Abstract
Petrosavia sakuraii (Petrosaviaceae) is a rare, mycoheterotrophic plant species that has a specific symbiotic interaction with a narrow clade of arbuscular mycorrhizal (AM) fungi. In the present study, we tested the hypothesis that the distribution and abundance of mycobionts in two P. sakuraii habitats, Nagiso and Sengenyama (central Honshu, Japan), determine the distribution pattern of this rare plant. Nagiso is a thriving habitat with hundreds of P. sakuraii individuals per 100 m2, whereas Sengenyama is a sparsely populated habitat with fewer than 10 individuals per 100 m2. AM fungal communities associated with tree roots were compared at 20-cm distances from P. sakuraii shoots between the two habitats by molecular identification of AM fungal partial sequences of the small subunit ribosomal RNA gene. The percentage of AM fungal sequences showing over 99 % identity with those of the dominant P. sakuraii mycobionts was high (54.9 %) in Nagiso, but low (13.2 %) in Sengenyama. Accordingly, the abundance of P. sakuraii seems to reflect the proportion of potential mycobionts. It is likely that P. sakuraii mycobionts are not rare in Japanese warm temperate forests since 11.2 % of AM fungal sequences previously obtained from a deciduous broad-leaved forest devoid of P. sakuraii in Mizuho, central Honshu, Japan, were >99 % identical to those of the dominant P. sakuraii mycobionts. Thus, results suggest that the abundant mycobionts may be required for sufficient propagation of P. sakuraii, and this quantitative trait of AM fungal communities required for P. sakuraii may explain the rarity of this plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 500 plant species have been identified to be mycoheterotrophic plants, which depend on mycorrhizal fungi from the Basidiomycota, Ascomycota, or Glomeromycota phyla for their carbon sources (Leake 1994; Merckx 2013). These plants usually show specificity to particular lineages of mycorrhizal fungi (Merckx et al. 2009; Waterman et al. 2013), and many of them are known to be rare plants (Merckx et al. 2013). The majority of land plant species form arbuscular mycorrhizas (AM) with Glomeromycota fungi (Smith and Read 2008), and more than 230 AM plant species in nine families are mycoheterotrophic (Merckx 2013). Although these mycoheterotrophs typically exhibit specificity towards particular AM fungal groups, specificity levels vary among different plant species (Bidartondo et al. 2002; Franke et al. 2006; Merckx and Bidartondo 2008; Merckx et al. 2010; Yamato et al. 2011a, b).
Petrosavia sakuraii (Makino) J.J.Sm. ex Steenis (Petrosaviaceae) is a mycoheterotrophic plant species that has symbioses with AM fungi. A highly specific interaction with Glomeraceae fungi has been revealed by Yamato et al. (2011b), in which quite high sequence identity of more than 99 % was found among the partial sequences of the small subunit ribosomal RNA gene (SSU rDNA) obtained from the dominant mycobionts of 17 P. sakuraii individuals in five habitats in Japan and China. P. sakuraii is found in warm temperate regions in East Asia such as the plantations of Chamaecyparis obtusa (Siebold & Zucc.) Endl. and secondary broadleaf forests (Yamato et al. 2011b). Although these vegetation types are common in Japan, P. sakuraii is an extremely rare species, categorized as “critically endangered” on the Red List of Japan (Ministry of the Environment 2012). P. sakuraii usually occurs with an extremely low abundance, and several populations are in decline or have already disappeared (Mizuno et al. 1974). The rarity of mycoheterotrophic plants seems to be related to specialized preference for biotic or abiotic factors (Merckx et al. 2013). Since P. sakuraii inhabits common vegetation types in warm-temperate regions in Japan, it does not seem to require specialized environmental factors. It is suggested that biotic factors such as pollination mutualism, seed dispersal, and mycorrhizal symbiosis may affect the rarity of mycoheterotrophic plants (Merckx et al. 2013). Though specialization in plant-pollinator interactions has been revealed in some mycoheterotrophic plants (Klooster and Culley 2009; Hentrich et al. 2010), Takahashi et al. (1993) showed a low specificity for the pollinators of P. sakuraii. With regard to seed dispersal characteristics, P. sakuraii produces large numbers of seeds, i.e., 138.5 ± 41.6 (mean ± SD) in a fruit under open pollination (Takahashi et al. 1993), and they are dust seeds suited to long-distance dispersal (Eriksson and Kainulainen 2011). Accordingly, these features may not correlate with the rarity of this species. Meanwhile, the high specificity towards mycorrhizal fungi may be responsible for the rarity of P. sakuraii, since mycoheterotrophic plants completely depend on carbon nutrients supplied by their fungal partners. Nevertheless, high specificity for mycorrhizal fungi does not necessarily result in the rarity of mycoheterotrophs. While a high specificity was found in the mycorrhizal symbiosis of a common, widely distributed mycoheterotroph, Eulophia zollingeri (Rchb.f.) J.J.Sm, the specificity for a wood-decomposer fungus Psathyrella candolleana (Fr.) Maire complex (Psathyrellaceae) of this orchid may not restrict its distribution due to the wide distribution and abundance of P. candolleana (Ogura-Tsujita and Yukawa 2008). As exemplified in the case of E. zollingeri, it is crucial to incorporate distribution patterns and the abundance of mycobionts as well as their specificity to test the impact of mycorrhizal symbiosis on the rarity of mycoheterotrophs.
