Abstract
Roots of terrestrial plants host a wide spectrum of soil fungi that form various parasitic, neutral and mutualistic associations. A similar trend is evident in freshwater aquatic plants and plants inhabiting salt marshes or mangroves. Marine vascular plants (seagrasses), by contrast, seem to lack specific root–fungus symbioses. We examined roots of two Mediterranean seagrasses, Posidonia oceanica and Cymodocea nodosa, in the northwestern Mediterranean Sea for fungal colonization using light and scanning and transmission electron microscopy. We found that P. oceanica, but not C. nodosa, is regularly associated with melanized septate hyphae in a manner resembling colonization by the ubiquitous dark septate endophytes (DSE) in roots of most terrestrial plants. P. oceanica roots were found to be colonized by sparse dematiaceous running hyphae as well as dense parenchymatous nets/hyphal sheaths on the root surface, intracellular melanized microsclerotia and occasionally also intra- and intercellular hyphae. The colonization was most prominent in the thick-walled hypodermis of the thinnest healthy looking roots, and the mycobiont seemed to colonize both living and dead host cells. Dark septate hyphae infrequently occurred also inside rhizodermal cells, but never colonized vascular tissues. The biological significance of this overlooked marine symbiosis remains unknown, but its morphology, extent, distribution across the NW Mediterranean Sea and absence in C. nodosa indicate an intriguing relationship between the dominant Mediterranean seagrass and its dark septate root mycobionts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A vast majority of terrestrial vascular plants form mutualistic root symbioses with a specialized group of soil fungi. These ancient associations (Redecker et al. 2000), called mycorrhizae (Smith and Read 2008), probably played a crucial role in the terrestrialization of vascular plants and their subsequent radiation on dry land (Selosse and Le Tacon 1998; Brundrett 2002). They represent one of the most intriguing plant adaptations for nutrient uptake, significantly affecting nutrient cycling in soils (Read and Perez-Moreno 2003; Read et al. 2004). Mycorrhizae vary in anatomy, morphology, ecophysiology and distribution (Read 1991; Brundrett 2004), but are functionally uniform, with a few exceptions, in that carbon flows from the photoautotrophic plant to the heterotrophic fungus and that water and mineral nutrients flow in the opposite direction. This requires the formation of specific exchange interfaces by the mycobiont inside the host root (Peterson and Massicotte 2004). It has been estimated that mycorrhizae are present in some 86 % of angiosperm species (Brundrett 2009), including freshwater plants (Søndergaard and Laegaard 1977; Beck-Nielsen and Madsen 2001; Sudová et al. 2011; Kohout et al. 2012) and plants from salt marshes (Radhika and Rodrigues 2007; Welsh et al. 2010; Eberl 2011) and mangroves (Sengupta and Chaudhuri 2002; Kothamasi et al. 2006). On the other hand, certain plant guilds do not require mycorrhizal symbioses for nutrient uptake (e.g. carnivorous plants, floating hydrophytes, parasites with haustoria, plants with cluster roots, etc.). Most of them belong to numerous families within the Brassicales, Caryophyllales, Lamiales, Poales and Santanales and also Alismatales, which comprise many hydrophytes including the only marine vascular plants—seagrasses (Brundrett 2009).
Besides mycorrhizal fungi, terrestrial plants live in symbioses with a wide spectrum of root endophytes that differ in their taxonomy, ecology and physiological significance for their hosts. In the Northern Hemisphere, their communities in temperate, boreal and subarctic plants are frequently dominated by dark septate endophytes (DSE), a group of miscellaneous soil fungi producing dematiaceous septate hyphae and melanized intracellular microsclerotia (Sieber 2002; Addy et al. 2005; Grünig et al. 2008). Additionally, some of them have been reported to form extra- and intraradical hyphal structures similar to those produced by mycorrhizal fungi for communication and nutrient exchange with their hosts, including dense parenchymatous hyphal nets on the root surface and intracellular hyphal loops (Vohník et al. 2003; Peterson and Massicotte 2004; Münzenberger et al. 2009; Vohník and Albrechtová 2011; Lukešová et al. 2015). Some authors even proved bi-directional nutrient flow between DSE and their host plants (Usuki and Narisawa 2007).
