Abstract
Two study plots, burned and control, were established in autumn 1998 in a Quercus ilex forest located in northern Spain, part of which had been affected by a low intensity fire in 1994. Soil samples for ectomycorrhizae (ECM) were taken over a 3-year period in each study plot in spring, summer, autumn and winter. ECM morphotypes were identified and the relative abundance of each morphotype in each soil sample calculated, along with species richness, Shannon diversity index and percentage of mycorrhization in each soil sample. The relative abundance of certain ECM morphotypes differed between burned and control plots, and the percentage of mycorrhizal tips was significantly lower in the burned than in the control plot. Nevertheless, there were no significant differences in the diversity, species richness or species composition of the ECM community in the burned and control plots. The dominant ECM morphotypes in both stands were Cenococcum geophilum and several thelephoroid fungi. Sphaerosporella brunnea and Pisolithus tinctorius thrived especially in the burned plot, whereas three ectomycorrhizal morphotypes assigned to the genus Hebeloma were especially abundant in the control plot. There was no significant variation in the relative abundance of the ECM morphotypes between seasons, but ECM community species richness was highest in autumn and lowest in summer. The percentage of mycorrhizal tips reached a maximum in winter, with its minimum in autumn. Collection of samples over the 3-year period also enabled us to detect a significant increase in percentage of ECM colonisation in the burned stand over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycorrhizal symbiosis has evolved as a mechanism of survival both for plants and fungi, enabling both to thrive in areas with poor soils, periodic droughts, extreme temperatures and other natural stresses (Gupta et al. 2000). For this reason, it is well known that mycorrhizae play an important role in the colonisation of new areas of land or in the regeneration of disturbed zones (Allen 1991; Baar et al. 1999).
As fire is one of the most common disturbances in natural communities, there is an increasing interest in analysing the effects of burning on the ectomycorrhizal (ECM) communities of different ecosystems worldwide and determining the extent to which ECM are involved in the regeneration of the area. However, most of the studies on the post-fire dynamics of the ECM community have been performed in conifer forests (Baar and Kuyper 1998; Baar et al. 1999; Dahlberg et al. 2001; Danielson 1984b; Grogan et al. 2000; Horton et al. 1998; Jonsson et al. 1999; Mah et al. 2001; Purdy et al. 2002; Stendell et al. 1999; Visser 1995), with one exception, a study carried out in a Eucalyptus regnans forest (Launonen et al. 1999).
All these studies were carried out in temperate and boreal areas, and little is known about the effect of fire on the ECM community of Mediterranean ecosystems, where fire is a common hazard. In these ecosystems, the only data available also come from gymnosperm-dominated forests (Horton et al. 1998; Martínez de Aragón et al. 2001; Torres and Honrubia 1997); studies dealing with host species such as Quercus ilex or Quercus suber have focused on the description of the ECM morphotypes encountered and have not investigated the effect of fire on these communities (Azul and Freitas 1999; García 2001; Sarrionandía et al., unpublished data).
Moreover, there is a lack of information on the seasonal and annual dynamics of the ECM community (Dahlberg et al. 1997), as most of the studies undertaken so far are based on single collections of ECM; very few reports present data gathered over 1 year or more (Azul and Freitas 1999; García 2001; Mineo and Majumdar 1996).
The aim of this work was therefore to determine the effects of a low-intensity wildfire on the ECM of a Mediterranean Q. ilex forest over a period of 3 years. Gathering data over this extended period also enabled the seasonal and annual variations in the ECM community of the Q. ilex-dominated ecosystem to be investigated.
Materials and methods
Field sampling
In September 1994, a low-intensity wildfire burned 38 ha of a Q. ilex forest at Nazar, Navarra, in northern Spain (latitude 42°38′29″N and longitude 2°16′37″W), while 30 ha of the forest remained undisturbed. We can describe the wildfire as being of low intensity because the loss of organic matter was minor, trees and shrubs were able to resprout easily, and the seed bank was not particularly affected. This study began 4 years later, when the trees from the burned stand had the appearance of 1.5- to 2-m-tall shrubs lacking a defined main trunk. Other shrubs and plants from the understorey had already attained heights of up to 1.5 m, making the stand difficult to enter due to the continuous vegetation cover. The burned and control plots were separated by a road and were 50 m apart.
The study site is located at an altitude of 746 m and has a south-facing aspect. The mean annual precipitation is 699 mm with peaks in April and November and a dry period in July and August, and the mean annual temperature is 11.7°C, varying between 4.7°C in the coldest month (January) and 20.8°C in the warmest month (August) (Departamento de Agricultura, Ganadería y Alimentación del Gobierno de Navarra, personal communication).
Both stands, burned and control, were visited in autumn, winter, spring and summer from autumn 1998 until summer 2001. After litter removal, five soil samples of ca. 450 g to a depth of 10 cm were randomly collected from each stand in each sampling date, placed in plastic bags, stored at 4°C and processed in the laboratory within 2 weeks. The soil was stony and usually dry, resulting in difficulties with the collection of a fixed volume using a soil corer as initially planned. A fixed soil weight was collected, therefore, using a spade instead. The type of soil in both stands was a lithic–calcixerollic Xerochrept according to the USA Soil Taxonomy, i.e., a calcareous and stony soil typical of areas with high slopes.
