Abstract
Truffles are edible hypogeous ascomycetes highly appreciated worldwide, especially the black truffle (Tuber melanosporum Vittad.). In recent decades, the cultivation of the black truffle has expanded across the Mediterranean climate regions in and outside its native range. Members of the Thelephoraceae (Thelephorales, Agaricomycetes, Basidiomycota) are commonly found in truffle plantations, but their co-occurrence with Tuber species and other members of the fungal community has been scarcely reported. Thelephoraceae is one of the most represented families of the ectomycorrhizal fungal community in boreal and Mediterranean forests. To reveal the diversity of these fungi in T. melanosporum-cultivated plantations, ten orchards located in the Navarra region (Northern Spain) were surveyed for 2 years. Morphological and molecular approaches were used to detect and identify the Thelephoraceae ectomycorrhizas present in those plantations. Ten different mycorrhizal types were detected and described. Four of them were morphologically identified as Tomentella galzinii, Quercirhiza cumulosa, Q. squamosa, and T39 Thelephoraceae type. Molecular analyses revealed 4–6 operational taxonomic units (OTUs), depending on the nucleotide database used, but similarities remained under 95 % and no clear species assignments could be done. The results confirm the diversity and abundance of this fungal family in the ectomycorrhizal community of black truffle plantations, generally established in Mediterranean areas. The occurrence and relative abundance of Thelephoraceae ectomycorrhizas is discussed in relation to their possible influence on truffle production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Truffles are edible hypogeous ascomycetes highly appreciated worldwide, especially the valuable black truffle (Tuber melanosporum Vittad.). In recent decades, the cultivation of the black truffle has expanded across all the Mediterranean climate regions, as well as to regions outside the European natural range including Australia, New Zealand, Chile, North America, and South Africa (Reyna and García-Barreda 2014). The harvesting of wild T. melanosporum plays an important role in the rural economy of Spain, with an estimated income of 7.5 million euros per year. The value that truffles add to forests is particularly interesting in Mediterranean forests. Given their low productivity, it could promote involvement of the rural communities in forest protection and management. In Spain, plantations have made up for the collapse of wild production over the past few decades. A current plantation surface is estimated to be around 10,000 ha which produces 60 % of the total truffle yield (Reyna and García-Barreda 2014).
Members of the Thelephoraceae (Thelephorales, Agaricomycetes, Basidiomycota) are commonly found in truffle plantations and old-growth Quercus forests (Richard et al. 2005), but their interactions with Tuber species and other members of the fungal community have been scarcely reported (De Miguel et al. 2014). The first studies carried out in truffle orchards revealed only two ectomycorrhizas belonging to Thelephoraceae types: Forma 3 (Gregori et al. 1990), corresponding to Tomentella sp.; and SB type (Giraud 1988), corresponding to Tomentella galzinii Bourdot (Kõljalg et al. 2001). Subsequent studies on ectomycorrhizal fungal diversity of black truffle plantations reported T. galzinii, T. subtestacea Bourdot & Galzin, T. ellisii (Sacc.) Jülich & Stalpers, T. badia (Link) Stalpers, T. coerulea (Bres.) Höhn. & Litsch., T. lateritia Pat., Quercirhiza squamosa (Palfner 1995) (Suz et al. 2010; Otsing and Tedersoo 2015), and many other Thelephoraceae morphotypes which only could be identified to family level. Murat et al. (2005) and Leonardi et al. (2013) concluded that Thelephoraceae is the most frequent ectomycorrhizal family in Tuber magnatum Pico. ecosystems. Similar results were found by Benucci et al. (2011) and Salerni et al. (2014) in T. aestivum Vittad. plantations and by Benucci et al. (2014) in T. macrosporum-productive sites. Napoli et al. (2010) and Mello et al. (2011) used 454 pyrosequencing to conclude that Thelephorales are one of the most highly represented orders in truffle ecosystems.
Thelephoroid fungi (specifically species of Thelephoraceae Chevall.) form resupinate sporocarps, mostly inconspicuous, and are considered one of the most abundant and diverse group of mycobionts in ectomycorrhizal fungal communities. Species in the genera Tomentella Pers. ex Pat., Pseudotomentella Svrček, Thelephora Ehrh. ex Willd., and Tomentellopsis Hjortstam are worldwide members of ectomycorrhizal fungal communities and are especially abundant in boreal forests (Erland and Taylor 1999; Kõljalg et al. 2000; Jakucs et al. 2005; Abarenkov et al. 2008; Suvi et al. 2010) but also occur in African, Asian, European, and South American ecosystems (Kõljalg et al. 2000; Dämmrich 2004–2015; Shiryaev 2008; Lee et al. 2010; Wei and Agerer 2010; Peintner and Dämmrich 2012; Yorou et al. 2012a, b). The systematic study of tomentelloid fungi by Kõljalg (1996) regarding distributions, new records, cladistic analyses, and microstructure is complemented by many other studies dealing with the geographic distribution of those species. In the Iberian Peninsula, Tellería (1980) described eight novel species for the Spanish mycological catalogue and Melo et al. (2004) compiled the approximately 50 species known.
