Abstract
Tomentella amyloapiculata and T. agbassaensis are described as new species within the genus Tomentella based on materials we collected in the West African, northern Guinean seasonal forests. We used a combination of anatomical characters, sequence analyses and phylogenetic inference of 71 ITS rDNA sequences to characterise the two new species. Anatomically, T. amyloapiculata is characterised by simple septate brown to dark brown, thick-walled subicular and subhymenial hyphae and triangular to slightly lobed brown basidiospores (in frontal view), with isolate aculei of 1–2 μm. Phylogenetically, T. amyloapiculata forms a sister species of T. fuscocinerea with a moderate bootstrap support of 70%. T. amyloapiculata deviates from T. fuscocinerea by 10.07–11.73% in their sequence similarities. As far as T. agbassaensis is concerned, it clusters phylogenetically together with T. bryophila with a strong bootstrap support of 99%. The species is characterised by slightly differentiated rhizomorphs with yellowish hyphae, clamped, thick-walled and yellow to dark yellow subicular hyphae and pale yellow, small basidiospores of 6–8(8.5) μm with aculei of up to 0.5 μm. Both new species deviated from each other by 11.0–11.60% with regard to the ITS rDNA nucleotides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Tomentella belongs to the order Thelephorales Corner ex. Oberw. which encompasses worldwide distributed species (Kõljalg 1996; Larsen 1964, 1968, 1974; Stalpers 1993; Yorou and Agerer 2008). Due to the paucity of strong and evident inter-specific discriminating anatomical features, the species concept has been unstable for a long time within the genus Tomentella, as attested by numerous synonymies and combinations (Kõljalg 1996; Larsen 1974; Wakefield 1966, 1969). The recent application of molecular systematics and DNA barcoding (Tedersoo et al. 2008, 2009) brought more insights into the understanding of species limits within Tomentella (Kõljalg et al. 2000, 2001; Yorou and Agerer 2007a, 2008; Yorou et al. 2007). Within the genus Tomentella, however, it is common that the search for a query identity in public databanks (NCBI, UNITE) results in a great number of best matches comprising different morphological species with an equal identity rate to the query. Though the similarity of a given tomentelloid species can be easily checked in public databanks such as UNITE (Kõljalg et al. 2005), it is still difficult to assign a specific name to a query with absolute certainty without referring to anatomo-morphological characters of either the basidiocarp (Kõljalg 1996; Larsen 1974; Stalpers 1993) or the ectomycorrhizae (Agerer 1987–2008). This problem is particularly recurrent when dealing with species/queries with different geographic origins (and consequently different mycorrhiza hosts) for which anatomical descriptions are lacking.
In this paper, two new tropical African species are described using a combination of anatomo-morphological characters and molecular data. The aim of the present and previous investigations (Yorou and Agerer 2007a, 2008, 2011a; Yorou et al. 2007) has been to contribute with our knowledge of species concepts within Tomentella by means of anatomical and molecular data.
Materials and methods
Specimen sampling
Specimens were collected in various vegetation types in the Northern Guinean seasonal forests of West Africa (White 1983) in the summers of 2005, 2006 and 2009. Preliminary notes were recorded using fresh materials that were afterwards dried using a propane gas heated field dryer (De Kesel 2001). Colour codes of the dried basidiocarps are given according to Kornerup and Wanscher (1978). All specimens used for descriptions and the holotypes are deposited in M (Holmgren et al. 1990).
Light microscopy and SEM investigations
We refer to Yorou and Agerer (2007a, 2007b, 2008, 2011a, 2011b, 2011c) for protocols of light microscopy and scanning electron microscopy.
DNA extraction and sequencing
DNA was extracted from dried basidiocarps. DNA extraction and PCR amplification are detailed in Yorou and Agerer (2008, 2011a). DNA sequencing was performed by the sequencing service of the Department Biology I (Ludwig-Maximilians-Universität, München), using BigDye Terminator Ready Reaction Cycles Sequencing Kit v3.1 (Applied Biosystems, Foster City, CA, USA). Sequencing was performed on 1 μl purified DNA probes plus 0.3 μl ITS1F (forward) and 0.3 μl ITS4B (reverse). Three sequences of the new species have been generated and deposited in GenBank (NCBI) under accession numbers EF507257, EF507262, EF507263.