Extraction and identification of ECM and calculation of abundance and diversity parameters
Approximately 300 g of each soil sample was randomly taken with a gardening spade to extract and identify the ECM morphotypes occurring in them. The samples were soaked in a 1% sodium metaphosphate solution for 24 h to clean the soil and debris from the roots before passing through a combination of 1.7- and 0.7-mm sieves. The roots extracted were kept in water in labelled tubes, stored at 4°C and analysed within 2 weeks.
Roots were observed under a stereomicroscope at a magnification of 15× in order to extract the mycorrhizal tips. Each mycorrhizal tip was analysed and assigned to an ECM morphotype according to morphological and anatomical features (Agerer 1986, 1987–2002, 1994, 1999). The descriptions of ECM morphotypes were then compared with other published descriptions of ECM to establish the identity of the fungal symbiont. In some cases, the identification was carried out through personal communications or by tracing the sporocarp to the ECM. ECM morphotypes which could not be identified were given a reference number. Voucher specimens of all ECM morphotypes were deposited in the Herbarium of the University of Navarra (PAMP).
After counting the number of mycorrhizal tips in each ECM morphotype, the relative abundance of each ECM morphotype in each soil sample was calculated (number of tips of a certain ECM morphotype/total number of mycorrhizal tips extracted in the sample), the species richness (number of different ECM morphotypes encountered in a given sample) and the Shannon diversity index (H=−∑p i lnp i , where p i is the relative abundance of ECM morphotype i in a sample; Barbour et al. 1999). The cumulative species richness over the 3 years of study was also calculated.
Percentage of total mycorrhization in each soil sample
The degree of ECM colonisation in the soil samples collected was calculated in order to compare quantities in the burned stand and the control plot. One hundred and fifty grams of each soil sample was taken randomly, cleaned and sieved as explained above to extract the roots. The percentage of total mycorrhization of a given sample (%M) was calculated using the gridline intersect method (Brundrett et al. 1996). The extracted roots were placed randomly in a grid divided into 1×1 cm squares, and the number of roots intersecting with the gridline counted under a stereomicroscope at 15× magnification. Whether the root at an intersection point was mycorrhizal or not (%M=100*n i /N, where n i is the number of intersections of mycorrhized roots and N the total number of root intersections) was also determined. All live secondary roots intersecting the grid were counted, not only root tips. (The Q. ilex roots were very fragile, and were mostly broken after processing the soil samples, so it was impossible to distinguish whether a piece was part of a root tip or not.)
Statistical analysis
The relative abundance of ECM morphotypes was analysed with the multivariate ordination technique Detrended Correspondence Analysis (DCA; Hill and Gauch 1980) due to the large number of soil samples and ECM morphotypes studied. The purpose was to analyse if there was any difference amongst soil samples regarding the relative abundance of the ECM morphotypes occurring in them. Variables included in the secondary matrix were: sampling stand (burned or control), sampling season (autumn, winter, spring or summer) and sampling year (first, second or third). Analyses were performed using the statistical programme PC-ORD version 4.25 (McCune and Mefford 1999). The Pearson correlation coefficient (r) was then calculated for each pair ECM morphotype-axis to determine if the relative abundance of each ECM morphotype was correlated with any group of soil samples (burned, control, autumn, winter, etc.). Rohlf and Sokal’s (1995) critical values were used to confirm that the correlations were not due to chance (P<0.01) according to the number of degrees of freedom.
The data on species richness and Shannon diversity index were tested for normal distribution by Kolmogorov–Smirnov test (Zar 1998). No normal distribution was found, and therefore differences between sampling plots, seasons and years were tested with Kruskal–Wallis and Mann–Whitney U tests by using the statistical programme SPSS for Windows version 11.0. Since the percentage of total mycorrhization was not a continuous variable, the same non-parametric tests were also applied to analyse these data.
Results
Dynamics of the relative abundance of ECM morphotypes
A total of 96 soil samples were collected over 11 seasonal samplings, 51 in the burned stand and 45 in the control plot; on some occasions only two or three, instead of the five soil samples initially planned, could be collected in each stand due to field sampling constraints. A total of 8,539 mycorrhizal tips, assigned to 32 different ECM morphotypes, were observed over the 3 years of study (see Table 1 for the most important characteristics; detailed descriptions can be obtained from the corresponding author). The mean number of mycorrhized tips analysed per 300 g soil sample was 89.5, ranging from 29 to 201. Although the identification of ECM morphotypes was exclusively based on morphology, and no molecular tools were used, the detailed analysis of morphological and anatomical characteristics was accurate enough to identify those ECM morphotypes showing significant distinguishing features. Eight ECM morphotypes were identified to species level, four to genus, one to family, three were unidentified ECM morphotypes whose description had been published, and the remaining 16 were unidentified ECM morphotypes. Sixteen of the 32 ECM morphotypes described were thelephoroid, i.e., attributed to the family Thelephoraceae (Tomentella galzinii, Tomentella pilosa, Pinirhiza dimorpha, Quercirhiza cumulosa, Quercirhiza stellata, and the ECM morphotypes 3, 4, 7, 15, 17, 31, 37, 39, 40, 41 and AD). Features shared by the thelephoroid ECM morphotypes included the dark colour (ranging between dark brown and black) of the ECM system, the pseudoparenchymatous structure of the outer mantle, and the presence of hyphae mostly coloured and with clamp connections, which in some cases formed slightly differentiated rhizomorphs (type C according to Agerer 1987–2002).