Some members of Thelephoraceae, such as the genus Tomentella (Basidiomycota, Thelephoraceae), were considered saprotrophic until the 1980s (Kõljalg 1996). Miller (1982) was the first to suggest that tomentelloid species likely form ectomycorrhizas with a broad range of hosts.
Morphological and anatomical descriptions of Thelephoraceae ectomycorrhizal morphotypes are scarce in the literature (Jakucs and Erős-Honti 2008; Wei and Agerer 2010; Jakucs et al. 2015). The first detailed descriptions of Tomentella were probably those of Danielson et al. (1984) and Danielson and Pruden (1989). According to Wei and Agerer (2010), Binder et al. (2013), and Jakucs et al. (2015), there are 50 Thelephoraceae ectomycorrhizas reported to date, but only 15 of them have been morphologically described: 12 Tomentella species: T. atroarenicolor Nikol., T. badia, T. brunneorufa M.J. Larsen, T. bryophila (Pers.) M.J. Larsen, T. ferruginea (Pers.) Pat., T. galzinii, T. lapida (Pers.) Stalpers, T. pilosa (Burt) Bourdot & Galzin, T. stuposa (Link) Stalpers, T. subclavigera Litsch., T. sublilacina (Ellis & Holw.) Wakef., and T. subtestacea Bourdot & Galzin; two Pseudotomentella: P. humicola M.J. Larsen and P. tristis (P. Karst.) M.J. Larsen; and one Tomentellopsis: T. submollis (Svrček) Hjortstam. More than 30 Thelephoraceae morphotypes still remain unidentified (Agerer et al. 1996–2008; Wei and Agerer 2010; Jakucs et al. 2015).
Mello et al. (2011) used pyrosequencing analysis to assess the soil fungal communities inside and outside truffle brûlés and found that 16 % of the total sequences inside the brûlé and 12 % outside belonged to Thelephorales. The rapid increase of powerful molecular tools to characterize fungal soil communities have yielded considerable information about mycorrhizal fungal species that coexist with valued truffles in natural plant communities and truffle plantations. However, these studies should be paired with accurate taxonomic and molecular studies of sporocarps to relate the deposited DNA sequences with the described ectomycorrhizal morphotypes.
The aim of the present work is to describe and identify the different Thelephoraceae morphotypes present in productive black truffle plantations established in Northern Spain. Molecular analysis was used as an identifying tool in cases of insufficient morphological characters in an attempt to identify unknown morphotypes to the species level. The relative abundance of Thelephoraceae ectomycorrhizas is discussed in relation to their possible influence on truffle production.
Materials and methods
Study area and sampling design
The study was carried out in ten productive black truffle plantations composed of previously inoculated Quercus ilex L. and Corylus avellana L. species established between 1990 and 1993 in the Navarra region (Northern Spain) in set-aside lands, formerly used for cereal production, at 400–800 m.a.s.l. All the soils are calcareous, shallow, stony, and well-aired. The macrobioclimate is Mediterranean: mean annual temperatures are between 11 and 13 °C and mean annual precipitation is 600–900 mm (Table 1). Current T. melanosporum sporocarp production averages between 6 and 12 kg/ha per season. Wild black truffles are traditionally hunted in the surrounding holm oak (Quercus ilex L. subsp. ballota (Desf.) Samp.) forests.
Root samples were taken with a soil corer (4 cm in diameter, 20 cm in depth), in spring and autumn of 2006 and 2007 within the burned area (brûlés) around six randomly selected holm oaks in each truffle plantation. The same trees were chosen in subsequent samplings. A total of 240 samples of approximately 250 cc in volume were each processed. Samples were preserved at 4 °C until examination. Soil samples were individually soaked and sieved to separate root fragments from the surrounding soil. Root tips were examined under the stereomicroscope, separating non-mycorrhizal and mycorrhizal roots.
Characterization of ectomycorrhizas
Thelephoraceae ectomycorrhizal morphotypes were identified according to the anatomical characteristics defined by Agerer (2006): heterogeneous mantle type assemblage, melanised hyphae with thelephoric acid, smooth or rough, incrustated or not, with or without clamp connections, mostly with cystidia, and frequent rhizomorphs, mainly with curled cells.
