Abstract
Background
Parastomal hernias can be prevented or repaired using synthetic mesh; however, reported complications include infection, fibrosis and potential bowel erosion. The study aim was to assess the safety, feasibility and potential efficacy of using a prophylactic collagen implant.
Methods
Twenty patients undergoing defunctioning stomas were randomised to a conventional procedure or reinforcement with the implant. Follow-up included regular symptom questionnaires, clinical examination, stoma site ultrasound, and serum inflammatory markers.
Results
Ten patients (four males; mean BMI 26.3) had a conventional stoma, and ten (three males; mean BMI 26.3) received the implant. At a median of 6.5 months follow-up, a parastomal hernia was clinically evident in three of ten patients without the implant, and in none of ten patients with the implant. There were no clinical complications, ultrasound evidence of chronic seromas or serological evidence of a systemic inflammatory response.
Conclusions
Xenogeneic collagen has been demonstrated to aid soft tissue reinforcement. In this study, in contrast to published data relating to the use of conventional synthetic mesh, there were no complications related to infection or the implant’s proximity to the bowel. This trial demonstrates that the implant is safe, feasible to use and has the potential to prevent parastomal herniation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parastomal hernias have a reported incidence of up to 50%, increasing with the length of follow-up [1, 2]. They can lead to complications ranging from poor cosmesis [3], mild discomfort and difficulty with appliance application (causing skin irritation and leakage of bowel contents) [4] to life-threatening complications such as strangulation, obstruction and perforation [5–7]. One in three require surgical repair [8] and, of the documented techniques, prosthetic mesh repair (to reinforce the edges of the stoma trephine) is the most efficacious, although it still has reported recurrence rates of up to 8%, as well as the associated morbidity and cost of a second procedure [1]. As such, certain surgeons have recognised that prevention of parastomal hernias may be the best approach [1, 9]. To date, a number of studies have reported encouraging results regarding the prophylactic placement of polypropylene mesh in an attempt to reduce the rate of parastomal herniation [8, 10, 11].
Polypropylene mesh strengthens the abdominal wall both by mechanical tension and by induction of a strong chronic inflammatory foreign body response [12]. This consequently results in mesh contraction and formation of an avascular fibrotic conglomerate [13], with the potential for bowel erosion, intraperitoneal adhesions, infection and, in the event of any of these complications, difficult removal of the mesh due to dense adhesions to the bowel and extraperitoneal tissue incorporation [14–18].
A more biocompatible alternative is an acellular cross-linked collagen sheet derived from porcine dermis (Permacol, Tissue Science Laboratories). It has been used successfully for laparoscopic inguinal and parastomal hernia repair [19, 20], repair of large abdominal wall defects [21, 22], and general surgical soft tissue augmentation in both animals and humans [23–26]. Comparative studies with polypropylene in rat models have demonstrated that it has better tissue compatibility, with less adhesion formation, more orderly collagen deposition and comparable tensile strength at 90 days after implantation [27].
The aim of this phase 1 study was to assess the safety, feasibility, and potential efficacy of preventing parastomal hernias using this cross-linked collagen implant.
Materials and methods
The study was approved by the local ethics committee (REC reference: P/02/263).
Patients
All patients requiring a defunctioning loop stoma, performed as part of an elective procedure, were prospectively invited to participate in the study on an intention-to-treat basis. After obtaining informed consent, patients were randomised, by means of opening consecutively numbered sealed envelopes, to receiving either a conventional loop stoma or the same procedure with addition of the collagen implant. Patients were blinded as to which arm of the trial they had been entered into. Patient age, sex, and body mass index (BMI) were recorded. Details on previous abdominal surgery and the primary procedure requiring a loop stoma were also recorded.
Materials
Permacol (Tissue science Laboratories, Aldershot, Hants, UK) is a porcine-derived acellular dermal sheet, predominately composed of Type I collagen (93–95%), with Type III collagen and a small amount of elastin comprising the remainder. Its manufacture involves trypsinisation (to remove all living cells and non-collagenous debris), solvent extraction (to remove all lipid and fat deposits), γ irradiation and cross-linkage with hexamethylene-di-isocyanate [25]. Implants were unconditionally donated by Tissue Science Laboratories Plc.