Sequence editing and similarity test between studied specimens
The sequences were edited with BioEdit v7.0.5 (Hall 2005). A rapid similarity test was run in BioEdit v7.0.5 in order to test the anatomo-morphological discrimination we made for the specimens. In addition, sequences of anatomo-morphologically close species were used to estimate the genetic distance between the studied specimens and species already described. Sequences were first aligned using “ClustalW Multiple Alignment” and the options “full multiple alignment”, “bootstrap NJ tree” and the “number of bootstrap = 1,000” activated. The 5’ and 3’ ends were excluded in order to get fully aligned segments. Identity/similarity of generated sequences was calculated using the “Pairwise alignment, calculation of the similarity/identity” option of BioEdit v7.0.5.
Molecular-phylogenetic analysis
The most similar sequences of each generated consensus sequence were searched for in UNITE (Kõljalg et al. 2005, http://unite.ut.ee) using the “Blastn” search option. Sequences of the top 10 most similar taxa identified to species level were downloaded. Similarly, we downloaded the top 10 best matches of the new generated sequences in Genbank NCBI (http://www.ncbi.nlm.gov) using the “Megablast search option” (Zhang et al. 2000). We excluded sequences of uncultured/environmental samples. The downloaded sequences were complemented with sequences used and published during previous investigations on tropical African resupinate Thelephorales (Yorou and Agerer 2007a, 2008, 2011a; Yorou et al. 2007). In addition, we added thelephoroid sequences from the Seychelles (Suvi et al. 2010; Tedersoo et al. 2007) to the dataset. All sequences were automatically aligned in BioEdit v7.0.5. The alignment was manually checked, improved and ambiguous columns that we could not align with absolute certainty were excluded. The final dataset included a total of 71 ITS rDNA thelephoroid sequences with a length of 506 characters. We used the final dataset to search for the most likely tree using RAxML (Stamatakis 2006). The GTR model of substitution was applied having Maximum Likelihood as optimal criterion. Robustness of individual branches was estimated using 1,000 bootstrap replicates (Stamatakis 2006). Simultaneously, the best scoring tree was obtained by running a fast bootstrap search of 100 pseudoreplicates in the program RAxML v.7.0.4 (Stamatakis et al. 2008). In addition, the most parsimonious tree was searched for by executing batch files generated by PAUPRat (Sikes and Lewis 2001) in PAUP* v4.0 (Swofford 2002), in order to check the topology of the most likely tree we obtained with the program RAxML (Stamatakis 2006; Stamatakis et al. 2008). The heuristic search was set with parsimony as the optimality criterion, multistate taxa interpreted as uncertainty, Zero-length branches set not to collapse, Maxtrees setting = 600 and Tree-Bisection-Reconnection (TBR) as branch-swapping alogarithm. All characters are unordered, of equal weight and gaps were treated as missing data. Consistency (CI) and Retention indices (RI) (Farris 1989) were calculated in order to estimate the amount of homoplasy.
Results
ITS rDNA-based differences between the new species
Specimen SYN 893 (accession number EF507263) deviates from SYN 930 (accession number EF507262) by 1.83%. Both specimens are accommodated under the new species Tomentella amyloapiculata. Higher deviation rates ranging from 11.00 to 11.60% could be calculated between T. agbassaensis (SYN 981; accession number EF507257) and above mentioned specimens.
According to “Blastn” search in UNITE, the most similar species of Tomentella amyloapiculata are T. fuscocinerea (Pers.) Donk (UDB003300) and T. cinerascens (P. Karst.) Höhn. & Litsch. (UDB003309) with identity rates ranging from 90 to 91%. Four different species, namely T. stuposa (Link) Stalpers, T. bryophila (Pers.) M.J. Larsen, T. lapida (Pers.) Stalpers and T. ramossisima (Berk. & M.A. Curtis) Wakef. present highest identity 91% with T. agbassaensis, according to the Megablast search (NCBI).