The mean relative abundance of each ECM morphotype in the burned and control stands over the 3 years is shown in Table 2. Only four ECM morphotypes had a relative abundance greater than 0.05 in each sampling stand over the 3 years of study, accounting for more than 5% of the mycorrhizal tips: Cenococcum geophilum, Q. cumulosa, morphotype 4 and type Hebeloma–Cortinarius in the control stand, and the same, except for morphotype 7 instead of Hebeloma–Cortinarius, in the burned stand.
DCA results showed that the data on relative abundance of the ECM morphotypes in the 96 soil samples were sufficiently dispersed at least along axis 1 (eigenvalue >0.5). The variable causing such dispersion appeared to be the sampling stand, because soil samples collected in the burned stand were grouped together to the right of axis 1 and on the upper part of axis 2 (positive correlation with both axes; Fig. 1), whereas those collected in the control stand appeared to the left of axis 1 and on the lower part of axis 2 (negative correlation with both axes). The other two variables studied, the sampling season and sampling year, did not cause any dispersion of data, and therefore were not considered further. Table 3 shows the ECM morphotypes correlated with axes 1 and 2 with Pearson correlation coefficient values greater than ∣0.267∣, which is Rohlf and Sokal’s (1995) critical value for 94 degrees of freedom (n−2) to confirm that the correlation is not due to chance (P<0.01). Those ECM morphotypes correlated positively with any of the two axes were more abundant in the burned stand, while the negatively correlated ECM morphotypes were more abundant in the control stand.
Ordination graph of the 96 soil samples collected over the 3-year period in the Nazar site after applying the multivariate ordination technique DCA to the relative abundance of the ectomycorrhizae morphotypes recorded in each soil sample. Soil samples collected in the burned stand are represented by a triangle, and those collected in the control stand are represented by a circle. Eigenvalue of axis 1=0.53
Dynamics of the species richness and Shannon diversity index of the ECM community
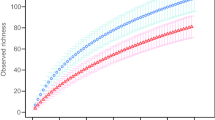
The number of ECM morphotypes encountered increased steadily with the number of samplings performed (Fig. 2). There were no significant differences in either species richness or the Shannon diversity index between the burned and control stands (P=0.18 and P=0.4, respectively, Mann–Whitney U). There was, however, a significant change in both parameters depending on the sampling season (Table 4). The species richness was significantly lower in summer than in any other season; the maximum values occurred in autumn, and were significantly greater than those in winter and summer. There was no significant difference, however, between species richness in the autumn and spring samples (P=0.08, Mann–Whitney U). The Shannon diversity index was only significantly greater in autumn than in summer, without showing any significant (P>0.05, Mann–Whitney U) difference in any other comparison between sampling seasons. Whereas the Shannon diversity index did not change significantly over the 3 years (P=0.07, Kruskal–Wallis), the species richness was significantly lower in the first year than in the second and third years. To test whether this variation over time was related to the differences between the burned and control stands, data on species richness from each stand were analysed separately (Table 5). The only significant change detected was in the burned stand, where the species richness was significantly lower the first year compared to the second year, while there was no significant difference (P>0.05, Mann–Whitney U) in any other comparison between years.
Dynamics of the degree of ECM colonisation
The percentage of total mycorrhization was significantly lower in the burned stand compared to the control plot (Table 4). There was also a significant change in the percentage of total mycorrhization in the samples collected in different sampling seasons, with the lowest and greatest values recorded in autumn and winter, respectively; no further differences were found between seasons in paired comparisons. The percentage of total mycorrhization observed in first year samples was significantly lower than in the third year, although the values from the second year were not significantly different (P>0.05, Mann–Whitney U) from any other. As described above for species richness, data from the burned and control stands were analysed separately (Table 5). There was a significant difference over the 3 years in the burned stand, with significantly lower values during the first year compared to the second year.
Discussion
Analysis of the ECM community of the Q. ilex forest at Nazar between 1998 and 2001 demonstrated that there was a change in the relative abundance of certain ECM morphotypes when comparing the stand affected by fire and the undisturbed plot. Although the number of mycorrhizal tips was significantly lower in the burned stand, there were no significant differences in diversity or species composition between plots. Caution is required in interpreting these results, but it is likely that fire played a significant role in the differences found between both plots.