In this study, AM fungal communities were investigated in tree roots at two habitats of P. sakuraii in the central part of Honshu, Japan. The first was Nagiso (Nagano Prefecture) which contains several hundred individuals of P. sakuraii per 100 m2, and the second is Sengenyama (Gifu Prefecture) which contains fewer than 10 individuals per 100 m2. The relationship between the relative abundance of the potential mycobionts of P. sakuraii in its habitats, estimated by molecular identification of AM fungi in tree roots, and the density of P. sakuraii individuals in these sites was evaluated. The relationship between AM fungal communities and the density of P. sakuraii individuals at the micro-habitat scale in the Nagiso population was also investigated. Furthermore, DNA databases and previous studies were surveyed to elucidate the distribution of potential mycobionts of P. sakuraii.
Materials and methods
Root sampling
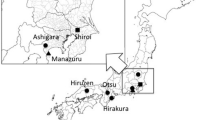
Two studies were performed, study 1 with sampling in 2011 and study 2 with sampling in 2012. In study 1, the two P. sakuraii habitats, Sengenyama and Nagiso, were selected to evaluate AM fungal communities associated with the different P. sakuraii populations. In Sengenyama, eight P. sakuraii individuals, which almost all appeared in 2011, were chosen from five sites (S1–S5; Tables 1 and 2) in a secondary forest, with Quercus variabilis Blume (Fagaceae), Chamaecyparis obtusa, Ilex pedunculosa Miq. (Aquifoliaceae), and Cleyera japonica Thunb. (Pentaphylacaceae) as the dominant trees or shrubs. In Nagiso, a site in which mycobionts of P. sakuraii were previously identified (Yamato et al. 2011b), eight individuals (N1a–h) were randomly chosen, with spacing of 50 cm, in a 5 × 5 m plot with 284 shoots of P. sakuraii. The plot was in a C. obtusa plantation with other tree species such as Quercus serrata Murray and Pinus densiflora Sieb. et Zucc. In both sampling sites, soil cores of 5 cm in diameter and 10 cm in depth, each containing tree roots, were collected at a 20-cm distance from the P. sakuraii shoot for each of the selected individuals, on 15 October 2011.
In study 2, five transects were marked from the center of the plot in a 30 × 40 m area, and soil cores were collected at 2- to 5-m intervals in each transect on 11 September 2012 to investigate the relationship between AM fungal communities and the distribution of P. sakuraii (Fig. 1). In each sampling position, P. sakuraii shoots were counted in a 1 m2 area around the sampling position and 25 soil cores were collected (Table 3).
Molecular analyses
Fine tree roots were collected from each soil core sample, and the total DNA was extracted from approximately 200 mg of fresh root using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). DNA was dissolved in 50 μL of elution buffer. For the samples collected from Sengenyama and Nagiso in 2011 (study 1), partial AM fungal SSU rDNA was amplified from the extracted DNA by polymerase chain reaction (PCR) with primers AML1 and AML2 (Lee et al. 2008) using TaKaRa Ex Taq, Hot Start Version (Takara Bio, Otsu, Japan). The PCR mixture contained 1 μL of the extracted DNA diluted at 1:10, 0.75 units of Taq polymerase, 0.25 μM of each primer, 200 μM of each deoxynucleotide triphosphate, and 3 μL of the supplied PCR buffer, in a total volume of 30 μL. The PCR program, performed on a PC-818S Program Temp Control System (Astec, Fukuoka, Japan), was as follows: initial denaturation at 94 °C for 2 min, followed by 35 cycles at 94 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min, and a final elongation step at 72 °C for 10 min. PCR products were purified using a Gel/PCR DNA Fragments Extraction Kit (RBC Bioscience, Taipei, Taiwan) and cloned using the pGEM-T Easy Vector System I (Promega, Madison, WI, USA). The DNA inserts were sequenced using a BigDye Terminator v3.1 Cycle Sequencing Kit on a 3130 Genetic Analyzer (Applied Biosystems, Tokyo, Japan).