Phylogenetic placement of the new species
Phylogenetic inference of the ITS rDNA sequence dataset generated a total of 4,341 most parsimonious trees with equal length of 572 steps (CI = 0.568, RI = 0.876). Of 506 unambiguously aligned characters, 199 were variable and 173 were parsimonious informative. All the most parsimonious trees were similar in topology. The likelihood of the most likely tree found is −5,215.909571 (tree length = 2.118876).
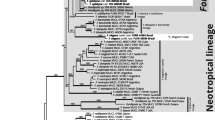
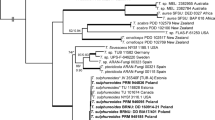
The topology of the trees generated by means of both RAxML and PAUP is quite similar. Thus, only the most likely tree obtained from RAxML is presented (Fig. 1). In general, the topology and bootstrap support of different clades of the most likely tree found is similar to the ones reported from previous studies (Kõljalg et al. 2000, Kõljalg et al. 2001; Yorou and Agerer 2007a; Yorou et al. 2007). However, in this study, the clade comprising T. punicea and T. botryoides form a sister clade of the T. stuposa complex, rather than forming a sister clade of T. ferruginea and T. umbrinospora.
The Maximum Likelihood tree showing the placement of the new species within Tomentella species. Bootstrap values higher than 50% are shown above the branches. Databank (UNITE or NCBI) accession numbers and country of origin of selected species are indicated after species names. The clades in which both new species fall are indicated in grey
In both RAxML and PAUP, T. agbassaensis clusters together with T. bryophila with a very strong bootstrap support of 99%. The clade comprising T. agbassaensis and T. bryophila forms a sister clade to T. lapida with a strong support of 93%. All three species, T. agbassaensis, T. bryophila and T. lapida, form a moderately supported (74% bootstrap) sister group of T. stuposa whose five representatives in this study cluster together with 91% bootstrap support. The phylogenetic placement of T. agbassaensis as sister species of T. bryophila, coupled with different anatomo-morphological characters (see below), support the erection of T. agbassaensis as a new species.
As far as T. amyloapiculata is concerned, it clusters together with T. fuscocinerea with a moderately bootstrap support of 70%. A sister clade of this group is a still undescribed Tomentella species from Benin (West Africa). These species together form a sister group to a clade comprising T. lilacinogrisea Wakef., T. cinerascens and T. subclavigera Litsch. with no bootstrap support at deeper roots. T. amyloapiculata is described here as new species based on sequence analyses and morpho-anatomical characters (see below).
Description of the two new species
Tomentella agbassaensis Yorou sp. nov. (Mycobank: MB 517983) Genbank NCBI, accession number EF507257
Material studied
Benin, central part, Borgou Province, region of Agbassa, forest reserve of Wari-Maro, 08°50′06.5″N, 002°17′15.9″E, leg and det. NS Yorou, 19.08.2006, herb. SYN 981 (M), holotype,
Etymology
In reference to Agbassa, one locality in the forest reserve of Wari-Maro where the type material has been collected.