Surgical technique
Stoma formation
All patients had a 2 × 2 cm trephine created through the layers of the anterior abdominal wall, including the rectus sheath, at a pre-marked skin site. In those receiving the implant, the potential space between the posterior layer of the rectus sheath and the peritoneal membrane (pre-peritoneal position) was dissected in all directions around the trephine to allow for the placement of the implant. Sterile sheets of 10 × 10 cm, 1.0 mm in thickness, were utilised. A cylindrical defect, approximately 2 cm in diameter, was fashioned in the centre of the collagen sheet, and the implant was inserted into the previously created plane. The central defect was sutured to the appropriate layer of the rectus sheath (at the 12, 3, 6 and 9 o’clock positions), using interrupted 3/0 prolene sutures, so as to encircle the abdominal trephine (Fig. 1). The outer four corners of the implant were also sutured to the rectus sheath in the same fashion. The cut edge of the peritoneum was sutured to the corresponding edge of the posterior layer of the rectus sheath to enclose the implant.
Central defect of implant sutured to the posterior layer of the rectus sheath (at the 12, 3, 6 and 9 o’clock positions), using interrupted 3/0 prolene sutures, so as to encircle the abdominal trephine. This photograph is a reprint from [28] (reproduced with permission by John Wiley & Sons on behalf of BJSS)
In all patients, the appropriate loop of bowel was brought through the peritoneum, the implant (if present), and the remaining layers of the anterior abdominal wall, without any tension. The stoma was fashioned in the standard manner using 3/0 vicryl rapide.
Stoma reversal
In those undergoing stoma reversal, the bowel was dissected down to the peritoneal cavity. If present, the collagen implant was biopsied, and the opening in the bowel either primarily closed with 3/0 vicryl or the surrounding bowel resected and anastomosed using a GIA stapler. The peritoneum, rectus sheath and, if present, the implant trephine, were closed using either 1/0 loop PDS or interrupted 1/0 nylon sutures. The skin was closed in the standard manner using staples.
Biopsy specimens were taken from the edge of the implant trephine, and immediately fixed in 4% formal saline. After fixation, appropriate samples were embedded in paraffin, 5 μm thick sections were cut and stained with haematoxylin and eosin (H&E).
Follow-up
Patients were followed-up until the time of stoma reversal or, in the event of the stoma not being reversed, until 12 months after stoma formation. Patients completed a questionnaire assessing for symptoms associated with parastomal herniation on a monthly basis, and underwent a clinical examination for signs of a parastomal hernia, and other complications, at 6 weeks postoperatively and then every 3 months until stoma reversal or 12-months post-stoma formation. In those patients whose stomas were reversed, at the time of the second procedure any evidence of stomal herniation was recorded. Serum white cell count, C-reactive protein levels and erythrocyte sedimentation rates were performed on a monthly basis, for 6 months, to establish whether there was any serological evidence of a systemic inflammatory response related to the presence of the implant. Ultrasound examination of the stoma site was performed at least 3 months after stoma formation, usually on the day prior to reversal, to detect for evidence of localised chronic seroma formation related to the presence of the implant.
Statistical analysis
Statistical analysis of the results was not performed on account of the small numbers involved in this phase1 study.
Results
Twenty patients were included in the study. Ten were randomised to receiving the mesh, and ten to a conventional stoma. All patient demographic and relevant surgical data is summarised in Table 1.
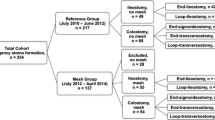
Clinical and operative
The clinical and operative findings are summarised in a flow chart (Fig. 2). Of the ten patients randomised to receiving a conventional stoma, five of ten had their stomas reversed at a median of 5 (range 3–8) months, and three of the ten patients had evidence of parastomal herniation. Of the ten patients recruited to receiving the implant, seven of ten had their stomas reversed at a median of 7 (range 1–10) months, and none of the ten patients had any evidence of parastomal herniation. At the time of stoma reversal, the collagen implant was found to be present and intact. The peritoneal and muscular surfaces of the implant had become bordered with non-fibrous, well-vascularised connective tissue with mild-to-moderate adherence, and fibrous scar tissue was only evident at the suture sites. Adherence to bowel serosa was non-existent to minimal. There was no difficulty in removing the implant, and the implant did not complicate reversal of the stoma. No patient (0 of 20) developed any infective complications, fistula formation or bowel erosion.