The intron of the trnL (UAA) gene of the plant chloroplast was also amplified with primers trnLF-cF and trnLF-dR to identify the tree root samples (Taberlet et al. 1991). The composition of the PCR mixture was the same as described above, except for the primers and the PCR program: initial denaturation at 94 °C for 2 min, followed by 30 cycles at 94 °C for 20 s, 55 °C for 30 s, 72 °C for 1 min, and a final elongation step at 72 °C for 10 min. The PCR products were purified and sequenced as described above. Eight DNA sequences were obtained for each sample from Sengenyama, and four sequences for each sample from Nagiso.
For all AM fungal sequences obtained with primers AML1 and AML2 (from the samples collected in 2011), multiple sequence alignments were conducted using ClustalW in MEGA 6 (Tamura et al. 2013) after the removal of non-AM fungal and chimera sequences suggested by the basic local alignment search tool (BLAST) (Altschul et al. 1997). The region for the alignment is a partial sequence that can be amplified with PCR primers NS31 (Simon et al. 1992) and AM1 (Helgason et al. 1998). Neighbor-joining analysis (Saitou and Nei 1987) was performed for the aligned sequence dataset using MEGA 6. Subsequently, AM fungal phylotypes were defined based on monophyletic units, in which all pairwise comparisons of sequences in the clade exhibited identities of greater than 97 %. Sequence identities were computed using Clustal X version 2.0.12 (Larkin et al. 2007). The 97 % sequence identity threshold of the NS31–AM1 region of SSU rDNA had been used for the definition of AM fungal taxa, designated as “virtual taxa (VTX),” in the MaarjAM database (Öpik et al. 2010).
For the samples collected from Nagiso in 2012 (study 2), partial AM fungal SSU rDNA was amplified with primers NS31 and AML2 using TaKaRa Ex Taq, Hot Start Version. To identify sequences from different samples, a set of 10 decamer Multiplex Identifier (MID) sequences for Roche/454 sequencing (Roche, Basel, Switzerland) was inserted at the 5′ end of NS31. The PCR mixture composition was as described above, and the PCR program was performed as follows: initial denaturation at 94 °C for 2 min, followed by 35 cycles at 94 °C for 30 s, 58 °C for 1 min, 72 °C for 1 min, and a final elongation step at 72 °C for 10 min. After purification using a Gel/PCR DNA Fragments Extraction Kit, the PCR products were pyrosequenced on a 454 GS Junior System (Roche Diagnostics, Basel, Switzerland). From all the sequences obtained by pyrosequencing, those with more than 350 base pairs (bps) were selected and sorted by MID sequences. After removing the MID and primer sequences, chimera reads were eliminated using the UCHIME program v. 4.2.40 (Edgar et al. 2011), with a minimum score of 0.1 to report a chimera. Sequence data were assembled using Assams v. 0.1.2013.01.01 (Tanabe and Toju 2013) with a minimum similarity setting of 97 % to set fungal operational taxonomic units (OTU). The longest sequences of each OTU were included as representative in the AM fungal sequence dataset obtained in 2011, and multiple sequence alignments were conducted using MEGA 6. The aligned sequences were cut at the shortest length (380 bps) among the representatives obtained by pyrosequencing. Neighbor-joining analysis was performed as described above to confirm the phylotype affiliation of the sequences. Those not affiliated to any of the phylotypes were considered to be representative sequences of novel phylotypes.
Representative DNA sequences arbitrarily selected from each AM fungal phylotype were deposited in the DNA Data Bank of Japan (DDBJ) with accession numbers LC027165–LC027221. These selected sequences were analyzed using a BLAST search against the GenBank database, and several sequences identified at the species level with more than 94 % identity were downloaded. Representative AM fungal sequences from P. sakuraii (Yamato et al. 2011b) and a deciduous, broad-leaved forest in Mizuho, Kyoto Pref., Japan (Yamato and Iwase 2005), were also downloaded. Furthermore, sequences with VTX codes from the MaarjAM database (Öpik et al. 2010) were downloaded for each phylotype. Claroideoglomus etunicatum (Y17639) in Claroideoglomeraceae was selected to be the outgroup because all AM fungi detected in this study belonged to Glomeraceae. The sequence data were subjected to multiple sequence alignments and a neighbor-joining analysis using MEGA 6 with a bootstrap analysis of 1000 replications (Felsenstein 1985). The phylogenetic tree obtained in the analysis was drawn using TreeView software (Page 1996).