Description
Basidiocarpis resupinatis, tenuibus, usque ad 0.5 mm altis, separabilibus, pelliculosis, continuis. Subiculo tenui, arachnoideo, pallidiore quam hymenium, flavo, marginibus sterilibus indeterminatis. Rhizomorphae in subiculo presentes, rhizomorphae crassiores in aqua et in 2.5% KOH distincte ochraceae, monomiticae; hyphis centralibus dilatatis, fibuligeris, raro septis simplicibus, 4–6(7) μm in diametro, crassitunicatis (0.5–1 μm), in marginibus cum hyphis tenuibus, tortuosis; hyphae distincte presentes ad conicae ramificationes laterales rhizomorpharum, 1–3 μm in diametro, interdum fibuligeris deficientibus. Hyphae subiculi fibuligerae, 4–6(–6.5) μm in diametro, in 2.5% KOH interdum sinuosae, crassitunicatae (0.5–1 μm). Hyphae subhymenii fibuligerae, septa simplicia absentia, 3–5(–7) μm in diametro, crassitunicatae (0.5–1 μm). Cystidia absentia. Basidia fibuligera, 25–45 μm longa, apicibus 7–9 μm, basalibus 5.5–7 μm, clavata, 4-sterigmatica, sterigmatibus 3–5(–7) μm longis, basaliter 1.5–2.5 μm latis. Basidiosporae (6–)6.5–8(–8.5) × (4.5)5–7(–7.5) μm in aspectu frontali, (5–)6–7(–7.5) × (5–)5.5–6.5(–7) μm in aspectu laterali, triangulares et partibus proximalibus dilatatis in aspectu frontali, ellipsoideae in aspectu laterali, echinulatae, aculeis plerumque isolatis, interdum duo vel plus quam duo, brevissimis (0.2–0.5 μm altis), in aqua et in 2.5% KOH flavidae, non cyanescentes, non congophilae, non cyanophilae, non amyloideae. Chlamydosporae absentes.
Basidiocarp resupinate, thin, up to 0.5 mm, separable from the substrate, pelliculose, continuous. Hymenophore smooth to finely granular, continuous, hymenium brown (6E4 to 6E5), subiculum thin, arachnoid, paler than the hymenium and yellowish, sterile margin indeterminate.
Rhizomorphs present in the subiculum, rare at the sterile margins, dark-brown under stereomicroscope, young rhizomorphs (up to 20 μm) yellow, old rhizomorphs deep yellow-brown in water and in 2.5% KOH, monomitic, slightly differentiated, of type C (according to Agerer 1987–2008; Fig. 2), composed of central large hyphae, covered by thinner peripheral ones, central individual hyphae clamped, simple septa rare, peripheral hyphae commonly tortuous mostly at the conical side branches, all hyphae yellow to deep yellow in water and in 2.5% KOH, central hyphae 4–6(–7) μm in diam., thick-walled (0.5–1 μm), peripheral hyphae thinner, 1–3 μm, some clampless.
Subicular hyphae clamped, simple septa rare, 4–6(–6.5) μm wide, regular, not inflated, commonly with sidebranch outgrowths, cross-shaped branches and closed anastomoses common (Fig. 3), sometimes sinuous in 2.5% KOH, thick-walled (0.5–1 μm), yellow to deep-yellow in water and in 2.5% KOH, without encrustations in water and in 2.5% KOH.
Subhymenial hyphae clamped, simple septa absent, 3–5(–7) μm wide, neither short nor inflated (Fig. 4), thin-walled (0.5–1 μm), colourless to pale yellow in water and in 2.5% KOH.
Cystidia absent.
Basidia clamped at base, 25–45 μm long, 7–9 μm at apex, 5.5–7 μm at base, clavate, not stalked, sometimes sinuous, without transverse septa, colourless to very pale yellow in water and in 2.5% KOH, slightly congophilous, slightly cyanophilous, 4-sterigmate, sterigmata 3–5(–7) μm long and 1.5–2.5 μm at base.
Basidiospores (6–)6.5–8(–8.5) x (4.5–)5–7(–7.5) μm in frontal face, (5–)6–7(–7.5) x (5–)5.5–6.5(–7) μm in lateral face, triangular with widened proximal part in frontal, ellipsoid in lateral view, (Fig. 5), oil drops absent, echinulate, aculei usually isolated, sometimes grouped in 2 or more, very short (0.2–0.5 μm), basidiospores pale yellow in water and in 2.5% KOH.
Chlamydospores absent.
Chemical reactions
No structure was found to be cyanescent, amyloid, congophilous or cyanophilous if not specifically indicated.
