyeoba et al. 2001).
A drug or medicine has been described as any substance or combination of substances presented as having properties for treating or preventing disease in human beings or animals (Meoni 2008). Drug discovery is anchored on a tripod stand namely efficacy, safety and risk/benefit assessment. The efficacy and safety of a drug determine the risks and benefits of its use. The safety of drugs and drug candidates is assessed by toxicological studies.
Toxicological tests in laboratory animals and humans (during clinical trials) are usually carried out on substances thought to have therapeutic potentials. This is more so necessary in ensuring the quality of herbal medicines since not all of them are safe as frequently claimed (Capasso et al. 2000). It can be harmful to take herbal medicines without being aware of their potential adverse effects. Although the public and some health care professionals believe that herbal medicines are relatively safe because they are ‘natural’, there are remarkably little data to support this assumption (Rodriguez-Fragoso et al. 2008). In addition, there are reports on the toxicities of some medicinal plants.
In the acute toxicity test, graded doses (250, 500, 1,000, and 2,000 mg/kg) of the extract was administered intraperitoneally to four groups of six mice each (Nweze et al. 2009). They were monitored for acute toxicity signs like behavioural changes or death for a period of 24 h. No deaths were recorded at the end of the experiment. Most toxicological studies stop at the acute toxicity test. An acute toxicity test should, however, be followed by a subacute toxicity test and then by a chronic toxicity study.
The aim of this work is to assess the subacute toxicity effects, if any, of daily oral dosing of the methanol extract of B. coriacea seed to rats of both sexes for a period of 28 days.
Materials and methods
Plant materials
Mature seeds of B. coriacea Engler were collected in February 2008 and identified by a taxonomist, Mr A. O. Ozioko of Bioresources Development and Conservation Centre, Nsukka. The seeds were pulverised into fine powder in a mill. The powdered plant materials were stored in sealed cellophane bags until extraction.
Preparation of extracts
Ground B. coriacea seeds were defatted with hexane at room temperature by cold maceration with intermittent shaking in a shaker for 72 h. The hexane extract was filtered out, while the marc was allowed to dry. The marc was re-extracted using 80% methanol in water. A tenfold quantity of solvent in relation to plant material was used for all extractions. All extracts were filtered using size 1 filter papers (Schleicher & Schuell, Germany). The solvents were allowed to evaporate under a hood. For the methanol extract, nitrogen gas was used to evaporate the solvent. To further ensure that all the water was removed, the extract was freeze-dried (Edwards's high vacuum Crawley, England). The extract was stored at 4°C until use.
Experimental animals
Forty-eight male and female albino wistar rats, weighing an average of 82 g and aged 6 weeks old, were used for the subacute toxicity test. The rats were obtained from the Department of Veterinary Pathology and Microbiology, University of Nigeria, Nsukka. They were divided into eight groups of six rats each. Four groups consisted of male rats, while the other four groups were females. They were housed under standard environmental conditions of temperature 25°C (±3°C) and 12-h light/12-h dark cycle. There was an acclimatization period of 7 days before the start of the experiment. They were fed with pelleted grower's mash containing 14.5% crude protein (Vital Feeds, Grand Cereals & Oil Mills Ltd. Jos, Plateau State, Nigeria) throughout the course of the study. The rats had free access to both feed and water ad libitum. They were maintained in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (DHHS, NIH Publication No. 85–23, 1985).
Subacute toxicity test
The rats were divided into four groups of 12 animals (6 males and 6 females). The animals were treated as follows: group A received 500 mg/kg/, group B 250 mg/kg, group C 125 mg/kg, while group D was the untreated control. All the animals in the treatment groups were treated once daily for 28 days consecutively. Toxic manifestations such as signs of toxicity and mortality were monitored daily. Body-weight changes were monitored weekly. Rats were fasted for 16–18 h and anaesthetized using chloroform vapour in a chamber on day 29. Blood samples were collected from the retro-orbital sinus for haematological and biochemical assays.