Similar results to those found in our study site have been reported in the ECM community of a Pinus sylvestris forest in Sweden (Jonsson et al. 1999) and a Picea forest in Canada (Mah et al. 2001), where it was concluded that fire induced a shift in the relative abundance of each species rather than a change in the species composition. Several studies have also reported a decrease in the number of mycorrhizal tips after fire (Dahlberg et al. 2001; Stendell et al. 1999; Torres and Honrubia 1997).
In contrast, several published studies report results that differ in several aspects from those reported here. For example, Baar et al. (1999) found a shift in the species composition of the ECM community of a Pinus muricata forest in California after fire, and in Scandinavia, Dahlberg et al. (2001) cited not only a change in the species composition but also a decrease in the ECM diversity of several conifer forests affected by fire.
Many factors would influence the impact of fire on the ECM community, of which the intensity of fire could be important (Dahlberg et al. 2001; Dahlberg 2002; Grogan et al. 2000). After a low-intensity fire, many trees survive, and the organic matter remains intact; in this situation, the effects of the fire on the ECM community are mostly small. The fire affecting the Q. ilex forest in Nazar was of low intensity, resembling those studied by Jonsson et al. (1999) and Mah et al. (2001). In contrast, the work of Baar et al. (1999) focused on forests severely affected by high-intensity fires.
Two other factors that may influence the effect of fire on the ECM community of the Q. ilex forest under study are: (1) Q. ilex is a host species which resprouts easily (Castell and Terradas 1993; Pausas 1997; Torres and Honrubia 1997), and is, therefore, probably able either to keep the ECM symbiosis while suffering the disturbance or reestablish it in a short time; (2) half of the area of the Q. ilex forest at Nazar remained undisturbed, which could act as a source of ECM inoculum readily dispersed into the burned stand by wind or small animals, leading to a rapid recovery of the ECM community (Goodman and Trofymow 1998).
C. geophilum was by far the most abundant ECM found in the 32 morphotypes encountered both in the burned and control stands. This species has very low host specificity, is highly competitive, and is considered to have the broadest host range of all ECM fungi (Molina et al. 1992). Most ECM studies published report the presence of C. geophilum (Horton and Bruns 1998); in many reports this species is the most abundant within the ECM communities analysed, with relative abundance ranging from 11 to 29% (Abourouh and Najim 1995; Al Sayegh Petkovsek and Kraigher 2000; Arveby and Granhall 1998; Baxter et al. 1999; Dahlberg et al. 1997; Durall et al. 1999; Kranabetter et al. 1999; Massicotte et al. 1999; Meotto et al. 1994; Trost et al. 1999). The mean relative abundance of C. geophilum recorded in both plots in the Nazar site was 40%. One possible explanation for such a high abundance could be the resistance of this species to drought (LoBuglio 1999; Pigott 1982) and its good adaptation to Mediterranean ecosystems. Possibly, the relative abundance recorded was never so high because previously reported work was conducted in boreal or temperate forests; however, more research is needed to confirm this hypothesis.
Some of the ECM morphotypes encountered were more abundant in the burned stand than in the unburned plot. Although it is not possible to draw any substantial conclusions, the higher abundance of these morphotypes in the burned stand might result from a greater tolerance of the disturbance or a more competitive re-colonization strategy than other ECM morphotypes under such unfavourable conditions. Sphaerosporella brunnea, an ascomycete fungus regarded as a pioneer in the colonisation of burned areas has this life strategy (Meotto and Carraturo 1988); it is also a severe contaminant in nurseries where plants have been deliberately inoculated with other ECM fungi (Bencivenga et al. 1995). S. brunnea forms ectendomycorrhizae (Molina et al. 1992), thereby increasing the range of host species with which it can establish mycorrhizal symbioses.
The presence of Pisolithus tinctorius also appeared to be linked to the burned stand, as it was totally absent from the undisturbed stand. This fungus is well adapted to disturbed sites and enhances the reestablishment of plants, but only in areas where other fungi are absent (McAfee and Fortin 1988). P. tinctorius has been used for inoculation of seedlings in reforestation projects, but these experiments were not always successful (Castellano 1996).
The abundance of the group of thelephoroid ECM morphotypes differed between the burned or control stands. T. galzinii and the ECM morphotypes 7 and AD were particularly abundant in the burned stand, whereas P. dimorpha, Q. cumulosa and ECM morphotype 4 thrived in the control stand. There is little published information on the behaviour of thelephoroid fungi after disturbance. On the one hand, Visser (1995) affirmed that fruitbodies of these fungi are more abundant in mature undisturbed forests, because of a dependence on the presence of woody debris and organic matter for fruiting. In contrast, Agerer (1999) supported the idea that thelephoroid ECM are well adapted to cope with environmental stresses because many species form rhizomorphs, enabling exploitation of a greater volume of soil to obtain water and nutrients (Harley and Smith 1983). In the Nazar site, the three thelephoroid ECM morphotypes which were especially abundant in the burned stand had rhizomorphs or very long emanating elements, whereas two of the three morphtypes linked with the control stand lacked such structures.