Rhizomorphs were classified according to Agerer (1999). Exploration types of ectomycorrhizas were also considered according to Agerer (2001). Samples of each morphotype were stored in formol, acetic, alcohol (FAA) fixative (Agerer 1986) and deposited in the Universidad de Navarra Herbarium PAMP-Mycorrhiza.
The morphotypes presenting Thelephoraceae features were separated, carefully cleaned with tap water, and stored at −21 °C until use. DNA extraction was performed with the PowerSoil™ DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA) from groups of 5–10 root tips from each morphotype, according to the manufacturer’s instructions. Nuclear ribosomic DNA internal transcribed spacer (ITS) regions, comprised between the 18S and 28S genes, were amplified by polymerase chain reaction (PCR), which contains 2 μl (from a 10-μM stock) of each of the universal fungal primers ITS1F (5′-TCCGTAGGTGAACCTGCGG-3′) (Gardes and Bruns 1993) and either ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. 1990) or ITS4B (5′-CAGGAGACTTGTACACGGTCCAG-3′) (Gardes and Bruns 1993), 25 μl of 2X PCR Solution Premix Taq™ (TaKaRa Ex Taq™ Version, TAKARA BIO INC., Japan), 2 μl of DNA template (corresponding to 20–40 ng of total DNA), and high performance liquid chromatography (HPLC) water to a final volume of 50 μl. PCR reactions were performed using a GeneAmp® 9700 PCR thermocycler (Applied Biosystems, Foster City, CA, USA) with an initial denaturation step of 95 °C for 3 min, followed by 35 cycles of amplification at 95 °C for 20 s, 55 °C for 30 s, and 72 °C for 40 s and a final extension step of 5 min at 72 °C. The PCR products were purified with a Roche® High Pure PCR Product Purification Kit (Roche Applied Science, Indianapolis, IN, USA) and sequenced in both directions with a 3730 DNA analyser (Applied Biosystems, Foster City, CA, USA). The obtained consensus sequences were registered in the NCBI GenBank® database (http://www.ncbi.nlm.nih.gov/nucleotide) (Benson et al. 2005). Accession numbers were HQ289861–HQ289867. Fungal identification was carried out by searching highly similar sequences in the GenBank and UNITE (http://unite.ut.ee/) (Kõljalg et al. 2013) databases using the megablast procedure.
Phylogenetic positions of the morphotypes studied within the genus Tomentella and Pseudotomentella were investigated by building a maximum likelihood tree with MEGA v6 (Tamura et al. 2013) using the available nrDNA ITS sequences from the UNITE database (http://unite.ut.ee/) (Kõljalg et al. 2013). Two sequences per species, if available, belonging to all the species of the two genera were included in the analysis, as well as the UNITE database reference sequences for Boletus appendiculatus Schaeff. and B. pinophilus Pilát & Dermek acting as outgroup. Kimura’s two-parameter model for substitution as well as Gamma distribution were selected based on an estimation performed on MEGA v6 (Tamura et al. 2013). Support of nodes was assessed using a 1000-replication bootstrap test.
The relationships between the occurrence of the Thelephoraceae and Tuber melanosporum mycorrhizas and the average truffle production within each of the ten plantations examined were determined by Pearson linear correlations.
Results
A total of 19 different ectomycorrhizal morphotypes were identified using anatomical and morphological features in all the studied plantations along the sampling periods. The most frequent ectomycorrhizas found in the plantations, with 45.6 % of occurrences, were those belonging to Ascomycetes. Morphotypes belonging to Basidiomycetes accounted for 37 % of the total, and 17.4 % remained unidentified. The occurrences of Thelephoraceae types were 27.9 %. Morphotypes with Thelephoraceae characteristics included T. galzinii, Quercirhiza cumulosa (De Román et al. 2002), Q. squamosa, and T39 (De Román and De Miguel 2005). Six different morphotypes were grouped together as Thelephoraceae type (incl. Tomentella type), due to the lack of information which limited further anato-morphological identification. Morphological characteristics of the ten Thelephoraceae morphotypes with peculiar and distinctive traits are described in Table 2 (some Thelephoraceae types could be different stages of maturity of the same species).
The ten morphotypes included in Table 2 were subjected to molecular analysis. The results of the megablast search including the seven morphotypes with sequences belonging to Thelephoraceae are shown in Table 3. The comparison of the ITS rDNA sequences with the UNITE and GenBank databases allowed the tentative identification of those morphotypes by the closest match. A match percentage of 97 % or higher was considered as a threshold to distinguish species (Peay et al. 2008). The scarce number of identified sequences of Thelephoraceae fungi in the GenBank and UNITE databases made it difficult to obtain conclusive taxonomic identities for the analysed morphotypes. Sequencing of Q. cumulosa and T39 morphotypes were not successful, probably because of the presence of contaminant fungal DNA in the mycorrhizas.
