Abstract
Background
Elective or emergency reconstruction of abdominal wall defects (AWD) is often difficult. Various techniques have been proposed for reconstructing AWD, including the use of synthetic implants. Porcine acellular dermal collagen (PermacolTM) is a biologic implant (PADCI) derived from porcine dermis. We report our experience with the use of PADCI in the management of large AWD in both emergency and elective surgery.
Methods
Twenty consecutive patients with chronic AWD (CAWD) arising from large incisional hernia or acute AWD (AAWD) arising from visceral edema or tumor resection were studied prospectively. After musculofascial mobilization, the AWD was closed using sheets (10 × 15 cm) of PADCI as an “underlay” interposition graft. Patients were followed up to a median of 18 months postoperatively.
Results
All 20 defects were closed without tension using PADCI. Eight and 12 patients had reconstruction for large AAWD and CAWD, respectively. The mean size of the defects was 180 cm2 (range = 96–850 cm2). The median number of PADCI used to repair the defects was one sheet (range = 1–7). Twelve patients (60%) had an uneventful recovery and were discharged within seven days. One patient (5%) died from multiple-organ failure. Seven patients (35%) developed a complication (two seromas, two minor wound infections, one wound hematoma, one skin edge necrosis, one superficial wound dehiscence, and wound sinus). Overall there were three recurrences (15%).
Conclusion
PADCI has the potential for reconstruction of large acute and chronic abdominal wall defects. Medium-term recurrence rate is comparable to synthetic mesh repairs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reconstruction of abdominal wall defects (AWD) is a challenging problem faced by many surgeons not only in the elective setting but also during emergency surgery. Deficiencies of the abdominal wall can be the result of sepsis, abdominal compartment syndrome, trauma, or primary herniation. The resultant defects differ in site and size and include varying degrees of loss of skin, muscle, and/or fascia. Incisional hernia is the most common cause of chronic abdominal wall defect (CAWD), with incidence rates as high as 11%–20% postlaparotomy [1]. On the other hand, acute abdominal wall defects (AAWD) may result from significant visceral edema secondary to fluid resuscitation for hemorrhagic and septic shock. This oedema often precludes abdominal wall closure after laparotomy. Closure under tension leads to fascial necrosis [2].
Synthetic and autologous materials have been used to achieve wound closure [2–5], but because of their inherent drawbacks, significant efforts have been made to identify new techniques and materials for the reconstruction of AWD. Among the biologic materials investigated, several have been produced from animal and human sources. Cadaveric allografts (e.g., AlloDerm®, LifeCell Corp., Suspend®, Mentor) are commercially available biologic materials, although availability of human tissue is organ bank dependent.
Porcine acellular dermal collagen implant (PADCI) (PermacolTM, TSL, UK) is a biologic material derived from processing porcine dermis, which is crosslinked with di-isocyanate, and has already been used for various urologic, gynecologic, and plastic surgery procedures [6–8].
In this study we report our experience with the use of PADCI in the management of large AWD in both emergency and elective surgery.
Materials and methods
Between January 2002 and June 2005, 20 consecutive patients with large AWD were enrolled in this study. Our inclusion criteria were patients with CAWD arising from large incisional hernia or recurrent incisional hernia and AAWD arising from visceral edema or extensive soft-tissue loss from various causes such as trauma or tumor resection. There is no standard definition of large AWD in the literature. We arbitrarily defined defects measuring 12 cm × 8 cm or greater as large AWD. Patients with small AWD and those with groin hernia were excluded from this study. The data were collected prospectively. Informed consent was obtained from all patients.
Preoperatively, all patients with CAWD were evaluated by full history and physical examination. Site and size of the defects were carefully assessed. In acute setting, the nature of the defect was measured during the operation. All received thromboprophylaxis with subcutaneous enoxaparin and broad-spectrum antibiotic prophylaxis preoperatively. All procedures were performed under general anesthesia.
We used Permacol™ as PADCI to reconstruct an abdominal wall defect. This is a biomaterial derived from porcine dermis by the enzymatic and chemical removal of cellular components, leaving a crosslinked collagen and its constituent elastin-rich matrix. It is presented as a sterile, off-white, moist, and tough but flexible flat sheet in sterile saline. It is packaged in double vacuum-packed aluminum foil/polyethylene sachets, which are impermeable to oxygen, and is sterilized by gamma irradiation. The size of each sheet used in this study was 10 cm wide × 15 cm long and 1.5 mm thick.