Hitherto, the ECM morphotype AD (“Angle Droit”; Giraud 1988) was only reported in truffle plantations or nurseries (Bencivenga et al. 1995; De Miguel et al. 2001; Giraud 1988; Granetti and Baciarelli Fallini 1997; Sáez and De Miguel 1995). In the current work, morphological and anatomical investigation suggested the morphotype could be attributed to the Thelephoraceae (R. Agerer, personal communication). This report marks the first time the morphotype has been partially identified. ECM morphotype AD appears to be a pioneer species as in the current work it was more abundant in the burned stand, and it is able to colonise roots of seedlings introduced into truffle plantations or grown in nurseries, as stated above (Bencivenga et al. 1995; De Miguel et al. 2001; Giraud 1988; Granetti and Baciarelli Fallini 1997; Sáez and De Miguel 1995).
Some ECM morphotypes were especially abundant in the undisturbed control stand. Amongst the most common were those in or closely related to the genus Hebeloma: Hebeloma cf. sinapizans, the type Hebeloma–Cortinarius, and ECM morphotype 30. It is possible that these ECM fungi are sensitive to fire, although further work is required for proof.
Only a few of the ECM morphotypes found were abundant and well distributed; most were rare species which occurred sporadically, a common pattern reported at any taxonomic level (Baar et al. 1999; Dahlberg et al. 2001; Durall et al. 1999; Gardes and Bruns 1996; Gehring et al. 1998; Grogan et al. 2000; Hagerman et al. 1999; Jonsson et al. 1999; Peter et al. 2001; Stendell et al. 1999; Taylor 2002). Most studies on ECM community structures suggest that C. geophilum and ECM in the Russulaceae, Thelephoraceae and other resupinate fungi dominate within all ECM communities (Dahlberg 2001; Horton and Bruns 2001; Peter et al. 2001). With the exception of the ECM of Russulaceae, which were not recorded in this study, the Q. ilex forest at Nazar was also dominated by the same ECM fungi.
The present results also follow a well-known trend for ECM communities: a steady increase in the number of ECM morphotypes encountered as more samplings are undertaken (Erland et al. 1999). Although the dominant ECM morphotypes are detected with less sampling effort, increasing number of samples help to gain a deeper insight into the true species diversity in the ECM community.
There was a significant change in the species richness of the ECM community and in the percentage of mycorrhizal tips depending on the season. The other parameters analysed, i.e. the relative abundance of ECM morphotypes and the Shannon diversity index, did not demonstrate any significant difference amongst seasons. The species richness was maximum in autumn and minimum in summer, whereas the degree of ECM colonisation was lowest in autumn and highest in winter. Azul and Freitas (1999) also recorded seasonal variations in the numbers of mycorrhizal tips in a Q. suber forest, with the lowest numbers also recorded in autumn. In contrast, Mineo and Majumdar (1996) found that the maximum amount of mycorrhizae in two Quercus alba and Quercus rubra forests occurred in autumn. García (2001) found no significant difference amongst seasons in the number of mycorrhizal tips occurring in a Q. ilex stand, with maximum diversity in winter and summer and the minimum in spring and autumn.
Due to the limitations of the present study and the contrasting reports in the literature, it is not possible to draw definitive conclusions on the seasonal dynamics of the ECM community in forests. Climatic conditions in different areas must play an important role, and only ecosystems sharing the same climatic, edaphic and tree species compositions should be compared in order to define the impact of stresses on ECM communities.
The collection of samples over 3 years enabled the increase in percentage of ECM colonisation in the burned stand from the first to the second and third sampling years to be detected. Species richness also appeared to increase over time, although the results are not clear. There seemed to be a progressive recovery of the ECM community in the burned stand, but more detailed investigations are required. In the undisturbed stand, no change was detected in the ECM community over the 3-year period.
Further studies on the potential effects of fire on the ECM communities of Mediterranean ecosystems are required, because these areas are particularly threatened by fire. Moreover, little information is available overall on seasonal variations in ECM and their succession, so far exclusively analysed through the study of the fruit bodies of ECM fungi.