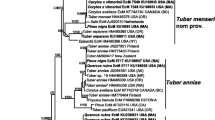
The result of the phylogenetic analysis (Fig. 1) shows the position within Tomentella and Pseudotomentella genera of the Thelephoraceae sequences obtained in this work. Quercirhiza squamosa and Thelephoraceae operational taxonomic units (OTUs 2 and 4 are clustered together next to T. badia sequences. Thelephoraceae OTU 1 is close to T. coerulea, and Thelephoraceae OTU 3 is placed among T. cinerascens, T. lilacinogrisea, and T. muricata. Thelephoraceae OTU 5 appears to have no homology in this tree. Thelephoraceae OTU 6 is close to P. tristis.
Maximum likelihood phylogram of fungal nrDNA ITS sequences for Tomentella and Pseudotomentella species from UNITE database (http://unite.ut.ee/) (Kõljalg et al. 2013). Two sequences per species, when available, were selected. Triangles indicate the sequences obtained from Thelephoraceae ectomycorrhizas found in Navarra black truffle plantations in this study. Boletus appendiculatus Sch. and B. pinophilus Pilát & Dermek sequences were used as outgroup. Nodes with bootstrap values less than 50 % from 1000 replications are not shown
The average production of T. melanosporum sporocarps in the surveyed plantations showed a weak positive correlation with the occurrence of Thelephoraceae mycorrhizas (r = 0.61, P = 0.05). No significant relationship was found between truffle production and black truffle mycorrhizas (r = −0.09, P = 0.79).
Discussion
The ectomycorrhizal diversity found in the ten plantations was relatively low as compared with the previous results obtained in Quercus ilex forests surrounding or nearby the areas surveyed in this study (De Román and De Miguel 2005; Clavería 2007). De Román and De Miguel (2005) revealed 32 morphotypes in the ectomycorrhizal community while Clavería (2007) found 47. Both studies showed a high proportion of Thelephoraceae morphotypes (16 and 17 Thelephoraceae types, respectively). A recent review of 85 papers showed more than 60 different types of ectomycorrhizas reported in black truffle orchards and plantations of different ages, with Thelephoraceae occurring as important members of those fungal communities, especially in stands greater than 10 years of age (De Miguel et al. 2014). However, we have to take into account that 64 out of the 85 reviewed papers identify the ectomycorrhizas of truffle plantations only according to morphological aspects (morphotypes). Although some of these descriptions have been made from controlled inoculations (De Román et al. 2005) or after very detailed studies by Agerer (1987–2012), the specific identifications of the fungal partner can be inaccurate without genetic information. .
Available data indicate that Thelephoraceae become one of the dominant groups of ectomycorrhizal fungi as truffle stands age (Águeda et al. 2010; Benucci et al. 2011; De Miguel et al. 2011; Leonardi et al. 2013; Benucci et al. 2014) with colonization from the natural surrounding woodlands (De Román and De Miguel 2005). Characters of ectomycorrhizas of Thelephoraceae species identified here fit well with those described by Agerer (2006) for Thelephoraceae. However, information about detailed ectomycorrhizal descriptions is insufficient. Our samples of Q. squamosa, according to the description of Palfner (1995) and Palfner and Agerer (1996), have been identified as Tomentella cf. badia by molecular techniques. This result agrees with the description of Binder et al. (2013), especially the epidermoid mantle, although Jakucs et al. (2015) suggest that Q. squamosa could correspond to T. bryophila, a species with angular cells in the outer mantle layer.
Molecular identification of different Tomentella species present in truffle stands have been carried out by several authors and include T. badia, T. bryophila, T. coerulea, T. ellisii, T. ferruginea, T. lateritia, T. lilacinogrisea Wakef., T. sublilacina T. subtestacea, and T. viridula Bourdot & Galzin (Pruett et al. 2008; Iotti et al. 2010; Suz et al. 2010; Benucci et al. 2011; De Miguel et al. 2011; Leonardi et al. 2013; Salerni et al. 2014). In spite of the 3382 Tomentella sequences deposited to date in the GenBank database (2405 corresponding to the ITS region) and 675 in the UNITE database, reliable specific identification is difficult because of the relatively low matching scores and the lack of described species.
Molecular analysis of Thelephoraceae OTUs 2 and 4 shows that they could belong to the same species as Q. squamosa mycorrhizas, although they show morphological differences which may correspond to different stages of development. Quercirhiza squamosa is one of the most common ectomycorrhizal types in T. melanosporum Spanish orchards, becoming more abundant as plantations ages (De Miguel and Sáez 2005; González-Armada et al. 2010a, b; De Miguel et al. 2014; Sánchez et al. 2014).