Reasons for patients not undergoing stoma reversal by 12 months included patient preference (n = 3), patient’s co-morbidities preventing further complex surgery (n = 2), recurrent anal carcinoma requiring proctectomy (n = 1), severe pouchitis (n = 1), and prolonged chemotherapy for advanced rectal cancer (n = 1).
Patient questionnaire
The results of the patient questionnaire are summarised in Table 2. Of the ten patients randomised to receiving a conventional stoma, three documented the presence of a parastomal bulge, which corresponded to the same three patients in whom a clinically detected parastomal hernia was evident. These three patients documented symptoms related to the presence of a parastomal hernia, including difficulty with bag application, leakage of stoma bag contents, nausea, vomiting, bloating, and parastomal discomfort. Of the ten patients recruited to receiving the implant, one of ten documented a parastomal bulge although there was no hernia evident on clinical examination, ultrasound examination or at the time of stoma reversal. One patient complained of symptoms of intermittent small bowel obstruction (nausea, vomiting, bloating and cessation of wind and stool per stoma) shortly after stoma formation, prompting early stoma reversal (1 month post initial stoma formation); no hernia was detected clinically, on ultrasound or at the time of stoma reversal, but a loop of small bowel proximal to the stoma was found to be wrapped around an intra-peritoneal adhesive band at a distance from the abdominal wall trephine and implant. Another patient (1 of 10) complained of regular nausea, vomiting and bloating but these symptoms were unaltered in frequency or severity compared to those experienced pre-stoma formation.
Stoma site ultrasonography
Of the ten patients randomised to receiving a conventional stoma, nine of ten underwent stoma site ultrasonography at a median of 5 (range 3–12) months post-operation, and none (0 of 9) had ultrasonographic evidence of a chronic seroma or fluid collection. Of the ten patients randomised to receiving the implant, seven of ten underwent stoma site ultrasonography at a median of 6 (range 1–10) months post-operation, and none (0 of 7) had ultrasonographic evidence of a chronic seroma or fluid collection.
Serology
Serology results are illustrated in Fig. 3. The white cell count was neither decreased nor elevated beyond the limits of the normal range in either group, with the exception of day 1 post-operation in the no-implant arm (Fig. 3a). The erythrocyte sedimentation rate (Fig. 3b) and C-reactive protein level (Fig. 3c) were elevated beyond the upper limit of the normal range in both groups post-operatively, but there was no apparent difference between the groups.
a–c Serology results at varying time points post-stoma formation: dark grey bars (red in online) results of patients who received the collagen implant, light grey bars (green in online) results of patients who had a conventional stoma. In each panel, the dotted line indicates the upper limit of the normal range. a Mean white cell count. b Mean erythrocyte sedimentation rate (ESR). c Mean C-reactive protein (CRP) level
Histology
Eight of ten patients recruited to receiving the implant underwent stoma reversal and had biopsies taken of the implant. These revealed a clear line of demarcation between the collagen implant and host connective tissue, with a mild mononuclear cell response and new vessel formation limited to the interface between the collagen implant and host connective tissue, and via native pores within the collagen implant [28]. No polymorphonuclear cell response was evident, and the only foreign body giant cells seen occurred in association with stitch granulomata [28].