References
Abourouh M, Najim L (1995) Les différents types d’ectomycorhizes naturelles de Cedrus atlantica Manetti au Maroc. Cryptogam Bot 5:332–340
Agerer R (1986) Studies on Ectomycorrhizae II. Introducing remarks on characterization and identification. Mycotaxon 26:473–492
Agerer R (1987–2002) Colour atlas of ectomycorrhizae. Einhorn-Verlag, Munich
Agerer R (1990a) Studies on ectomycorrhizae XXIV. Ectomycorrhizae of Chroogomphus helveticus and C. rutilus (Gomphidiaceae, Basidiomycetes) and their relationship to those of Suillus and Rhizopogon. Nova Hedwig 50(1–2):1–63
Agerer R (1990b) Chroogomphus helveticus ssp. tatrensis. In: Agerer R (ed) Colour atlas of ectomycorrhizae, plate 37. Einhorn Verlag, Schwäbisch Gmünd
Agerer R (1994) Characterization of ectomycorrhizas. In: Norris JR, Read D, Varma AK (eds) Techniques for mycorrhizal research. (Methods in Microbiology, vol 23). Academic Press, London, pp 25–73
Agerer R (1999) Anatomical characteristics of identified ectomycorrhizas: an attempt towards a natural classification. In: Varma A, Hock B (eds) Mycorrhiza. Structure, function, molecular biology and biotechnology, 2nd edn. Springer, Berlin Heidelberg New York, pp 633–682
Agerer R, Gronbach E (1988) Cenococcum geophilum. In: Agerer R (ed) Colour atlas of ectomycorrhizae, plate 11. Einhorn Verlag, Schwäbisch Gmünd
Al Sayegh-Petkovsek S, Kraigher H (2000) Types of ectomycorrhizae from Kocevska Reka. Phyton-Annales Rei Botanicae 40(4):37–42
Allen MF (1991) The ecology of mycorrhizae. Cambridge University Press, Cambridge
Arbevy AS, Granhall U (1998) Occurrence and succession of mycorrhizas in Alnus incana. Swed J Agric Res 28:117–127
Azul AM, Freitas H (1999) Mycorrhizal fungi and their application to forestation programmes with cork oak (Quercus suber L.). In: Micorrización en áreas mediterráneas de la Península Ibérica. Junta de Extremadura, Badajoz, pp 75–82
Baar J, Kuyper TW (1998) Restoration of aboveground ectomycorrhizal flora in stands of Pinus sylvestris (Scots Pine) in the Netherlands by removal of litter and humus. Restor Ecol 6(3):227–237
Baar J, Horton TR, Kretzer AM, Bruns TD (1999) Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire. New Phytol 143:409–418
Barbour MG, Burk JH, Pitts WD, William FS, Schwartz MW (1999) Terrestrial plant ecology, 3rd edn. Benjamin/Cummings, California
Baxter JW, Pickett STA, Carreiro MM, Dighton J (1999) Ectomycorrhizal diversity and community structure in oak forest stands exposed to contrasting anthropogenic impacts. Can J Bot 77:771–782
Bencivenga M, Di Massimo G, Donnini D, Tanfulli M (1995) Micorrize inquinanti frequenti nelle piante tartufigene. Nota 1—Inquinanti in vivaio. Micol Ital 2:167–178
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. ACIAR Monograph 32, Canberra, pp 374
Castell C, Terradas J (1993) Ecofisiología de la encina (Quercus ilex) en la Serra de Collserola (Barcelona). Diferencias entre rebrotes e individuos adultos. In: Actas del I Congreso Forestal Español, Lourizán 1993. Ponencias y Comunicaciones, Tomo 1, pp 233–238
Castellano MA (1996) Outplanting performance of mycorrhizal inoculated seedlings. In: Mukerji KG (ed) Concepts in mycorrhizal research. Handbook of vegetation science, vol 19/2. Kluwer Academic Publishers, Dordrecht, pp. 223–301
Dahlberg A (2001) Community ecology of ectomycorrhizal fungi: an advancing interdisciplinary field. New Phytol 150:555–562
Dahlberg A (2002) Effects of fire on ectomycorrhizal fungi in fennoscandian boreal forests. Silva Fenn 36(1):69–80
Dahlberg A, Jonsson L, Nylund JE (1997) Species diversity and distribution of biomass above and below ground among ectomycorrhizal fungi in an old-growth Norway spruce forest in South Sweden. Can J Bot 75:1323–1335
Dahlberg A, Schimmel J, Taylor AFS, Johannesson H (2001) Post-fire legacy of ectomycorrhizal fungal communities in the Swedish boreal forest in relation to fire severity and logging intensity. Biol Conserv 100:151–161
Danielson RM (1984a) Ectomycorrhiza formation by the operculate discomycete Sphaerosporella brunnea (Pezizales). Mycologia 76:454–461
Danielson RM (1984b) Ectomycorrhizal associations in jack pine stands in northeastern Alberta. Can J Bot 62:932–939
De Miguel AM, De Román M, Etayo ML (2001) Mycorrhizal fungi competing with Tuber melanosporum Vitt. in cultivated truffle beds in northeastern Spain. In: Proceedings of the Second International Conference on Edible Mycorrhizal Mushrooms, Christchurch, New Zealand. CD-ROM
De Román M, Agerer R, De Miguel AM (2002) “Quercirhiza cumulosa” + Quercus ilex L. subsp. ballota (Desf.) Samp. Descr Ectomycorrhizae 6:13–18