To compare the proportion of AM fungal phylotypes to those in Mizuho (Yamato and Iwase 2005), all AM fungal sequences obtained from this site were incorporated into the above dataset and analyzed using the neighbor-joining method. The geographic distribution of AM fungi closely related to the mycobionts of P. sakuraii was inferred from the available sequences classified into the VTX00194 taxon, in which the sequences of P. sakuraii mycobionts are included. All sequences covering the NS31–AM1 region under this VTX code were analyzed using the neighbor-joining method. In this analysis, the localities and vegetation types of sampling sites were retrieved for all sequences.
Correlation between read abundance and genic abundance
Though read abundance has been often used as a measure of the phylotype abundance in culture-independent analyses of DNA amplified from environmental samples, technological artifacts such as DNA extraction, primer selectivity, and DNA sequencing could bias relative quantification of biological abundance (Amend et al. 2010). Therefore, it was necessary to confirm correlation between the read abundance and the genic abundance in the experimental system of this study. For this, the effect of the ratio of Glo1 fungal DNA (the phylotype including the dominant mycobionts of P. sakuraii—Yamato et al. (2011b)) in PCR templates on the relative read abundance of Glo1 fungi was examined. To prepare the DNA templates, a series of mixtures was made of two DNA extracts in different ratios, one with high detection of Glo1 fungi and the other with lower detection. Among the DNA extracts obtained from the samples in 2011, N1c was selected for high detection of Glo1 fungi and S5a for lower detection of the fungi. The DNA concentration was adjusted to 1 ng μl−1, and six different mixtures, M1–M6, were prepared as follows: M1 (0 μl of N1c and 5 μl of S5a), M2 (1 μl of N1c and 4 μl of S5a), M3 (2 μl of N1c and 3 μl of S5a), M4 (3 μl of N1c and 2 μl of S5a), M5 (4 μl of N1c and 1 μl of S5a), and M6 (5 μl of N1c and 0 μl of S5a). An AM fungal SSU rDNA fragment was amplified from 1 μl of the each DNA mixture using the primers AML1 and AML2 and cloned as described. A part of the DNA fragment was subsequently sequenced using NS31 as sequencing primer. After assigning the obtained AM fungal sequences into AM fungal phylotypes, the relation between the ratio of N1c in the PCR templates and the ratio of Glo1 fungi detected was investigated by Pearson’s coefficient test.
Data analysis
A rarefaction analysis was performed based on the number of AM fungal phylotypes detected against the number of sequences in each soil core sample using the Analytic Rarefaction program v. 1.3 (Hooland 2003). χ 2 analysis was used to examine the differences between the AM fungal communities in Sengenyama and Nagiso on the basis of the 2011 data. For the samples collected in Nagiso in 2012, the correlation between the proportion of Glo1 fungal sequences in the root samples and the number of P. sakuraii shoots counted in a 1 m2 area around the sampling position was examined using Pearson’s coefficient test.
Results
Community of arbuscular mycorrhizal fungi in two P. sakuraii habitats (study 1)
Molecular identification of tree roots in soil cores using the DNA sequence of the trnL intron revealed six plant taxa Chamaecyparis, Clethra, Cleyera, Ilex, Symplocos, and Araliaceae in Sengenyama (Table 1). Meanwhile, only Chamaecyparis was found in Nagiso. The vegetation survey at these sites suggests that these taxa represent Chamaecyparis obtusa (Cupressaceae), Clethra barvinervis Sieb. et Zucc. (Clethraceae), Cleyera japonica (Pentaphylacaceae), Ilex pedunculosa (Aquifoliaceae), and Symplocos coreana (Lev.) Ohwi (Symplocaceae). Eleutherococcus sciadophylloides (Fr. & Sav.) H. Ohashi and Evodiopanax innovans (Sieb. et Zucc.) Nakai were the most likely species to have been sampled among the Araliaceae.