Thelephoraceae type 1 has a very close and consistent relation with some T. coerulea sequences, but BLAST score (95 %) has not reached the minimum to consider its identity solved. T. coerulea mycorrhizas have not been described yet, so we cannot confirm molecular results using morphological descriptions. Basidiomes of this species were described in Melo et al. (2006) under Quercus species in the Iberian Peninsula. Thelephoraceae type 3 shares the clade with T. cinerascens and T. lilacinogrisea, agreeing with BLAST results in UNITE and GenBank databases, respectively, and with T. muricata. Its identity is yet to be confirmed but its position within the Tomentella clade is well defined. On the contrary, Thelephoraceae type 5 does not correspond to any group. Its low BLAST score (92 % in both databases with 98 % query coverage in GenBank) could mean that this species is not represented in the databases used. One morphotype, appearing in different plantations and dates, has been identified as P. tristis. A previously detailed description is available for this species (Agerer 1994a, b). However, some morphological characteristics of our morphotype, such as color or presence of Thelephoraceae rhizomorphs, do not match Agerer’s descriptions. The low matching percentage (88 %) with P. tristis sequences included in the UNITE database could suggest that this morphotype corresponds to other Pseudotomentella species not included in molecular databases.
Previous studies indicate that the most abundant ectomycorrhizal fungi in black truffle bed communities were those with rhizomorphs (Hebeloma spp., Pisolithus spp., Scleroderma spp., and Tomentella-like types) or those with abundant emanating hyphae (Hebeloma spp., Tomentella spp., and Trichophaea spp.) (De Miguel et al. 2001; De Miguel and Sáez 2005; Baciarelli-Falini et al. 2006; Águeda et al. 2010; Suz et al. 2010). Most Thelephoraceae ectomycorrhizas belong to the medium-distance exploration type (Agerer 2006), with rhizomorphs and emanating hyphae. Duddridge et al. (1980) showed that mycorrhizal rhizomorphs can absorb water and facilitate its transport over ecologically significant distances. The presence of this character in ectomycorrhizal morphotypes would increase their competitive ability. The development of rhizomorph-forming mycorrhizas could be favoured in mature black truffle plantations when brûlés are well developed and tillage is not necessary (Sánchez 2012). These characteristics could allow Thelephoraceae to coexist with already established Tuber ectomycorrhizas which would be favoured by a priority effect due to nursery inoculation, as suggested by Kennedy (2010). Many Thelephoraceae morphotypes have brown or dark hyphae. Pigott (1982) suggested that melanised cell walls in some ectomycorrhizal fungi are mechanisms to tolerate soil drying. Tomentelloid fungi live in the upper organic horizons and may have some advantages over non-melanised fungi when the organic matter is subject to desiccation during dry periods, characteristic of the Mediterranean environments where black truffles occur (Kõljalg et al. 2000).
Thelephoraceae are some of the most common taxa in ectomycorrhizal fungal communities worldwide. This study shows that Thelephoraceae species are also well represented in the ectomycorrhizal fungal community of black truffle plantations of northern Spain. Moreover, our results show a weak, although significant (P = 0.05), correlation between the abundance of Thelephoraceae morphotypes and the average truffle production. Increasing our knowledge on the ectomycorrhizal fungi-sharing habitat with truffles can be a critical step to understand the competitive interactions of T. melanosporum within this community and to enhance the domestication of truffle production.