Clinical haematology and biochemistry
Blood samples were collected in eppendorf vials containing disodium ethylene diamine tetraacetate for erythrocyte count (Coles 1968), total and differential leukocyte count (Coles 1968) and red cell indices such as haemoglobin (Hb) (Coles 1968), packed cell volume (PCV) (Coles 1968) and glucose (On-Call® Plus blood glucose test strips and glucose metres, Acon laboratories Inc, 4108 Sorrento Valley Boulevard, San Diego, CA 92121, USA) at the end of the test period. Sera from clotted blood were collected for blood chemistry and enzyme analysis. Parameters such as sodium (Maruna 1958), potassium (Terri and Sesin 1958), total cholesterol (Roeschlaw et al. 1974), urea (Fawcett and Scott 1960), creatinine (Fossati et al. 1983), total protein (Randox total protein test kit, Randox laboratories Ltd, Ardmore, Diamond Road, Crumlin, Co. Antrim, UK, BT29 $QY), albumin (Doumas et al. 1971), alanine amino transferase (ALT) (Reitman and Frankel 1957) and aspartate amino transferase (AST) (Reitman and Frankel 1957) were monitored. The difference between the total protein and albumin values was taken as the globulin value. All rats were euthanized after blood collection.
Clinical observations, body weight and temperature
Clinical observations were made in the morning and evening every day for moribundity and mortality. Detailed clinical observations were made on the study animals at 1, 4 and 6 h after the first dose on the first study day and once daily thereafter. Body weights were recorded before dosing and weekly thereafter using an electronic weighing balance. Body temperature was determined weekly using a rectal thermometer.
Necropsy
Terminal necropsy was performed on all animals by day 29. Animals were euthanized and necropsied. Some organs like the lungs, heart, liver, spleen, kidney, brain and testes/ovaries were observed for gross lesions and then weighed to determine their relative organ weights (weight of the organ divided by the weight of the rat). Tissues showing lesions were collected. All tissues were preserved in 10% formal saline.
Analysis of data
Data are presented as mean ± standard error of mean. All data were subjected to statistical analysis using one-way analysis of variance to determine significant difference between the means. Differences were considered significant at p < 0.05.
Results
The result of the mean group rectal temperature readings for males is shown in Table 1. At the onset and third week of the experiment, the mean group rectal temperature of the untreated control was significantly (p < 0.05) lower than that of the 125 mg/kg-treated group. But by the first and last weeks of the study, the mean group temperature of the untreated control group was significantly (p < 0.05) lower than those of all the experimental groups. During the second week, the mean group temperature reading of the males in the untreated control group D was only significantly (p < 0.05) lower than the mean group temperature of the rats dosed with 250 mg/kg. But for the females (Table 2), the mean group temperature of the rats in the untreated group D was significantly (p < 0.05) lower than those of all the treatment groups at the onset and last week of the experiment. By the first week, the mean group temperature of the untreated control was significantly (p < 0.05) lower than that of the rats dosed with 500 mg/kg. By the second week, the mean group temperature of the untreated control was significantly lower than those of the rats dosed with 250 and 125 mg/kg of the extract. No significant difference in mean group temperature was seen in all the experimental groups by week 3.
The result of the body weight changes in the males is shown in Table 3. Weight gain was significantly (p < 0.05) highest in the 500 mg/kg group and higher in the 250 and 125 mg/kg groups when compared with the untreated control group. By the second and third weeks, mean weight gain was significantly (p < 0.05) lower in the control group than in all the treatment groups. During the fourth week, the mean weight gain of the control rats was significantly lower than those of the 500 and 125 mg/kg-dosed groups. For the females (Table 4), there was no significant difference in mean weight gain throughout the study.
The result of the mean group haematological profile of the male rats is shown in Table 5. The mean group RBC count of the untreated control rats was significantly higher (p < 0.05) than those of the rats in groups A (500 mg/kg-treated) and B (250 mg/kg-treated). There was, however, no significant difference between the RBC counts of rats in the untreated control group and those in group C (125 mg/kg-treated). No significant differences were observed for all the other haematological parameters like PCV, Hb, platelet count, total and differential leucocyte counts.