Three hundred forty-two AM fungal sequences were obtained from the tree roots collected in 2011 (study 1): 189 from Sengenyama (17–35 sequences per soil core sample) and 153 from Nagiso (15–25 sequences per sample). These sequences were divided into seven phylotypes (Glo1–Glo7) in Glomeraceae (Fig. 2, Tables 2 and 3). The rarefaction analysis confirmed that, within ±0.5 phylotypes, the number of sequences reached a confidence level of more than 95 % for each soil core sample. Among the seven phylotypes, Glo5 was not found in Nagiso, but the remaining six types were found in both sites. The percentage of Glo1, including the dominant mycobionts of P. sakuraii reported by Yamato et al. (2011b) (Fig. 2), was above half (54.9 %) in Nagiso, but only 13.2 % in Sengenyama.The all Glo1 sequences obtained showed greater than 99 % sequence identity with the P. sakuraii mycobionts.
Neighbor-joining phylogenetic tree based on partial sequences (446–453 bps) of the small subunit of nuclear ribosomal RNA gene (SSU rDNA) of AM fungi in the Glomeraceae obtained from tree roots in soil cores collected at a 20-cm distance from P. sakuraii shoots in Sengenyama and Nagiso. Each code corresponds to the sample and clone numbers. Phylotypes of the detected fungi (Glo1–9) are shown (see Table 3). The sequence used for Glo1–Glo7 is from the 2011 samplings, whereas those of Glo8 (A4-01) and Glo9 (A4-02) were obtained from the 2012 sampling. Sequences obtained from the MaarjAM database are shown with fungal virtual taxon (VTX) codes. GenBank accession numbers are given for all sequences. The tree is rooted to Claroideoglomus etunicatum (Y17639) in the Claroideoglomeraceae. Bootstrap values with 1000 replications are shown where they exceed 50 %. The scale bar shows the number of substitutions per site
Correlation between read abundance and genic abundance
The correlation between the read abundance and the genic abundance was confirmed experimentally. The concentration of the purified PCR products amplified from M1–M6 mixtures of the two DNA extracts N1c and S5a (see “Materials and Methods” section) was 6.3–12.0 ng μl−1 (Table S1). From each of the DNA mixture, 18–27 partial AM fungal sequences were obtained, and the sequences were assigned to the AM fungal phylotypes (Table S1). Pearson’s coefficient test revealed a statistically significant (P < 0.001) strong correlation (R 2 = 0.954) between the ratio of N1c in the PCR templates and the ratio of Glo1 fungi detected (Fig. S1).
Relation of mycobiont abundance to P. sakuraii density at the micro-habitat scale (study 2)
In study 2 in Nagiso, 0–52 P. sakuraii shoots (9.16 ± 15.6; mean ± SD) were found in a 1 m2 area surrounding the sampling positions. The pyrosequencing of 25 samples generated 1964 sequences longer than 350 bps. These sequences were divided into seven phylotypes, among which two phylotypes, Glo8 and Glo9, were newly discovered (Fig. 2, Tables 2 and 3). All sequences in Glo1 showed greater than 99 % sequence identity with the dominant P. sakuraii mycobionts. The proportion of Glo1 in the total AM fungal sequences was 59.0 % (1158/1964) (Table 3), and Glo1 fungi were detected in all soil core samples around P. sakuraii shoots. However, the proportion of Glo1 in each sample did not correlate with the number of shoots (R = −0.093).
Comparisons with Mizuho and database sequences
All AM fungal sequences obtained from Mizuho (Yamato and Iwase 2005), with the exception of one sequence, were affiliated with the six phylotypes from the present study, i.e., 16 sequences in Glo1, 47 in Glo2, 8 in Glo3, 14 in Glo4, 1 in Glo6, and 2 in Glo7 (Table 3). Among the 16 Glo1 sequences, 10 coincided with the dominant mycobiont of P. sakuraii with more than 99 % identity.
Almost all of the 30 sequences downloaded from the MaarjAM database covering the region between the primers NS31 and AM1 in VTX00194 were obtained from forest environments (Fig. 3). Among these, the 21 sequences belonging to the clade of P. sakuraii mycobionts were collected from Japanese forests in Nagano, Kyoto, Gifu, and Amami. The one exception was AB601898, which was obtained from P. sakuraii roots found in a Chinese forest.