Durall DM, Jones MD, Wright EF, Kroeger P, Coates KD (1999) Species richness of ectomycorrhizal fungi in cuttblocks of different sizes in the interior Cedar–Hemlock forests of northwestern British Columbia: sporocarps and ectomycorrhizae. Can J For Res 29(9):1322–1332
Erland S, Jonsson T, Mahmood S, Finlay RD (1999) Below-ground mycorrhizal community structure in two Picea abies forests in southern Sweden. Scand J For Res 14:209–217
García G (2001) Aproximación a las dinámicas poblacionales de las ectomicorrizas de los ecosistemas forestales ibéricos. Montes 63:5–15
Gardes M, Bruns TD (1996) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Can J Bot 74:1572–1583
Gehring CA, Theimer TC, Whitham TG, Keim P (1998) Ectomycorrhizal fungal community structure of pinyon pines growing in two environmental extremes. Ecology 79(5):1562–1572
Giraud M (1988) Prélèvement et analyse de mycorhizes. In: CTIFL (ed) La truffe. FNPT 10. Congrès de la trufficulture, Saintes, 27–28 novembre 1987, pp 49–63
Golldack J, Münzenberger B, Hüttl R (1999) “Pinirhiza dimorpha” + Pinus sylvestris L. Descr Ectomycorrhizae 4:73–78
Goodman DM, Trofymow JA (1998) Comparison of communities of ectomycorrhizal fungi in old-growth and mature stands of Douglas fir at two sites on Southern Vancouver Island. Can J For Res 28:574–581
Granetti B (1995) Caratteristiche morfologiche, biometriche e strutturali delle micorrize di Tuber di interesse economico. Micol Ital 2:101–117
Granetti B, Baciarelli Falini L (1997) Competizione tra le micorrize di Tuber melanosporum Vitt. e quelle di altri funghi in una tartufaia coltivata a Quercus ilex L. Micol Ital 3:45–59
Grogan P, Baar J, Bruns TD (2000) Below-ground ectomycorrhizal community structure in a recently burned bishop pine forest. J Ecol 88:1051–1062
Gupta V, Satyanarayana T, Garg S (2000) General aspects of mycorrhiza. In: Mukerji KG, Chamola BP, Singh J (eds) Mycorrhizal biology. Kluwer Academic/Plenum Publishers, New York, pp 27–44
Hagerman SM, Jones MD, Bradfield GE, Gillespie M, Durall DM (1999) Effects of clear-cut logging on the diversity and persistence of ectomycorrhizae at a subalpine forest. Can J For Res 29:124–134
Harley JL, Smith SE (1983) Mycorrhizal symbiosis. Academic Press, London
Hill MO, Gauch HG (1980) Detrended correspondence analysis, an improved ordination technique. Vegetatio 42:47–58
Horton TR, Bruns TD (1998) Multiple host-fungi are the most frequent and abundant ectomycorrhizal types in a mixed stand of Douglas fir (Pseudotsuga menziesii) and bishop pine (Pinus muricata). New Phytol 139:331–339
Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Mol Ecol 10:1855–1871
Horton TR, Cázares E, Bruns TD (1998) Ectomycorrhizal, vesicular–arbuscular and dark septate fungal colonization of bishop pine (Pinus muricata) seedlings in the first months of growth after wildfire. Mycorrhiza 8:11–18
Jakucs E, Agerer R (1999) Tomentella pilosa (Burt) Bourdot & Galzin+Populus alba L. Descr Ectomycorrhizae 4:135–140
Jakucs E, Bratek Z (1998) Genea verrucosa. In: Agerer R (ed) Colour atlas of ectomycorrhizae, plate 120. Einhorn Verlag, Schwäbisch Gmünd
Jakucs E, Agerer R, Bratek Z (1997) “Quercirhiza fibulocystidiata”+Quercus spec. Descr Ectomycorrhizae 2:67–71
Jakucs E, Bratek Z, Agerer R (1998a) Genea verrucosa Vitt. + Quercus spec. Descr Ectomycorrhizae 3:7–11
Jakucs E, Agerer R, Bratek Z (1998b) Quercirhiza fibulocystidiata. In: Agerer R (ed) Colour atlas of ectomycorrhizae, plate 132. Einhorn Verlag, Schwäbisch Gmünd
Jonsson L, Dahlberg A, Nilsson MC, Zackrisson O, Karen O (1999) Ectomycorrhizal fungal communities in late-successional Swedish boreal forests, and their composition following wildfire. Mol Ecol 8:205–215
Kõljalg U, Jakucs E, Bóka K, Agerer R (2001) Three ectomycorrhiza with cystidia formed by different Tomentella species as revealed by rDNA ITS sequences and anatomical characteristics. Folia Cryptogam Est 38:27–39
Kranabetter JM, Hayden S, Wright EF (1999) A comparison of ectomycorrhiza communities from three conifer species planted on forest gap edges. Can J Bot 77:1193–1198
Launonen TM, Ashton DH, Keane PJ (1999) The effect of regeneration burns on the growth, nutrient acquisition and mycorrhizae of Eucalyptus regnans F. Muell. (mountain ash) seedlings. Plant Soil 210:273–283
LoBuglio KF (1999) Cenococcum. In: Cairney JWG, Chambers SM (eds) Ectomycorrhizal fungi. Key genera in profile. Springer, Berlin Heidelberg, New York pp 287–310