The result of the haematological analysis for the female animals is shown in Table 6. The mean group PCV, mean group haemoglobin concentration (Hbc) and total white blood cell (WBC) counts of the untreated control group D rats were significantly (p < 0.05) higher than those of all the treatment groups. The RBC counts of the rats in the untreated control group D were also significantly (p < 0.05) higher than those of the rats in groups A (500 mg/kg-treated) and B (250 mg/kg-treated) but not the rats in group C (125 mg/kg-treated).
The result of the clinical chemistry at terminal bleed for the males is shown in Table 7. No significant differences were observed between the treatment and control groups. The result of the clinical chemistry at terminal bleed for the female rats is shown in Table 8. No significant differences were observed between the control and treatment groups.
The result of the mean group relative organ weights of the male rats are shown in Table 9. The relative weights of the lungs and hearts of the untreated control group D animals were significantly (p < 0.05) lower than those of all the treatment groups. Also the relative weights of the liver in the untreated control group D animals were significantly (p < 0.05) higher than those of the treatment groups. For all the other organs (spleen, testes, kidney and brain), there was no significant difference in their relative organ weights.
The result of the mean group relative organ weights of the female rats is shown in Table 10. The relative weights of the lungs in the untreated control group D rats were significantly (p < 0.05) lower than those of all the other treatment groups. For all other organs, there were no significant differences in their relative organ weights.
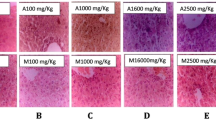
On necropsy, lungs from the extract-treated groups appeared severely congested when compared with the controls (Fig. 1). Also the spleens obtained from the extract-treated rats appeared pale with bile stains and signs of atrophy (Fig. 2). Another lesion observed was in the liver of the extract-treated animals. The caudal lobes of the liver were congested in the rats dosed with the methanol extract of B. coriacea seed (Fig. 3).
Discussion
From physical observation, there was no moribundity or mortality recorded in the course of the daily oral dosing of rats with the methanol extract of B. coriacea seed for 28 days. The treated rats appeared normal, though with signs of hyperactivity when compared with the untreated control animals. Perhaps the extract had some stimulatory effects on the central nervous system. This finding is not out of place since the extract used in this work was crude. Crude extracts have so many different constituents with diverse activities.
There were no observed changes in feed and water consumption. This implies that dosing with the extract may not have affected their appetite and feed conversion rates adversely. There were no digestive disturbances like diarrhoea or vomiting/regurgitation observed equally. However, on postmortem, some of the animals dosed with the different tested doses of the methanol extract of B. coriacea seed had slight catarrhal and haemorrhagic enteritis.
Body weight gain in the untreated male rats was significantly (p < 0.05) lower than that in the treated groups during the first, second and third weeks of the experiment. During the last week of the experiment, weight gain in the control rats was significantly (p < 0.05) lower than those of the 500 and 125 mg/kg-treated animals. In the female rats, there was no significant difference in body weight gain throughout the experiment. This observed difference in weight gain of the male rats may be due to the effect of the extract.
The body temperatures of all the animals fluctuated throughout the study period. This could be due to differences in the environmental temperature. The mean group temperature of the untreated rats were significantly (p < 0.05) lower than those of the different treated groups at different times starting from the beginning to the end of this work. This increase in temperature may not be due to the extract, since it was observed even before dosing with the extract commenced.
This prolonged dosing of rats with B. coriacea seed extract appeared to have deleterious side effects on the haematopoietic system. This was seen in the lower RBC counts of both sexes of treated animals which was significant (p < 0.05) when compared to the untreated controls. This effect appeared to be more serious in the females where all the RBC indices (PCV, Hb and RBC counts) were significantly (p < 0.05) lower than in the untreated animals. This effect was dose-dependent especially for RBC count and is indicative of anaemia. This anaemia was also evident on postmortem. The carcasses of treated animals were pale in colour unlike those of the untreated rats. Also their spleens had bile stains and were pale with signs of atrophy when compared to the untreated controls. In addition, the total mean group WBC count of the extract treated rats was significantly (p < 0.05) lower than that of the untreated controls. This is suggestive of immunosuppression.