Habitat
On the undersides of dead logs and barks, under native Caesalpiniaceae (Isoberlinia doka Craib and Stapf, I. tomentosa Craib & Stapf, Burkea africana Hook. F. & Afzelia africana Smith) and Euphorbiaceae (Uapaca togoensis Pax).
Tomentella amyloapiculata Yorou sp. nov. (Mycobank: MB 517984) GenBank NCBI, accession numbers EF507263 (holotype), EF507262.
Material studied
Benin, central part, Borgou Province, region of Wari-Maro, forest reserve of Wari-Maro, 08°22′20.7″N, 002°48′32.5″E, leg and det. NS YOROU, 06.08.2005, herb. SYN 893 (M), holotype, Genbank NCBI, accession number EF507263. — Benin, central part, Borgou Province, region of Wari-Maro, forest reserve of Wari-Maro, 08°23′19.8″N, 002°47′25.6″E, 09.08.2005, herb. SYN 930 (M), Genbank NCBI, accession number EF507262. — Guinea, upper Guinea, Province of Kakan, Prefecture of Kouroussa, Forest reserve of Baro, 10°57′09.2″N, 009°01′45.0″W, on dead logs, leg. and det. NS. Yorou, 18.07.2009, herb. SYN 2291 (M). — Guinea, upper Guinea, Province of Kakan, Prefecture of Kouroussa, on the road between Kouroussa and Dabola, forest reserve of Moussaya, 10°00′56.7″N, 009°59′47.1″W, on dead logs, leg. and det. NS. Yorou, 19.07.2009, herb, SYN 2325 (M).
Etymology
In reference to the typically amyloid apiculi.
Description
Basidiocarpis resupinatis, tenuibus, ad 0.5 mm altis, adhaerentibus usque ad separabilibus, crustosis, continuis. Rhizomorphae in subiculo et in margine absentes. Hyphae subiculi septis simplicibus, 4–6(–7) μm in diametro. Hyphae subhymenii septis simplicibus, 4–7(–8) μm in diametro, crassitunicatae (0.5–1 μm). Cystidia absentia. Basidia septis simplicibus, 40–65 μm longa, apicibus 7.5–13 μm, basibus 6.5–8 μm, utriformia, 4-sterigmatica, sterigmatibus 7–10(–11) μm longis, basaliter 2–3 μm latis. Basidiosporae (8–)8.5–11(–12) x (7.5–)8–9(–10) μm in aspectu frontali, (8–)8.5–11.5(–12) x (7–)7.5–8.5(–9) μm in aspectu laterali, triangulares, partibus proximale dilatatis usque ad sublobatis in aspectu frontali, ellipsoideae in aspectu laterali, echinulatae, aculeis isolatis, 1–2 μm longis, in aqua et in 2.5% KOH pallide brunneae usque ad brunneae, non congophilae, non cyanophilae, apiculo distincte amyloideo. Chlamydosporae absentes.
Basidiocarp resupinate, thin, up to 0.5 mm, adherent to separable from the substrate, crustose, continuous. Rhizomorphs lacking. Hymenophore smooth to finely granular, continuous, hymenium dark brown (6F4), subiculum thin, arachnoid, dark brown to almost black, darker than the hymenium, sterile margin indeterminate.
Rhizomorphs absent.
Subicular hyphae simple septate, clamps completely absent, 4–6(–7) μm wide, usually regular, commonly with short side branch outgrowths, sometimes sinuous in 2.5% KOH, cross-shaped branching and anastomoses rare, thick-walled (0.5–1 μm), brown to dark-brown in water and in 2.5% KOH, without encrustations in water and in 2.5% KOH.
Subhymenial hyphae simple septate, 4–7(–8) μm wide, neither short nor inflated (Fig. 6), tortuous, thick-walled (0.5–1 μm), brown to dark-brown in water and in 2.5% KOH.
Cystidia absent.
Basidia simple septate at base, 40–65 μm long, 7.5–13 μm at apex, 6.5–8 μm at base, utriform, not stalked, sometimes sinuous, without transverse septa, colourless to very pale brown in water and in 2.5% KOH, slightly congophilous, cyanophilous, 4-sterigmate, sterigmata 7–10(11) μm long and 2–3 μm at base.