Surgical technique
All patients with CAWD underwent exposure of the hernia sac and fascial margins of the defect through preexisting incision lines or open wounds. In all cases with CAWD, the cutaneous scars were excised. In patients with AAWD where potential wound contamination was suspected, wounds were irrigated with saline. All these patients underwent abdominal wall reconstruction done as primary closure after dealing with the primary pathology.
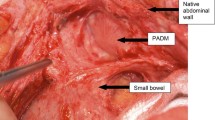
In all cases the fascial margins were defined clearly. Lysis of adhesions and any intra-abdominal procedures were performed as appropriate. Skin and subcutaneous tissues were then undermined in the prefascial plane above the anterior rectus sheath to facilitate skin closure. PADCI was rehydrated in normal saline. The number of PADCI sheets used depended on the size of the defect. The rehydrated PADCI was affixed as an underlay retrofascial patch and fixed with interrupted horizontal mattress sutures using 0 polypropylene. At least 2 cm of the implant was allowed to overlap the defect. If necessary, multiple sheets of PADCI were combined and sewn with running 0 polypropylene sutures to achieve a tension-free closure of the defect.
Because skin was mobilized extensively to close primarily over porcine dermis, at least two drains were used to minimize seroma formation. Subcutaneous tissue was reapproximated with interrupted absorbable sutures and skin was primarily closed with clips in all patients. The operating time and intraoperative blood loss were recorded. Any intraoperative complications were also recorded.
Postoperative protocol
Patients were assessed closely for any postoperative complications. Patients were further evaluated at six weeks, three months, and six months postoperatively in the outpatient clinic. Follow-up data consisted of details of postdischarge course. Final review was done by telephone survey, during which patients and their family physicians were asked to provide information on clinical course and the occurrence of any recurrence. Only patients with complaints suggestive of recurrence were invited for further hospital visit.
The incidence of complications was determined by using homogeneous definitions. We defined seroma as fluid collections that required drainage or caused symptoms. The definition of wound infection was based on clinical signs of infection and microbiological culture. Recurrence was defined as any abnormal protrusion at the site of the prior repair.
Results
Overall 20 abdominal wall defects were reconstructed. It was possible to close all the defects without tension using PADCI. Demographic characteristics of the patients in our series include a median age of 51 years (range = 22–85 years), a male:female ratio of 11:9, and a median weight of 72 kg (range = 62–110 kg).
Eight patients had reconstruction for AAWD and 12 had an elective procedure performed for CAWD. Details of the patient characteristics in each group are shown in Table 1. Of the 12 patients with CAWD, three had first-time repair of a large incisional hernia, five had had one previous repair, and four had had two or more previous repairs. One patient in the AAWD group had bowel resection due to Crohn’s disease and was on steroids and two had bowel resection due to irreversible ischemia secondary to strangulated incisional hernia. A single graft (10 cm × 15 cm) was used in 15 patients, two grafts in three patients, and three and seven grafts in one patient each.
Twelve patients (60%) had an uneventful recovery and were discharged from the hospital within seven days. The patient in hospital for 35 days was an 85-year-old female awaiting placement in a nursing home. Complications and recurrences are shown in the Table 2.
There was one death (5%) in this series. This patient was a 77-year-old female with a background history of congestive heart failure and steroid-dependent chronic obstructive airway disease. She underwent emergency laparotomy and small-bowel resection for intestinal obstruction due to strangulated incisional hernia. She died of multiple organ failure on postoperative day 6.
Seven patients (35%) developed a complication. One patient who underwent repair of a giant incisional hernia developed necrosis of the edges of the skin flaps. This patient needed excision and resuturing of skin leaving the graft in situ. The same patient also developed urinary tract infection, which was treated by oral antibiotics. Two patients with localized wound infections were managed conservatively with antibiotics. One patient with Crohn’s disease and on steroids had a superficial wound dehiscence on postlaparotomy day 7. He was managed initially by wound dressing and then by secondary suture without removing the implant. The same patient also developed lower respiratory tract infection. Patients with seromas were managed by outpatient percutaneous aspiration. One patient with a strangulated hernia who developed wound seroma, also subsequently developed a wound sinus, which healed spontaneously over a three-week period. There were no cases of intestinal fistula or problems related to intestinal adhesion or chronic wound pain.