There were no observed differences between the treated and untreated control animals with regard to their clinical chemistry. Starting from the liver enzymes ALT and AST to the other parameters associated with liver function like albumin, globulin and total protein, there were no significant differences. Likewise parameters indicative of renal function (urea, creatinine, sodium and potassium) did not show any malfunctioning of the kidney. Even the fasting blood glucose and cholesterol levels were within their normal ranges.
The mean group relative weights of the lungs and hearts of males in all the treatment groups were significantly (p < 0.05) higher than those of the untreated control group. The increased lung weights could be due to the congestion observed at postmortem. The increased heart weights could also be due to the increased activity of the heart muscles in order to compensate for anaemia. The mean group weights of the liver in the treatment groups were also significantly (p < 0.05) lower than those of the untreated control animals. This is indicative of atrophy, but it appears that the liver function was not compromised going by the results of the blood chemistry parameters. In the females, it was only the mean group relative weights of the lungs of the rats in all treatment groups that were significantly (p < 0.05) higher than those of the untreated controls. This means that the males were more severely affected in their relative organ weights than the females.
In conclusion, the 28-day oral dosing of the methanol extract of B. coriacea seed to rats did not cause obvious physical effects except signs of hyperactivity in the treated animals. However, on haematological analyses and necropsy, signs of toxicity like anaemia, congestion of the liver and lungs and signs of atrophy were observed in the liver of male rats.
References
Ajaiyeoba EO, Onocha DA, Olarenwaju OT (2001) In vitro anthelmintic properties of Buchholzia coriacea and Gynandsopsis gynandra extracts. Pharm Biol 39:217–220
Capasso R, Izzo AA, Pinto L, Bifulco T, Vitobello T, Mascolo N (2000) Phytotherapy and quality of herbal medicines. Fitoterapia 71:S58–S65
Coles EH (1968) Veterinary Clinical Pathology, 3rd Edition. W. B. Saunders Co. Philadelphia, pp 145–151.
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta 31:87–96
Ezekiel OO, Onyeoziri NF (2009) Preliminary studies on the antimicrobial properties of Buchholzia coriacea (wonderful kola). Afr J Biotechnol 8:472–474
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Fossati P, Prencipe L, Berti G (1983) Enzymatic creatinine assay: a new colorimetric method based on hydrogen peroxide measurement. Clin Chem 29:1494–1496
Maruna RFL (1958) Beitrag zur serum-natrium-bestimmung Eine kritische studie iiber kolorimetrische bestimmungen und angabe einer einfachen fotometrischen methode. Clin Chim Acta 2:581–585
Meoni P (2008) Pharmacology and toxicology of drug discovery and development, a paper presented at a workshop organized by CSIR titled ‘Drug Design and Development’ held at the University of Pretoria, South Africa, October 27–29.
Nweze NE, Asuzu IU (2006) The anthelmintic effects of Buchholzia coriacea seed. Niger Vet J 27:60–65
Nweze NE, Fakae LB, Asuzu IU (2009) Trypanocidal activity of the ethanolic extract of Buchholzia coriacea seed. Niger Vet J 29:1–6
Reitman S, Frankel S (1957) A colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvate transaminases. Am J Clin Pathol 28:56–63
Rodriguez-Fragoso L, Reyes-Esparza J, Burchiel SW, Herrera-Ruiz D, Torres E (2008) Risks and benefits of commonly used herbal medicines in Mexico. Toxicol Appl Pharmacol 227:125–135
Roeschlaw P, Bernt E, Gruber JW (1974) Enzymatic determination of total cholesterol in serum. J Clin Chem Clin Biochem 12:226–403
Terri AE, Sesin PG (1958) Colorimetric determination of potassium in human serum and plasma using sodium tetraphenyl boron. Am J Clin Pathol 29:86
Acknowledgements
The authors thank the Phytomedicine Programme, University of Pretoria, South Africa and Strathclyde Institute for Drug Research, University of Strathclyde, Glasgow, UK for the use of their facilities in the extraction of the plant material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nweze, N.E., Anene, B.M., Asuzu, I.U. et al. Subacute toxicity study of the methanolic seed extract of Buchholzia coriacea (Capparaceae) in rats. Comp Clin Pathol 21, 967–974 (2012). https://doi.org/10.1007/s00580-011-1210-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-011-1210-y