Discussion
The view that prevention is the best approach to the management of parastomal hernias has been expressed in two review articles [1, 2], and there is good clinical evidence to date for placing a mesh at the time of stoma formation in order to achieve this aim [8, 10, 11]. However, the ideal material for this purpose has yet to be determined, although the specific success determining characteristics of such a material would intuitively include: avoidance of a foreign body inflammatory response (biocompatibility), and therefore fibrosis, contraction and potential bowel erosion; adequate mechanical strength; and prolonged biodegradation, thereby avoiding herniation following early implant resorption. Furthermore, it has been proposed that both recurrent and incisional hernias (which by definition include parastomal hernias [3]) can be regarded as a consequence of a pathological shift of the collagen ratio within the healed wound, from “mature” type I collagen to “immature” type III collagen, which may result in a loss of tensile strength, and predispose to hernia formation [29]. It is therefore reasonable to hypothesise that any material used to reinforce an abdominal wall stoma should either be predominantly composed of type I collagen or correct the balance of collagen metabolism. Permacol is composed of up to 95% type I collagen, and has been shown in both in vitro and animal studies to possess the aforementioned qualities. In vitro studies have shown that the cross-linking confers resistance to collagenase degradation [30], and when implanted into the abdominal wall of rat models, Permacol induced a mild chronic inflammatory response with no evidence of significant fibrosis [26, 27].
The results of this prospective randomised pilot study to prevent parastomal herniation demonstrate that this novel procedure seems safe to use: there were no complications related to infection or the proximity of the implant to the bowel; there was no ultrasonographic evidence of localised seroma formation or serological evidence of a systemic inflammatory response related specifically to the implant; and the histological data revealed only a mild chronic inflammatory response to the implant. Technically, the procedure is easy to perform and, more importantly, the implant is easy to remove should any hitherto unrecognised complications arise. Most importantly, the data suggests that this technique has the potential to prevent parastomal hernias, in that none of the ten patients who received the implant developed a parastomal hernia compared with three of ten patients who underwent a conventional stoma.
The decision to pilot the technique on defunctioning loop stomas was based on a number of factors. Previous studies have demonstrated a 6% herniation rate in loop stomas at 3 months [1], and it is reasonable to assume that the rate increases with the duration of follow-up, as has been shown with end stomas [2]. Moreover, the construction of loop stomas requires a comparatively larger abdominal trephine than end stomas, which theoretically places them at greater risk of developing a parastomal hernia in the longer term. These are important points when considering that the median time to stoma reversal in the 12 of 20 patients who underwent stoma reversal was 6.5 (range 1–10) months, and that the remainder, either being unsuitable or unwilling to undergo reversal, are therefore at increased risk of herniation in the longer term. Other factors included the greater technical ease of reversing loop stomas, compared to end stomas, in the event of complications, and the unique opportunity this study model provided for histological assessment of the human host response to the implant (more detailed assessment is not within the remit of this paper and is the subject of another study [28]).
In conclusion, this procedure, using a cross-linked collagen implant, seems safe to use in close proximity to the bowel (in contrast to published data relating to the use of conventional synthetic mesh [14–18]), is technically feasible and has the potential to prevent parastomal hernias. Further study, employing appropriately powered sample sizes, longer-term follow-up and cost–benefit analysis, is now required to establish whether this cross-linked collagen implant is at least as effective as synthetic mesh at preventing parastomal herniation, and which method is associated with the fewest complications. An additional challenge will be to identify whether all patients undergoing stoma formation should undergo prophylactic primary mesh placement or if the procedure should be targeted at those most at risk of such a complication.