Median follow-up was 18 months (range = 6–36 months). There were three recurrences (15%), one at three months and two at six months. The patient with recurrence that appeared at three months had already had three polypropylene mesh repairs. The three recurrences included a patient who had a background of Crohn’s disease, a second patient who had reconstruction for multiple stab wounds, and a third patient following a fourth repair for recurrent incisional hernia. In the first two instances the recurrent defect was broad-based at the upper part of the wound; in the third case recurrence affected the whole length of the midline wound. In the first two cases, as the hernia was asymptomatic and was not affecting their daily activities, patients did not want further operation. The third patient declined further intervention.
There were no differences in complication rate with the use of single sheet vs. multiple sheets.
Discussion
Various techniques have been described to create a tension-free reconstruction of abdominal wall defects, including prosthetic mesh insertion, tissue expansion, pedicled or free tissue flaps, vacuum-assisted wound closure, and component separation fascial release [2–5, 9]. The optimal method must protect the bowel from unnecessary adhesions, provide a homeostatic environment that aids in the resolution of visceral edema, and promote a safe and reliable fascial closure [9]. Recurrence rates following primary repair in excess of 50% have been reported [10, 11].
Prosthetic implantation has significantly improved the results of primary fascial closure by minimizing tension on the repair. Synthetic biomaterials such as polypropylene are the mainstays of reconstruction of CAWD today [9, 12]. However, many surgeons would hesitate to repair a large chronic incisional hernia with polypropylene mesh if the mesh would be in direct contact with intestine. Moreover, there are many clinical situations in which the use of such materials is ill-advised. These situations include, but are not limited to, recent intra-abdominal infections, sites with previous wound infections, operative fields in which a stoma will be located near the suture line, enterocutaneous fistulae, and in patients who are immunocompromised. Furthermore, such materials can increase the risk of adhesions to intra-abdominal viscera and enterocutaneous fistula formation [13]. Expanded polytetrafluoroethylene (ePTFE) has also been used for AAWD or CAWD. This material is strong and biocompatible and less likely to stimulate adhesions to viscera. ePTFE, however, is largely intolerant of infection and its use in the presence of contamination, infection, and enteric fistulae is limited [14, 15].
Biologic grafts have the potential advantage over synthetic materials because of less tendency toward infection, erosion, extrusion, and rejection [16]. Moreover, there is no intra-abdominal adhesion formation and/or fistula formation. Autologous tissue grafts have been used to reconstruct defects in the presence of contaminated wounds where prosthetic materials were contraindicated [17]. Despite this advantage, autologous tissues are not always available in sufficient quantity, and their use can greatly increase operative time, complexity, and morbidity and are generally outside the scope of practice of many general surgeons [5, 17].
Ramirez et al. [2] described the technique of musculofascial “component separation” for repair of abdominal wall defects. After the separation of the external oblique fascia and muscle with incisions lateral to the linear semilunaris and the creation of a plane between the external oblique and internal oblique muscles, a rectus abdominus–internal oblique–transversus abdominus complex is mobilized. With this technique, abdominal wall integrity is reestablished without prosthetic materials or a donor site. Despite the clear advantages, this operation necessitates extensive dissection and is not always sufficient, requiring the need for additional prosthetic implantation [18]. Moreover, de Vries Reilingh et a1. [19] reported a 32% reherniation rate in a series of 43 patients following component separation repairs.
With increasing experience and interest in biologic fascial substitutes such as Alloderm Acellular Tissue Matrix (Lifecell, Branchburg, NJ) and Permacol (TSL), utilization of synthetic mesh may decline. Availability of cadaveric allograft is dependent on organ donation banks. On the other hand, porcine dermis is much more abundant and structurally very similar to human dermis [6].