Neighbor-joining phylogenetic tree based on partial sequences (506–508 bps) of the small subunit of nuclear ribosomal RNA gene (SSU rDNA) of fungal virtual taxon (VTX) 00194 in Glomeraceae. The accession number of the type sequence is shown in bold. The tree is rooted to Glomus macrocarpum (FR750376). Vegetation types and GenBank accession numbers are shown for the analyzed sequences. Bootstrap values with 1000 replications are shown where they exceed 50 %. The scale bar shows the number of substitutions per site
Discussion
Only nine AM fungal phylotypes in the Glomeraceae were detected in the two P. sakuraii habitats, Sengenyama and Nagiso, even though pyrosequencing was applied for study 2. Since fungal DNA of the other Glomeromycota families could be amplified by the primer sets AML1–AML2 or NS31–AML2 in previous studies (Lee et al. 2008; Van Geel et al. 2014), the exclusive detection of Glomeraceae fungi in the tree roots seems to reflect the actual fungal communities of the habitats. Among the nine phylotypes, it is reasonable to consider the Glo1 fungi as the potential mycobionts of P. sakuraii, because the predominant root mycobionts of P. sakuraii previously reported in Nagiso samples (Yamato et al. 2011b) belong to this phylotype with over 99 % identity. A comparison of AM fungal communities in tree roots of the two P. sakuraii habitats, a thriving one in Nagiso and a sparsely populated one in Sengenyama, showed that the percentage of Glo1 fungi was high (54.9 %) in Nagiso but low (13.2 %) in Sengenyama. Although the rarity of P. sakuraii limited sampling replications, these data indicate that the abundance of potential mycobionts seems to be important for the vigor of P. sakuraii populations. Likewise, McCormik et al. (2009) have shown that the level of abundance of Tomentella (Thelephoraceae, Basidiomycota), exclusive mycobionts for the mycoheterotrophic orchid Corallorhiza odontorhiza var. odontorhiza (Willd.) Nutt., correlates with the number of plant partners in the habitats. These results suggest that not only the presence but also the relative abundance of potential mycobionts may determine the distribution pattern of mycoheterotrophic plants. This is further endorsed by the fact that mycorrhizal specificity is not necessarily the main reason for the limited distribution of rare mycorrhizal specialists, as previously shown by Ogura-Tsujita and Yukawa (2008). It may also be reasonable to assume that the number of P. sakuraii individuals affects the distribution pattern of the mycobionts. However, the effect of P. sakuraii on the abundance of the mycobionts seems to be negligible, because the relative abundance of Glo1 fungi did not correlate with the number of P. sakuraii shoots at the micro-habitat scale, as shown in the 2012 investigation in Nagiso.
Dumbrell et al. (2010) have suggested that AM fungal communities are typically dominated by a single phylotype, representing on average 40 % of the total abundance within the community, but that the dominant AM fungal type varies among communities. In the present study, the proportion of Glo1 detection was more than 50 % in Nagiso. Since all published data of VTX00194, a fungal virtual taxon in which Glo1 fungi are included, are recorded from forest sites, this fungal group is likely to be mainly distributed in forest environments. However, little is known about the abundance and optimal growth conditions of Glo1 in Japanese tree stands.
The Nagiso site is primarily composed of planted Chamaecyparis obtusa trees, and molecular identification of mycorrhizal tree roots revealed that all mycorrhiza in association with Glo1 are formed in C. obtusa roots. In Sengenyama, Glo1 was detected from 66.6 % (4/6) of soil core samples containing C. obtusa roots. Though little is known about the influence of tree species on the community structure of AM fungi, these results seem to indicate a tripartite relationship among P. sakuraii, Glo1 fungi, and C. obtusa, which may be similar to the specific relationship between Rhizanthella (a mycoheterotrophic orchid), Ceratobasidium (a basidiomycete fungus), and Melaleuca (a tree in Myrtaceae), found in Australia (Bougoure et al. 2009).
In many studies with sequences amplified from environmental samples, it is assumed that read abundance are semi-quantitative: the difference in the proportional abundance of a given species across the samples generally reflects the actual proportional abundance of that species in the environment (Amend et al. 2010). However, some technological artifacts in DNA extraction, PCR, and sequencing could affect the quantification of the read abundance, as well as various copy numbers of ribosomal genes (Amend et al. 2010). The confirmation of a strong correlation between the ratio of N1c, a DNA extract with high Glo1 detection in the PCR templates and the ratio of Glo1 fungi detected (Table S1, Fig. S1), supports the validity of using the DNA read abundance as a measure of genic abundance in the experimental system of the present study.