Basidiospores (8–)8.5–11(–12) x (7.5–) 8–9(–10) μm in frontal face, (8–)8.5–11.5(–12) x (7–)7.5–8.5(–9) μm in lateral face, triangular with widened proximal part to slightly lobed in frontal, ellipsoid in lateral view (Fig. 7), oil drops rare, echinulate, aculei isolated, 1–2 μm, basidiospores pale brown to brown in water and in 2.5% KOH, apiculus typically amyloid, turning dark blue in Melzer’s reagent.
Chlamydospores absent.
Reaction in chemicals
No structure was found to be cyanescent, amyloid, congophilous or cyanophilous if not specifically indicated.
Habitat
At the underside of dead bark and wood debris, in woodlands and forests dominated by Euphorbiaceae (Uapaca togoensis) and Ceasalpiniaceae (Isoberlinia doka, I tomentosa, Burkea africana, Afzelia africana and Anthonota crassifolia).
Discussion
Tomentella agbassaensis is characterised by colourless to yellowish subicular and subhymenial hyphae and by yellowish triangular basidiospores of up to 8.5 μm in frontal view. Only a limited number of resupinate Thelephorales present yellowish hyphae and basidiospores (Dämmrich 2006; Kõljalg 1996; Stalpers 1993). Yellowish subicular/subhymenial hyphae and/or basidiospores have been reported for T. italica (Sacc.) M.J. Larsen (Dämmrich 2006; Kõljalg 1996), T. furcata Yorou & Agerer (Yorou and Agerer 2007a) and for T. bryophila ( Dämmrich 2006; Kõljalg 1996; Yorou and Agerer 2011a). T. italica differs anatomically from T. agbassaensis by its irregularly globose bigger basidiopores of up to 10 μm with bifurcate ornaments, whilst those of T. agbassaensis are mostly triangular, up to 8(8.5) μm. T. furcata is reported from the same forest reserve but presents a crustose thin basidiocarp (Yorou and Agerer 2007a). It differs however by its simple septate brown to dark brown subicular hyphae, absence of rhizomorphs and distinctly yellow to deep yellow, bigger subglobose to globose basidiospores of up to 13 μm with longer bifurcate aculei. In a similar way, T. bryophila differs from T. agbassaensis by its bigger, distinctly globose basidiospores (up to 10 μm) with long, isolate and conical aculei of up to 2.5 μm.
Molecular-phylogenetically, T. agbassaensis is close to T. bryophila. One of the synapomorphic characters that could explain such proximity is the yellowish colour of the basidiospores in both species, though basidiospores of T. bryophila present amyloid apiculi (Dämmrich 2006; Yorou and Agerer 2011b). Tomentella lapida forms a sister species of both T. agbassaensis and T. bryophila (Fig. 1). In this group, T. agbassaensis presents quite different anatomical features, among others the smaller (up to 8.5 μm) triangular to slightly lobed basidiospores with short aculei (up to 0.5 μm) and the presence of slightly differentiated rhizomorphs, unlike both the previously mentioned species that lack them (Yorou and Agerer 2011c).