References
Abarenkov K, Kõljalg U, Suvi T, Jairus T, Saar I, Tedersoo L (2008) High global diversity of Tomentella and Thelephora (Thelephorales, Basidiomycota). 21st New Phytologist Symposium. The ecology of ectomycorrhizal fungi. Montpellier (France)
Agerer R (1986) Studies on ectomycorrhizae II. Introducing remarks on characterization and identification. Mycotaxon 26:473–492
Agerer R (ed) (1987–2012) Colour atlas of ectomycorrhizae. 1st–15th delivery, Einhorn-Verlag + Druck GmbH, Schwäbisch Gmünd
Agerer R (1994a) Pseudotomentella tristis (Thelephoraceae). Eine Analyse von Fruchtkörper und Ektomykorrhizen. Z Mykol 60:143–158
Agerer R (1994b) Pseudotomentella tristis. In: Agerer R (ed) Colour atlas ot ectomycorrhizae, plate 84. Einhorn-Verlag, Sshwäbisch Gmünd
Agerer R (1999) Never change a functionally successful principle: the evolution of Boletales s.l. (Hymenomycetes, Basidiomycotina) as seen from below-ground features. Sendtnera 6:5–91
Agerer R (2001) Exploration types of ectomycorrhizae. A proposal to clasify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107–114. doi:10.1007/s005720100108
Agerer R (2006) Fungal relationships and structural identity of their ectomycorrhizae. Mycol Prog 5:67–107. doi:10.1007/s11557-006-0505-x
Agerer R, Danielson RM, Egli S, Ingleby K, Luoma D, Treu R (1996–2008) Descriptions of ectomycorrhizae. 1st-12th del., Einhorn-Verlag + Druck GmbH, Schwäbisch Gmünd
Águeda B, Fernández-Toirán LM, De Miguel AM, Martínez-Peña F (2010) Ectomycorrhizal status of a mature productive black truffle plantation. For Syst 19:89–97
Baciarelli-Falini L, Rubini A, Riccioni C, Paolocci F (2006) Morphological and molecular analyses of ectomycorrhizal diversity in a man-made Tuber melanosporum plantation: description of novel truffle-like morphotypes. Mycorrhiza 16:475–484. doi:10.1007/s00572-006-0066-5
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2005) GenBank. Nucleic Acids Res 33:D34–D38
Benucci GMN, Raggi L, Alberini E, Grebenc T, Bencivenga M, Falcinelli M, Di Massimo G (2011) Ectomycorrhizal communities in a productive Tuber aestivum Vittad. orchard: composition, host influence and species replacement. FEMS Microbiol Ecol 76:170–184
Benucci GMN, Raggi L, Albertini E, Gógán Csorbai A, Donnini D (2014) Assessment of ectomycorrhizal biodiversity in Tuber macrosporum productive sites. Mycorrhiza 24:281–292
Binder A, Peršoh D, Yorou NS, Verma R, Bässler C, Agerer R (2013) Ectomycorrhizae of Tomentella badia: description and molecular identification. Acta Mycol 48:155–171
Clavería V (2007) Estudio de la comunidad ectomicorrícica de un bosque maduro de Quercus ilex subsp. ballota, su caracterización y dinámica espacio-temporal. Doctoral dissertation, University of Navarra
Dämmrich F (2004–2015) Tomentella and related genera. Online-key for European species of tomentelloid fungi: (with special regard of distribution in Germany) http://www.tomentella.de/Pages/index_e.htm
Danielson RM, Pruden M (1989) The ectomycorrhizal status of urban spruce. Mycologia 81:335–341
Danielson RM, Zak JC, Parkinson D (1984) Mycorrhizal inoculum in a peat deposit formed under a white spruce stand in Alberta. Can J Bot 63:2557–2560
De Miguel AM, Sáez R (2005) Algunas micorrizas competidoras de plantaciones truferas. Pub Biol Univ Nav Serie Botánica 16:1–18
De Miguel AM, De Román M, Etayo ML (2001) Mycorrhizal fungi competing with Tuber melanosporum Vitt. in cultivated truffle beds in NE Spain. Proceedings of the II International Workshop on Edible Mycorrhizal Mushrooms, Christchurch, New Zealand
De Miguel AM, Parladé J, Águeda B, Pera J, González-Armada B, Sáez R (2011) Thelephoroid ectomycorrhizae in black truffle plantations. In: Sixth International Workshop on Edible Mycorrhizal Mushrooms (IWEMM6). Rabat, Morocco
De Miguel AM, Águeda B, Sánchez S, Parladé J (2014) Ectomycorrhizal fungus diversity and community structure with natural and cultivated truffle hosts: applying lessons learned to future truffle culture. Mycorrhiza 24:S5–S18. doi:10.1007/s00572-013-0554-3
De Román M, De Miguel AM (2005) Post-fire, seasonal and annual dynamics of the ectomycorrhizal community in a Quercus ilex L. forest over a 3-year period. Mycorrhiza 15:471–482. doi:10.1007/s00572-005-0353-6
De Román M, Agerer R, De Miguel AM (2002) Quercirhiza cumulosa + Quercus ilex L. subsp. ballota (Desf.) Samp. Descr Ectomyc 6:13–18