More intensive sampling was conducted in the expanded sampling area in Nagiso in 2012 (study 2). Since the percentage of Glo1 fungi was similar in the two consecutive sampling years (54.9 % in 2011 and 59.0 % in 2012), the high abundance of Glo1 fungi in this thriving P. sakuraii habitat seems to be stable. Moreover, Glo1 fungi are likely to be widespread at this site because this phylotype was detected in all the samples. However, the abundance of Glo1 fungi did not correlate with the number of P. sakuraii shoots at the micro-habitat scale. This indicates that other factors such as seed dispersal may affect the distribution pattern of P. sakuraii at the micro-habitat scale. Furthermore, the long root system of P. sakuraii (Watanabe 1944), unusual in mycoheterotrophs, may also contribute to a low correlation between mycobiont abundance and the number of P. sakuraii shoots at the micro-habitat scale. In typical mycoheterotrophs with reduced roots or rhizomes, the exact positions of plant individuals should overlap with the distribution of fungal partners. Meanwhile, P. sakuraii can thrive in positions irrespective of the existence of its mycobionts because the extensive root system provides greater opportunities to contact fungal partners.
Among the nine AM fungal phylotypes detected in this study, six types were shared by the two habitats, Sengenyama and Nagiso, separated by 52 km. The AM fungal community of these sites was compared with that of Mizuho, a secondary deciduous broad-leaved forest in Kyoto Prefecture, devoid of P. sakuraii, based on the comprehensive data of AM fungal diversity in this site published by Yamato and Iwase (2005). This forest is separated from Sengenyama and Nagiso by a distance of 159 and 207 km, respectively, and comprises AM trees such as Ilex pedunculosa (Aquifoliaceae), Clethra barvinervis (Clethraceae), Rhus trichocarpa Miq. (Anacardiaceae), Eurya japonica Thunb. (Pentaphylacaceae), and Chamaecyparis obtuse, as well as several ectomycorrhizal tree species. The Mizuho forest also shares the same six AM fungal phylotypes with Sengenyama and Nagiso. These results suggest that AM fungal communities are quite similar in the three tree stands in Sengenyama, Nagiso, and Mizuho, although the composition of tree species differs. Among the 89 AM fungal sequences obtained in Mizuho (Yamato and Iwase 2005), 10 sequences coincided with those of the P. sakuraii mycobionts with more than 99 % identity. The relative abundance of potential mycobionts of P. sakuraii in Mizuho (11.2 %) is similar to that in Sengenyama (13.2 %). Thus, it is likely that the potential mycobionts of P. sakuraii are not rare in Japanese temperate forests.
In conclusion, the results reported here suggest that abundance of the mycobionts in tree roots may affect the distribution of Petrosavia sakuraii. As mentioned above, P. sakuraii produces large numbers of seeds (Takahashi et al. 1993), thus in situ seed sowing with consideration of AM fungal communities may be a promising method for the reintroduction or reinforcement of P. sakuraii populations. However, further studies on the prosperity and decline of the AM fungi in forest ecosystems are required for the conservation of this plant.
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amend AS, Seifert KA, Bruns TD (2010) Quantifying microbial communities with 454 pyrosequencing: does read abundance count? Mol Ecol 19:5555–5565
Bidartondo MI, Redecker D, Hijri I, Wiemken A, Bruns TD, Domínguez L, Sérsic A, Leake JR, Read DJ (2002) Epiparasitic plants specialized on arbuscular mycorrhizal fungi. Nature 419:389–392
Bougoure JJ, Ludwig M, Brundrett M, Grierson PF (2009) Identity and specificity of the fungi forming mycorrhizas with rare, mycoheterotrophic Rhizanthella gardneri (Orchidaceae). Mycol Res 113:1097–1106
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: is there a role for stochastic processes? J Ecol 98:419–428
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Eriksson O, Kainulainen K (2011) The evolutionary ecology of dust seeds. Perspect Plant Ecol 13:73–87
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Franke T, Beenken L, Döring M, Kocyan A, Agerer R (2006) Arbuscular mycorrhizal fungi of the Glomus-group A lineage (Glomerales; Glomeromycota) detected in myco-heterotrophic plants from tropical Africa. Mycol Prog 5:24–31
Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW (1998) Ploughing up the wood-wide web? Nature 394:431
Hentrich H, Kaiser R, Gottsberger G (2010) The reproductive biology of Voyria (Gentianaceae) species in French Guiana. Taxon 59:867–880
Hooland SM (2003) Analytic rarefaction 1.3. User’s guide and application. https://www.uga.edu/_strata/software/AnRare/Readme.html. Accessed 10 Jan 2011