Anatomically, the simple septate subicular and subhymenial hyphae and the triangular brown basidiospores of up to 12 μm with isolated aculei (of up to 2 μm) make the identification of T. amyloapiculata very easy. Among all the already described thelephoroid species, T. fuscocinerea is anatomically the closest. Like T. amyloapiculata, it presents an adherent crustose brown basidiocarp, simple septate subicular hyphae and brownish basidiospores with similar size. Anatomically, T. fuscocinerea is characterised by colourless to only very pale brown subicular hyphae of maximum 5 μm diam. (Dämmrich 2006; Kõljalg 1996), whilst those of T. amyloapiculata are wider (up to 7 μm) with brown to dark brown colour in water and in 2,5% KOH. According to Kõljalg (1996), T. fuscocinerea may present monomitic rhizomorphs in the subiculum. We were not able to observe rhizomorphs in both specimens of T. amyloapiculata we examined. Subhymenial hyphae of T. fuscocinerea are thin-walled, partly cyanescent (Dämmrich 2006; Kõljalg 1996; Melo et al. 2000). In addition, basidia of T. fuscocinerea may present a cyanescent reaction. Subhymenial hyphae of T. amyloapiculata are always thick-walled and no cyanescent reaction is observed neither in subhymenial hyphae nor in the basidia. Unlike T. fuscocinerea, apiculus of basidiospores of T. amyloapiculata are distinctly amyloid. Such a character has never been reported for T. fuscocinerea. The amyloid reaction is a rare character within resupinate Thelephorales (Dämmrich 2006). It is only known within a few species, namely on the hyphae of T. subamyloidea Agerer (Agerer and Bougher 2001). The apiculus of T. bryophila and T. lapida are partly amyloid (Dämmrich 2006; Yorou and Agerer 2011c). The two latter species differ by their typically globose basidiospores with long conical aculei from T. amyloapiculata (see above).
Molecular-phylogenetically, T. amyloapiculata forms a sister species of T. fuscocinerea with a moderate bootstrap support of 70%. Such phylogenetic proximity is supported by some synapomorphies that are highlighted in previous paragraphs.
References
Agerer R (1987–2008) Colour Atlas of Ectomycorrhizae, 1st–14th delivery. Schwäbische Gmünd, Einhorn
Agerer R, Bougher NL (2001) Tomentella subamyloidea sp. nov. and Tomentella radiosa (Thelephorales, Hymenomycetes, Basidiomycota) from Australia. Aust Syst Bot 14:607–614
Dämmrich F (2006) Studien der tomentelloiden Pilze in Deutschland-Unter besonderer Berücksichtigung der Zeichnungen von Frau Dr. H. Maser aus den Jahren 1988-1994; Teil 1: Die Gattung Tomentella. Z Mykol 72:167–212
De Kesel A (2001) A Mushroom dryer for the travelling mycologist. Field Mycol 2:131–133
Farris JS (1989) The retention index and rescaled consistency index. Cladistics 5:417–419
Hall T (2005) BioEdit: Biological sequence alignment editor for Win95/98/NT/2 K/XP. Ibis therapeutic, Carlsbad, CA
Holmgren PK, Holmgren NH, Barnet LC (1990) Index Herbariorum. Part I. Herbaria of the World. 8th edn. Regnum Vegetabile 120. New York botanical Garden, New York
Kõljalg U (1996) Tomentella (Basidiomycota) and related genera in Temperate Eurasia. Synop Fungorum 9:1–213
Kõljalg U, Dahlberg A, Taylor AFS, Larsson E, Hallenberg N, Stendil J, Larsson K-H, Fransson PM, Kårén O, Jonsson L (2000) Diversity and abundance of resupinate thelephoroid fungi as ectomycorrhizal symbionts in swedish boreal forests. Mol Ecol 9:1985–1996
Kõljalg U, Jakucs E, Bóka K, Agerer R (2001) Three ectomycorrhizae with cystidia formed by different Tomentella species as revealed by rDNA ITS sequences and anatomical characteristics. Folia Cryptogam Est 38:27–39
Kõljalg U, Larsson K-H, Abarenkov K, Nilsson RH, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T, Sen R, Taylor AFS, Tedersoo L, Vrålstad T, Ursing BM (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166:1063–1078
Kornerup A, Wanscher JH (1978) Methuen handbook of colour, 3rd edn. Eyre Methuen, London
Larsen MJ (1964) Tomentella and related genera in Northern America. I. Studies of nomenclatural types of species of Hypochnus described by Burt. Can J Bot 43:1485–1510
Larsen MJ (1968) Tomentelloid fungi of North America. –State Univ. College of Forestry, Syracuse Univ., Tech. Publ 93:1–157
Larsen MJ (1974) A contribution to the taxonomy of the genus Tomentella. Mycol Mem 4:1–145
Melo I, Salcedo I, Telleria MT (2000) Contribution to the knowledge of tomentelloid fungi in the Iberian Peninsula. II. Karstenia 40:93–101
Sikes DS, Lewis PO (2001). PAUPRat: A tool to implement Parsimony Ratchet searches using PAUP*. (http://viceroy.eeb.uconn.edu/paupratweb/pauprat.htm)
Stalpers JA (1993) The Aphyllophoraceous Fungi I. Key to the species of the Thelephorales. Stud Mycol 35:1–168
Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stamatakis A, Hoover P, Rougemont J (2008) A Rapid Bootstrap Algorithm for the RAxML Web-Servers. Syst Biol 75:758–771
Suvi T, Tedersoo L, Abarenkov K, Beaver K, Gerlach J, Kõljalg U (2010) Mycorrhizal symbionts of Pisonia grandis and P. seychellarum in Seychelles: Identification of mycorrhizal fungi and description of new Tomentella species. Mycologia 102:522–533
Swofford DL (2002) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer, Sunderland, Mass.