De Román M, Clavería V, De Miguel AM (2005) A revision of the descriptions of ectomycorrhizas published since 1961. Mycol Res 109:1063–1104
Duddridge JA, Malibari A, Read DJ (1980) Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature 287:834–836
Erland S, Taylor AFS (1999) Resupinate ectomycorrhizal Fungi. In: Cairney JWG, Chambers SM (eds) Ectomycorrhizae fungi: key genera in profile. Springer, Berlin, pp 347–364
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for Basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Giraud M (1988) Prélèvement et analyse de mycorrhizas. In: Parra C (ed) CTIFL – La truffe. FNPT, Paris, pp 49–63
González-Armada B, De Miguel AM, Cavero RY (2010a) Ectomycorrhizae and vascular plants growing in brulés as indicators of below and above ground microecology of black truffle production areas in Navarra (Northern Spain). Biodivers Conserv 19:3861–3891
González-Armada B, De Miguel AM, Cavero RY (2010b) Estudio de la flora vascular y la micobiota micorrícica en quemados truferos de Navarra (España). Atti 3°Congresso Internazionale di Spoleto sul tartufo, pp 152–162
Gregori G, Lo Bue G, Maggiorotto G (1990) Caratterizzazione di ectomicorrize in tartufaie naturali e di impianto e valutazione della loro competitivitá nei confronti di Tuber magnatum. Atti del Secondo Congresso Internazionale di Spoleto sul Tartufo, pp 219–246
Iotti M, Lancellotti E, Hall I, Zambonelli A (2010) The ectomycorrhizal community in natural Tuber borchii grounds. FEMS Microbiol Ecol 72:250–260. doi:10.1111/j.1574-6941.2010.00844.x
Jakucs E, Erős-Honti Z (2008) Morphological-anatomical characterization and identification of Tomentella ectomycorrhizas. Mycorrhiza 18:277–285. doi:10.1007/s00572-008-0183-4
Jakucs E, Kovács GM, Szedlay G, Erős-Honti Z (2005) Morphological and molecular diversity and abundance of tomentelloid ectomycorrhizae in broad-leaved forest of the Hungarian Plain. Mycorrhiza 15:459–470
Jakucs E, Erős-Honti Z, Seress D, Kovács GM (2015) Enhancing our understanding of anatomical diversity in Tomentella ectomycorrhizas: characterization of six new morphotypes. Mycorrhiza 25:419–429. doi:10.1007/s00572-014-0622-3
Kennedy P (2010) Ectomycorrhizal fungi and interspecific competition: species interactions, community structure, coexistence mechanisms, and future research directions. New Phytol 187:895–910. doi:10.1111/j.1469-8137.2010.03399.x
Kõljalg U (1996) Tomentella (Basidiomycota) and related genera in temperate Eurasia. Synopsis Fungorum 9. Fungiflora, Oslo
Kõljalg U, Dahlberg A, Taylor AFS, Larsson E, Hallenberg N, Stenlid J, Larsson KH, Fransson PMA, Kårén O, Jonsson L (2000) Diversity and abundance of resupinate thelephoroid fungi as ectomycorrhizal symbionts in Swedish boreal forests. Mol Ecol 9:1985–1996. doi:10.1046/j.1365-294X.2000.01105.x
Kõljalg U, Jakucs E, Bóka K, Agerer R (2001) Three ectomycorrhiza with cystidia formed by different Tomentella species as revealed by rDNA ITS sequences and anatomical characteristics. Folia Cryptog Estonica 30:27–39
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Tellería MT, Weiß M, Larsson K-H (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Lee SS, Thi BK, Patahayah M (2010) An ectomycorrhizal thelephoroid fungus of Malaysian dipterocarp seedlings. J Trop For Sci 22:355–363
Leonardi M, Iotti M, Oddis M, Lalli G, Pacioni G, Leonardi P, Maccherini S, Perini C, Salerni E, Zambonelli A (2013) Assessment of ectomycorrhizal fungal communities in the natural habitats of Tuber magnatum (Ascomycota, Pezizales). Mycorrhiza 23:349–358
Mello A, Napoli C, Murat C, Morin E, Marceddu G, Bonfante P (2011) ITS-1 versus ITS-2 pyrosequencing: a comparison of fungal populations in truffle grounds. Mycologia 103:1184–1193
Melo I, Salcedo I, Tellería MT, Blanco M, Illana C (2004) Bases corológicas de Flora Micológica Ibérica Adiciones y números 2179–2238. Cuad Trab Flora Micol Ibérica 20:15–56
Melo I, Salcedo I, Tellería MT (2006) Contribution to the knowledge of tomentelloid fungi in the Iberian Peninsula. V. Nova Hedwigia 82:167–187
Miller OK (1982) Taxonomy of ecto- and ectendomycorrhizal fungi. In: Schenck NC (ed) Methods and principles of mycorrhzial research. The American Phytopatological Society, St Paul Minn, pp 91–101