Mah K, Tackaberry LE, Egger KN, Massicotte HB (2001) The impacts of broadcast burning after clear-cutting on the diversity of ectomycorrhizal fungi associated with hybrid spruce seedlings in central British Columbia. Can J For Res 31:224–235
Martínez de Aragón J, Bonet JA, Colinas C (2001) Potencial de inóculo micorrícico en bosques quemados de la comarca del Solsonès (Lleida) un año después del incendio . In: Actas del III Congreso Forestal Español, Granada, Mesa 6, pp 402–407
Massicotte HB, Molina R, Tackaberry LE, Smith J, Amaranthus MP (1999) Diversity and host specificity of ectomycorrhizal fungi retrieved from three adjacent forest sites by five host species. Can J Bot 77:1053–1076
McAfee BJ, Fortin JA (1988) Comparative effects of the soil microflora on ectomycorrhizal inoculation of conifer seedlings. New Phytol 108:443–449
McCune B, Mefford MJ (1999) PC-ORD. Multivariate analysis of ecological data, version 4. MjM Software Design, Gleneden Beach, OR, USA
Meotto F, Carraturo P (1988) Ectomicorrizia di Sphaerosporella brunnea (A. & S.) Svrcek & Kubicka in piantine tartufigene. Allionia 28:109–116
Meotto F, Pellegrino S, Craddock JH (1994) Funghi ectomicorrizici del castagno con particolare referimento ai funghi eduli. Italus Hortus 1(2):58–64
Mineo L, Majumdar SK (1996) Ectomycorrhizae in oaks (Quercus alba, Q. rubra) in Northeastern Pennsylvania woodlands: morphology, frequency and implied physiology and ecology. In: Mukerji KG (ed) Concepts in mycorrhizal research. Handbook of vegetation science, vol 19/2. Kluwer Academic Publishers, Dordrecht, pp 315–331
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbioses: community–ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning. An integrative plant–fungal process. Chapman & Hall, London, pp 357–423
Pausas JG (1997) Resprouting of Quercus suber in NE Spain after fire. J Veg Sci 8:703–706
Peter M, Ayer F, Egli S, Honegger R (2001) Above- and below-ground community structure of ectomycorrhizal fungi in three Norway spruce (Picea abies) stands in Switzerland. Can J Bot 79:1134–1151
Pigott CD (1982) Fine structure of mycorrhiza formed by Cenococcum geophilum Fr. on Tilia cordata Mill. New Phytol 92:501–512
Purdy BG, Macdonald SE, Dale MRT (2002) The regeneration niche of white spruce following fire in the mixedwood boreal forest. Silva Fenn 36(1):289–306
Rohlf FJ, Sokal RR (1995) Statistical tables, 3rd edn. Freeman, New York, 199 pp
Sáez R, De Miguel AM (1995) La trufa negra. Tuber melanosporum Vitt. Guía práctica de truficultura. ITGA-Universidad de Navarra, Pamplona, 94 pp
Stendell ER, Horton TR, Bruns TD (1999) Early effects of prescribed fire on the structure of the ectomycorrhizal fungus community in a Sierra Nevada ponderosa pine forest. Mycol Res 103(10):1353–1359
Taylor AFS (2002) Fungal diversity in ectomycorrhizal communities: sampling effort and species detection. Plant Soil 244:19–28
Torres P, Honrubia M (1997) Changes and effects of a natural fire on ectomycorrhizal inoculum potential of soil in a Pinus halepensis forest. For Ecol Manag 96:189–196
Trost T, Agerer R, Urbancic M, Kraigher H (1999) Biodiversity of ectomycorrhizae in a Norway spruce stand on Pokljuka. Phyton-Annales Rei Botanicae 39(4):225–232
Urban A, Weiss M, Bauer R (2003) Ectomycorrhizas involving sebacinoid mycobionts. Mycol Res 107(1):3–14
Visser S (1995) Ectomycorrhizal fungal succession in jack pine following wildfire. New Phytol 129:389–401
Weiss M (1991) Pisolithus tinctorius. In: Agerer R (ed) Colour atlas of ectomycorrhizae, plate 63. Einhorn Verlag, Schwäbisch Gmünd
Weiss M (1992) Mycorrhizae formed by Pisolithus tinctorius (Basidiomycetes) on Norway spruce. Cryptogam Bot 2:337–344
Zambonelli A, Salomoni S, Pisi A (1995) Caratterizzazione anatomo-morfologica delle micorrize di Tuber borchii, Tuber aestivum, Tuber mesentericum, Tuber brumale, Tuber melanosporum su Pinus pinea. Micol Ital 2:119–137
Zar JH (1998) Biostatistical analysis, 4th edn. Pearson-Prentice Hall, New Jersey
Acknowledgements
This research was supported by a Ph.D. grant from the Spanish Instituto Nacional de Investigaciones Agrarias y Alimentarias and by two projects funded by Gobierno de Navarra and PIUNA. We would also like to thank Steve Woodward for revising the manuscript and two anonymous referees for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Román, M., de Miguel, A.M. Post-fire, seasonal and annual dynamics of the ectomycorrhizal community in a Quercus ilex L. forest over a 3-year period. Mycorrhiza 15, 471–482 (2005). https://doi.org/10.1007/s00572-005-0353-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-005-0353-6