References
Carne PW, Robertson GM, Frizelle FA (2003) Parastomal hernia. Br J Surg 90:784–793
Israelsson LA (2005) Preventing and treating parastomal hernia. World J Surg 29:1086–1089
Pearl RK (1989) Parastomal hernias. World J Surg 13:569–572
McGrath A, Porrett T, Heyman B (2006) Parastomal hernia: an exploration of the risk factors and the implications. Br J Nurs 15:317–321
Cuthbertson AM, Collins JP (1947) Strangulated para-ileostomy hernia. Aust N Z J Surg 47:86–87
Gabriel WB, Lloyd-Davies OV (1935) Colostomy. Br J Surg 22:520–538
Goligher JC, Lloyd-Davies OV, Robertson CT (1951) Small-gut obstructions following combined excision of the rectum with special reference to strangulation round the colostomy. Br J Surg 38:467–473
Janes, Cengiz Y, Israelsson LA (2004) Randomised clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surg 91:280–282
Martin L, Foster G (1996) Parastomal hernia—review. Ann R Coll Surg Engl 78:81–84
Marimuthu K, Vijayasekar C, Ghosh D, Mathew G (2006) Prevention of parastomal hernia using preperitoneal mesh: a prospective observational study. Colorectal Dis 8:672–675
Gogenur I, Mortensen J, Harvald T, Rosenberg J, Fischer A (2006) Prevention of parastomal hernia by placement of a polypropylene mesh at the primary operation. Dis Colon Rectum 49:1131–1135
Stoppa R (2003) About biomaterials and how they work in groin hernia repairs. Hernia 7:57–60
Rodgers BM, Maher JW, Talbert JL (1981) The use of preserved human dura for closure of abdominal wall and diaphragmatic defects. Ann Surg 193:606–611
Kaufman Z, Engelberg M, Zager M (1981) Fecal fistula: a late complication of Marlex mesh repair. Dis Colon Rectum 24:543–544
Stone HH, Fabian TC, Turkelson ML, Jurkiewicz MJ (1981) Management of acute full-thickness losses of the abdominal wall. Ann Surg 193:612–618
Schneider R, Herrington JL, Granda AM (1979) Marlex mesh in repair of a diaphramatic defect later eroding into the distal esophagus and stomach. Am Surg 45:337–339
Voyles CR, Richardson JD, Bland KI (1981) Emergency abdominal wall reconstruction with polypropylene mesh. Short-term benefits versus long-term complications. Ann Surg 194:219–223
Helton WS, Fisichella PM, Berger R, Horgan S, Espat NJ, Abcarian H (2005) Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch Surg 140:549–562
Smart N, Immanuel A, Mercer-Jones M (2007) Laparoscopic repair of a Littre’s hernia with porcine dermal collagen implant (Permacol). Hernia 11:373–376
Inan I, Gervaz P, Hagen M, Morel P (2007) Laparoscopic repair of parastomal hernia using a porcine dermal collagen (Permacol) implant. Dis Colon Rectum 50:1465
Adedeji OA, Bailey CA, Varma JS (2002) Porcine dermal collagen graft in abdominal-wall reconstruction. Br J Plast Surg 55:85–86
Liyanage SH, Purohit GS, Frye JN, Giordano P (2006) Anterior abdominal wall reconstruction with a Permacol implant. J Plast Reconstr Aesthetic Surg 59:553–555
MacLeod TM, Williams G, Sanders R, Green CJ (2003) Prefabricated skin flaps in a rat model based on a dermal replacement matrix Permacol. Br J Plast Surg 56:775–783
MacLeod TM, Sarathchandra P, Williams G, Sanders R, Green CJ (2004) Evaluation of a porcine origin acellular dermal matrix and small intestinal submucosa as dermal replacements in preventing secondary skin graft contraction. Burns 30:431–437
Macleod TM, Williams G, Sanders R, Green CJ (2005) Histological evaluation of Permacol as a subcutaneous implant over a 20-week period in the rat model. Br J Plast Surg 58:518–532
Kaleya RN (2005) Evaluation of implant/host tissue interactions following intraperitoneal implantation of porcine dermal collagen prosthesis in the rat. Hernia 9:269–276
Zheng F, Lin Y, Verbeken E, Claerhout F, Fastrez M, De Ridder D, Deprest J (2004) Host response after reconstruction of abdominal wall defects with porcine dermal collagen in a rat model. Am J Obstet Gynecol 191:1961–1970
Hammond TM, Chin-Aleong J, Navsaria H, Williams NS (2008) Human in vivo cellular response to a cross-linked acellular collagen implant. Br J Surg 95:438–446
Jansen PL, Mertens PR, Klinge U, Schumpelick V (2004). The biology of hernia formation. Surgery 136:1–4
Jarman-Smith ML, Bodamyali T, Stevens C, Howell JA, Horrocks M, Chaudhuri JB (2004) Porcine collagen crosslinking, degradation and its capability for fibroblast adhesion and proliferation. J Mater Sci Mater Med 15:925–932
Author information
Authors and Affiliations
Corresponding author
Additional information
Declaration: The experiments performed comply with the current laws of the country in which they were performed.
Rights and permissions
About this article
Cite this article
Hammond, T.M., Huang, A., Prosser, K. et al. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia 12, 475–481 (2008). https://doi.org/10.1007/s10029-008-0383-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-008-0383-z