Tedersoo L, Suvi T, Beaver K, Kõljalg U (2007) Ectomycorhizal fungi of the Seychelles: Diversity patterns and host shifts from the native Vateriopsis seychellarum (Dipterocarpaceae) and Intsia bijuga (Ceasalpiniaceae) to the introduced Eucalyptus robusta (Myrtaceae) but not Pinus caribea (Pinaceae). New Phytol 175:321–333
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Kõljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490
Tedersoo L, Suvi T, Jairus T, Ostonen I, Põlme S (2009) Revisiting ectomycorrhizal fungi of the genus Alnus: differential host specificity, diversity and determinants of the fungal community. New Phytol 182:727–735
Wakefield EM (1966) Some extra-European species of Tomentella. Trans Br Mycol Soc 49:357–362
Wakefield EM (1969) Tomentelloideae of the British Isles. Trans Br Mycol Soc 53:161–206
White F (1983) The vegetation of Africa - A descriptive memoire to accompany the UNESCO/AETFAT/UNSO vegetation map of Africa. Natural Resources Research, No. 20. Paris, UNESCO
Yorou SN, Agerer R (2007a) Tomentella furcata, a new species from Benin (West Africa) with basidia forming internal hyphae. Mycol Prog 6:239–247
Yorou SN, Agerer R (2007b) Type Studies of three tomentelloid fungi (Basidiomycota, Thelephorales): Tomentella radiosa, Tomentella cinereoumbrina and Tomentella punicea. Nova Hedwig 85:521–539
Yorou SN, Agerer R (2008) Tomentella africana, a new species from Benin (West Africa) identified by morphological and molecular data. Mycologia 100:68–80
Yorou SN, Agerer R. (2011a). Anatomical and ITS rDNA-based phylogenetic identification of two West African resupinate Thelephorales. Mycoscience (revised version submitted)
Yorou SN, Agerer R (2011b) Non rhizomorphic resupinate Thelephorales from Italy. Nova Hedwigia (in press)
Yorou SN, Agerer R (2011c) Rhizomorphic resupinate Thelephorales from Italy. Nova Hedwigia (in press)
Yorou NS, Kõljalg U, Sinsin B, Agerer R (2007) Studies in African thelephoroid fungi: 1. Tomentella capitata and Tomentella brunneocystidia, two new species from Benin (West Africa) with capitate cystidia. Mycol Progress 6:7–18
Zhang Z, Schwarz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214
Aknowledgment
We are much indebted to the Deutsche Forschungsgemeinschaft (DFG) for financial support (project Ag7/19-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yorou, N.S., Diabate, M. & Agerer, R. Phylogenetic placement and anatomical characterisation of two new West African Tomentella (Basidiomycota, Fungi) species. Mycol Progress 11, 171–180 (2012). https://doi.org/10.1007/s11557-011-0739-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-011-0739-0