Murat C, Vizzini A, Bonfante P, Mello A (2005) Morphological and molecular typing of the below-ground fungal community in a natural Tuber magnatum truffle-ground. FEMS Microbiol Lett 245:307–313. doi:10.1016/j.femsle.2005.03.019
Napoli C, Mello A, Borra A, Vizzini A, Sourzat P, Bonfante P (2010) Tuber melanosporum, when dominant, affects fungal dynamics in truffle grounds. New Phytol 185:237–247. doi:10.1111/j.1469-8137.2009.03053.x
Otsing E, Tedersoo L (2015) Temporal dynamics of ectomycorrhizal fungi and persistence of Tuber melanosporum in inoculated Quercus robur seedlings in North Europe. Mycorrhiza 25(1):61–66. doi:10.1007/s00572-014-0591-6
Palfner G (1995) Quercirhiza squamosa + Quercus. In: Agerer R (ed) Colour atlas of ectomycorrhizae, plate 86
Palfner G, Agerer R (1996) “Quercirhiza squamosa” eine nichtidentifizierte Ektomykorrhiza an Quercus robur. Sendtnera 3:137–145
Peay K, Kennedy P, Bruns T (2008) Fungal community ecology: a hybrid beast with a molecular master. Bioscience 58:799–810
Peintner U, Dämmrich F (2012) Tomentella alpina and other tomentelloid taxa fruiting in a glacier valley. Mycol Prog 11:109–119
Pigott CD (1982) Survival of mycorrhiza formed by Cenococcum geophilum Fr. in dry soils. New Phytol 92:513–517
Pruett G, Bruhn J, Mihail J (2008) Temporal dynamics of ectomycorrhizal community composition on root systems of oak seedlings infected with Burgundy truffle. Mycol Res 112:1344–1354
Reyna S, García-Barreda S (2014) Black truffle cultivation: a global reality. For Syst 23:317–328
Richard F, Millot S, Gardes M, Selosse MA (2005) Diversity and specificity of ectomycorrhizal fungi retrieved from an old-growth Mediterranean forest dominated by Quercus ilex. New Phytol 166:1011–1023
Salerni E, D’Aguanno M, Leonardi P, Perini C (2014) Ectomycorrhizal communities above and below ground and truffle productivity in a Tuber aestivum orchard. For Syst 23:329–338
Sánchez S (2012) Ectomicorrizas en el cultivo de trufa negra: ecología, diversidad y gestión. Doctoral dissertation, University of Zaragoza
Sánchez S, Ágreda T, Águeda B, Martín M, de Miguel AM, Barriuso J (2014) Persistence and detection of black truffle ectomycorrhizas in plantations: comparison between two field detection methods. Mycorrhiza 24:S39–S46. doi:10.1007/s00572-014-0560-0
Shiryaev A (2008) Diversity and distribution of thelephoroid fungi (Basidiomycota, Thelephorales) in the Sverdlovsk region, Russia. Folia Cryptogamica Estonica 44:131–141
Suvi T, Tedersoo L, Abarenkov K, Beaver K, Gerlach J, Kõljalg U (2010) Mycorrhizal symbionts of Pisonia grandis and P. sechellarum in Seychelles: identification of mycorrhizal fungi and description of new Tomentella species. Mycologia 102:522–533. doi:10.3852/09-147
Suz LM, Martín MP, Fischer CR, Bonet JA, Colinas C (2010) Can NPK fertilizers enhance seedling growth and mycorrhizal status of Tuber melanosporum-inoculated Quercus ilex seedlings? Mycorrhiza 20:349–360. doi:10.1007/s00572-009-0289-3
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tellería MT (1980) Contribución al estudio de los Aphyllophorales españoles. J Cramer Bibliotheca Mycol 74
Wei J, Agerer R (2010) Three Ectomycorrhizae of Thelephoraceae on Chinese Pine (Pinus tabulaeformis) and a key to thelephoroid Ectomycorrhizae. Nova Hedwigia 91:165–186
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols. A guide to methods and applications. Academic Press, London, pp 315–322
Yorou NS, Diabate M, Agerer R (2012a) Phylogenetic placement and anatomical characterisation of two new West African Tomentella (Basidiomycota, Fungi) species. Mycol Prog 11:171–180
Yorou NS, Gardt S, Guissou ML, Diabaté M, Agerer R (2012b) Three new Tomentella species from West Africa identified by anatomical and molecular data. Mycol Prog 11:449–462
Acknowledgments
Authors would like to thank Navarra’s truffle plantation owners for their invaluable collaboration through the years. This study was supported by Gobierno de Navarra—Educación (2005 Project) and the Spanish Ministry of Economy MINECO project AGL 2012-40035-C03.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Miguel, A.M., Águeda, B., Sáez, R. et al. Diversity of ectomycorrhizal Thelephoraceae in Tuber melanosporum-cultivated orchards of Northern Spain. Mycorrhiza 26, 227–236 (2016). https://doi.org/10.1007/s00572-015-0665-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0665-0