Klooster M, Culley T (2009) Comparative analysis of the reproductive ecology of Monotropa and Monotropsis: two mycoheterotrophic genera in the Monotropoideae. Am J Bot 96:1337–1347
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Leake JR (1994) The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol 127:171–216
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbial Ecol 65:339–349
McCormick MK, Whigham DF, O’Neill JP, Becker JJ, Werner S, Rasmussen HN, Bruns TD, Taylor DL (2009) Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae. Ecol Monogr 79:619–635
Merckx V (2013) An Introduction. In: Merckx V (ed) Mycoheterotrophy. Springer, New York, pp 1–17
Merckx V, Bidartondo MI (2008) Breakdown and delayed cospeciation in the arbuscular mycorrhizal mutualism. Proc R Soc B 275:1029–1035
Merckx V, Bidartondo MI, Hynson NA (2009) Mycoheterotrophy: when fungi host plants. Ann Bot 104:1255–1261
Merckx V, Stöckel M, Fleischmann A, Bruns TD, Gebauer G (2010) 15N and 13C natural abundance of two mycoheterotrophic and a putative partially mycoheterotrophic species associated with arbuscular mycorrhizal fungi. New Phytol 188:590–596
Merckx V, Smet EF, Specht CD (2013) Biogeography and conservation. In: Merckx V (ed) Mycoheterotrophy. Springer, New York, pp 103–155
Ministry of the Environment (2012) Red list. http://www.biodic.go.jp/rdb/rdb_f.html. Accessed 9 Janurary 2014
Mizuno M, Tanaka M, Hukuhara H, Suzuki T (1974) On the habitat of Protolirion sakuraii Dandy studies on the plants in Gifu prefecture and adjacent prefecture IV. J Geobotany 21:70–81 (in Japanese)
Ogura-Tsujita Y, Yukawa T (2008) High mycorrhizal specificity in a widespread mycoheterotrophic plant Eulophia zollingeri (Orchidaceae). Am J Bot 95:93–97
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier Ü, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241
Page RDM (1996) An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Simon LM, Lalonde TD, Bruns TD (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58:291–295
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Takahashi H, Nishio E, Hayashi H (1993) Pollination biology of the saprophytic species Petrosavia sakuraii (Makino) van Steenis in central Japan. J Plant Res 106:213–217
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanabe AS, Toju H (2013) Two new computational methods for universal DNA barcoding: a benchmark using barcode sequences of bacteria, archaea, animals, fungi, and land plants. Plos One 8(10):e76910
Van Geel M, Busschaert P, Honnay O, Lievens B (2014) Evaluation of six primer pairs targeting the nuclear rRNA operon for colonization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. J Microbiol Methods 106:93–100
Watanabe K (1944) Morphologish-biologische studien uber Miyoshia sakuraii Makino. J Jpn Bot 20:85–93
Waterman RJ, Klooster MR, Hentrich H, Bidartondo MI (2013) Species interactions of mycoheterotrophic plants: specialization and its potential consequences. In: Merckx V (ed) Mycoheterotrophy. Springer, New York, pp 267–296
Yamato M, Iwase K (2005) Community analysis of arbuscularmycorrhizal fungi in a warm-temperate deciduous broad-leaved forest and introduction of the fungal community into the seedlings of indigenous woody plants. Mycoscience 46:334–342
Yamato M, Yagame T, Iwase K (2011a) Arbuscular mycorrhizal fungi in roots of non-photosynthetic plants, Sciaphila japonica and Sciaphila tosaensis (Triuridaceae). Mycoscience 52:217–223
Yamato M, Yagame T, Shimomura N, Iwase K, Takahashi H, Ogura-Tsujita Y, Yukawa T (2011b) Specific arbuscular mycorrhizal fungi associated with non-photosynthetic Petrosavia sakuraii (Petrosaviaceae). Mycorrhiza 21:631–639
Acknowledgments
We thank the Education Board of Kani City for permission for sampling in the Sengenyama habitat. This study was supported by the Global COE Program “Advanced utilization of fungus/mushroom resources for sustainable society in harmony with nature” and a Grant-in-Aid for Scientific Research (15H04417 and 15K14442 to T.Y.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Correlation between the ratio of N1c, a DNA extract with much Glo1 detection, in the PCR templates and the ratio of Glo1 sequences among AM fungal sequences obtained in the PCR products to confirm a validity of using the DNA read abundance as the measure of genic abundance in the experimental system of this study. (GIF 69 kb)
Table S1
(XLSX 9 kb)
Rights and permissions
About this article
Cite this article
Yamato, M., Takahashi, H., Shimono, A. et al. Distribution of Petrosavia sakuraii (Petrosaviaceae), a rare mycoheterotrophic plant, may be determined by the abundance of its mycobionts. Mycorrhiza 26, 417–427 (2016). https://doi.org/10.1007/s00572-016-0680-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0680-9