In a rat model comparing biomechanical properties of PADCI and polypropylene in the reconstruction of a full-thickness AWD, Zheng et al. [20] showed that PADCI induces a milder inflammatory response, less adhesion formation, more orderly collagen deposition, and neovascularisation than polypropelene and reaches a comparable tensile strength in 90 days. Unlike polypropylene mesh, PADCI can be in contact with the bowel wall because it does not form a biofilm [21]. In humans PADCI has been used in the surgical repair of inguinal hernia and a number of urologic and gynecologic procedures [6–8]. In this study we have shown that successful closure of large AWD is possible with the use of PADCI, not only in the clean wound but also in potentially contaminated wound. Di-isocyanate crosslinking of porcine dermal collagen prevents biodegradation and mineralization in the presence of infection. Furthermore, systemic antibiotic can easily reach the implant because of neovascularisation and thereby helps eradicate infective organisms in the implant. This property allows its use in contaminated wounds [6].
Sharma and Holl-Allen [22] reported use of ZenodermTM for elective reconstruction of 11 incisional hernias. The initial porcine dermal grafts such as ZenodermTM were crosslinked using aldehyde. The problem with long-term use of aldehyde-crosslinked implants is that foci of calcification may develop, which can be extensive [6]. Our study differs from the above study in two aspects. First, we used di-isocyanate (to prevent calcification of the graft) crosslinked porcine dermis (Permacol) and, second, in addition to reconstruction of the CAWD, we also used the implant to reconstruct AAWD. Besides two isolated case reports on the use of Permacol in the reconstruction of an abdominal wall defect [23], to our knowledge this is the largest series to report the usefulness of PADCI in the reconstruction of AAWD.
Seroma formation has been reported in 1%–15% of cases following incisional hernia repair. Most of the seromas can be managed on an outpatient basis, as was done in our study. Wound infection is another common complication (4%–12%), in some cases requiring removal of the synthetic mesh [4, 21, 24]. Bauer et al. [3] reported a 3% incidence of fistula, and Martin-Duce et al. [24] reported persistent pain beyond six months in 28% of patients. Wound infection occurred in two of our cases and both resolved with antibiotics. None of our patients required removal of the implant due to complication.
Heavy contamination at the time of surgery significantly increases the risk of severe complications when mesh is used. One study [12] using polypropylene mesh described gross contamination in 29 of 31 patients, 14 of whom ultimately extruded the mesh. The authors recommend use of PADCI in these circumstances.
The only death in our series, from multiple-organ failure, was not related to the implant or the operation technique. Multiple-organ failure was due to comorbidities and late presentation. There were no disabling complications directly related to the use of PADCI, such as wound contracture, adhesions, fistula, intestinal obstruction, or persistent wound pain in this study.
Luijendijk et al. [4], in a multicenter randomized prospective trial comparing open suture repair and synthetic mesh repair of incisional hernia, reported a recurrence rate of 46% in the suture group and 23% in the mesh group after a mean follow-up of 26 months. Although our overall recurrence rate at median follow-up of 18 months was 15%, in the CAWD repair group, recurrence rate was 8% (1 in 12). Our recurrence rate is comparable to that of other methods of reconstruction in the medium term. Like all promising new materials and innovations, the theoretical advantages and early encouraging results of this material need to withstand the test of time. Evidence from contemporary literature comparing complications and recurrence rates following repair of varying-size abdominal defects using polypropylene mesh, ePTFE, and PADCI are shown in the Table 3 [3, 4, 25]. There is no study in the literature that has reported long-term (all patients followed for at least 5 years) results and complications in large abdominal wall defects. We do not know the exact cause of recurrence in our patients since none of our patients underwent reoperation. One possibility is implant diastasis, secondary to the stretching of the mesh itself. This has been suggested in the literature with the use of Alloderm mesh [26]. Whether this is the case with the use of PADCI should be looked at in future studies, particularly in the long-term follow-up.
The cost of any implant material may be significant. The cost of a 10–cm × 15-cm PADCI (Permacol) graft is approximately 2073.00 euro. With the increasing availability and use of biologic implant materials, this cost is likely to decrease. In a recent cost analysis study comparing suture repair vs. mesh repair for incisional hernia, Israelsson et al. [27] showed that the total costs of a mesh repair were 1,898 Swedish kronor lower than a suture repair. In any cost-benefit analysis, one should take into account the operation time, length of hospital stay, and morbidity associated with the use of any implant material.
Conclusions
PADCI has the potential for reconstruction of large acute and chronic abdominal wall defects. This material is useful in situations in which delayed wound healing is anticipated or when large quantities of prosthetic material are used. Medium-term recurrence rate is comparable to synthetic mesh repair.
References
Mudge M, Hughes LE (1985) Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg 72:70–71
Ramirez OM, Ruas E, Dellon AL (1990) “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 86: 519–526
Bauer JJ, Harris MT, Kreel I, et al. (1999) Twelve-year experience with expanded polytetrafluoroethylene in the repair of abdominal wall defects. Mt Sinai J Med 66:20–25
Luijendijk RW, Hop WC, van den Tol MP, et al. (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343:392–398
Caffee HH (1983) Reconstruction of the abdominal wall by variations of the tensor fasciae latae flap. Plast Reconstr Surg 71:348–353
Harper C (2001) Permacol: clinical experience with a new biomaterial. Hosp Med 62:90–95
Giri SK, Drumm J, Saunders JA, et al. (2005) Day-case sling surgery for stress urinary incontinence: feasibility and safety. BJU Int 95:827–832
Saray A (2003) Porcine dermal collagen (Permacol) for facial contour augmentation: preliminary report. Aesthetic Plast Surg 27:368–375
Brock WB, Barker DE, Burns RP (1995) Temporary closure of open abdominal wounds: the vacuum pack. Am Surg 61:30–35
George CD, Ellis H (1986) The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl 68:185–187
Paul A, Korenkov M, Peters S, et al. (1998) Unacceptable results of the Mayo procedure for repair of abdominal incisional hernias. Eur J Surg 164:361–367
Voyles CR, Richardson JD, Bland KI, et al. (1981) Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg 94:219–223
Butler CE, Prieto VG (2004) Reduction of adhesions with composite AlloDerm/polypropylene mesh implants for abdominal wall reconstruction. Plast Reconstr Surg 114:464–473
Bleichrodt RP, Simmermacher RK, van der Lei B, et al. (1993) Expanded polytetrafluoroethylene patch versus polypropylene mesh for the repair of contaminated defects of the abdominal wall. Surg Gynecol Obstet 176:18–24
Brown GL, Richardson JD, Malangoni MA, et al. (1985) Comparison of prosthetic materials for abdominal wall reconstruction in the presence of contamination and infection. Ann Surg 201:705–711
Hodde J (2002) Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng 8:295–308
Disa JJ, Goldberg NH, Carlton JM, et al. (1998) Restoring abdominal wall integrity in contaminated tissue-deficient wounds using autologous fascia grafts. Plast Reconstr Surg 101:979–986
Lowe JB 3rd, Lowe JB, Baty JD, et al. (2003) Risks associated with “components separation” for closure of complex abdominal wall defects. Plast Reconstr Surg 111:1276–1283
de Vries Reilingh TS, van Goor H, Rosman C, et al. (2003) “Components separation technique” for the repair of large abdominal wall hernias. J Am Coll Surg 196:32–37
Zheng F, Lin Y, Verbeken E, et al. (2004) Host response after reconstruction of abdominal wall defects with porcine dermal collagen in a rat model. Am J Obstet Gynecol 191:1961–1970
Leber GE, Garb JL, Alexander AI, et al. (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Sarmah BD, Holl-Allen RT (1984) Porcine dermal collagen repair of incisional herniae. Br J Surg 1:524–525
Liyanage SH, Purohit GS, Frye JN, et al. (2005) Anterior abdominal wall reconstruction with a Permacol implant. J Plast Reconstr Aesthet Surg 59:553–555
Martin-Duce A, Noguerales F, Villeta R, et al. (2001) Modifications to Rives technique for midline incisional hernia repair. Hernia 5:70–72
de Vries Reilingh TS, van Geldere D, Langenhorst B, et al. (2004) Repair of large midline incisional hernias with polypropylene mesh: comparison of three operative techniques. Hernia 8:56–59
Gupta A, Zahriya K, Mullens PL, et al. (2006) Ventral herniorrhaphy: experience with two different biosynthetic mesh materials, Surgisis and Alloderm. Hernia 10:419–425
Israelsson LA, Jonsson L, Wimo A (2003) Cost analysis of incisional hernia repair by suture or mesh. Hernia 7:114–117
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaikh, F.M., Giri, S.K., Durrani, S. et al. Experience with Porcine Acellular Dermal Collagen Implant in One-stage Tension-free Reconstruction of Acute and Chronic Abdominal Wall Defects. World J Surg 31, 1966–1972 (2007). https://doi.org/10.1007/s00268-007-9174-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-007-9174-4