Seagrasses are a narrow ecological and taxonomic group of marine sessile macrophytes that evolved from terrestrial ancestors some 100 million years ago (Les et al. 1997). They comprise about 50–70 species (Hemminga and Duarte 2000; den Hartog and Kuo 2007) which have adapted to marine life by adopting several characteristic ecophysiological traits such as hydrophily and clonal growth. Seagrasses constitute less than 0.02 % of the angiosperm flora, yet they inhabit ca. 10 % of the coastal ocean area, represent ca. 1 % of the total marine plant biomass and are responsible for ca. 15 % of the total carbon storage in marine ecosystems (Hemminga and Duarte 2000). A recent estimate indicates that seagrasses can store up to twice as much carbon per square kilometre as temperate and tropical forests (Fourqurean et al. 2012). Seagrasses possess certain morphological, physiological and ecological traits which reduce the chance for symbioses with symbiotic fungi. Firstly, they take up nutrients through leaves (Khan and Belik 1995). Secondly, many species possess root hairs, and their roots develop extensive aerenchymatous systems in the cortex (Kuo and McComb 1989; Kuo and den Hartog 2007), i.e. in the tissue with the highest rates of arbuscular mycorrhizal (AM) colonization, the most common type of mycorrhiza, in terrestrial plants. Finally, many seagrasses live in muddy substrates low in available oxygen (Hemminga and Duarte 2000), which suppresses the development of extensive extraradical mycelia characteristic of many types of mycorrhiza (Nielsen et al. 1999). Congruently, the few studies focusing on seagrass root mycobionts (e.g. Cuomo et al. 1985; Nielsen et al. 1999; Panno et al. 2013; Torta et al. 2014) detected fungal hyphae or mycelia inside or on the surface of seagrass roots, but provided no clear evidence that seagrasses enter mycorrhizae or root endophytic associations similar to those occurring on dry land.
The genus Posidonia (Alismatales) is the evolutionary oldest genus of seagrasses, with the earliest fossil record from the Cretaceous (den Hartog 1970). It has a uniquely discontinuous distribution with eight of its nine species occurring in the Southern Hemisphere along the coast of Australia; the single Northern Hemisphere species Posidonia oceanica is endemic to the Mediterranean Sea (Green and Short 2003). Despite being a phanerogam, P. oceanica mostly relies on vegetative reproduction, which can be quite effective. Large climax meadows of the species dominate many sublittoral (depth of ca. 0–40 m) habitats along the Mediterranean coast, and the largest clones were estimated to spread over some 15 km while being hundreds to thousands of years old (Arnaud-Haond et al. 2012). Rhizomes, roots and senescent leaf sheaths of P. oceanica are exceptionally resistant to decay, which results in the formation of a characteristic peat-like sediment. This unique organic seabed layer (matte) can be several metres thick and thousands of years old (Hemminga and Duarte 2000). Vascular plants cannot effectively access such stocks of organically bound nutrients without the aid of symbiotic bacteria or fungi. However, the possibility that seagrasses in general and the matte-forming P. oceanica in particular host mycorrhizal or endophytic fungal symbionts has so far been examined only to a limited extent and with inconclusive results.
P. oceanica is sometimes accompanied by Cymodocea nodosa (Alismatales), a seagrass common in, but not restricted to, the Mediterranean Sea (den Hartog and Kuo 2007). This species usually occupies shallower waters and often grows in muddy substrates unsuitable for P. oceanica. Unlike P. oceanica, C. nodosa does not produce a matte, and its root system is characterized by vigorous production of root hairs (Kuo and McComb 1998). To our knowledge, possible root–fungus associations in C. nodosa have not yet been investigated.
In their pioneering work, Nielsen et al. (1999) did not find AM symbiosis in the seagrasses Thalassia testudinum and Zostera marina along the coasts of Denmark, Mexico and the USA. They nevertheless concluded that “it would be interesting to see data on possible AM colonization in seagrass species without root hairs from P-limited habitats, especially for fast growing species with hfaceigh nutrient demands” and encouraged other researchers “to investigate species like Cymodocea nodosa and Posidonia oceanica in the Mediterranean Sea”. Here, we report the results of our screening of these two Mediterranean seagrasses for mycorrhizal/root endophytic colonization.
Materials and methods
Root sampling
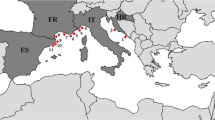
Root samples of P. oceanica (L.) Delile were collected using free and scuba diving between June and September 2012 at 11 localities in the NW Mediterranean Sea (Fig. 1, Table 1). We also sampled roots of the co-occurring seagrass C. nodosa (Ucria) Asch. where present (two localities; Table 1). The collection depths varied from 0.5 m in Antibes where leaves of P. oceanica emerged from the water at low tide to 31 m at Borak (Table 1). C. nodosa was found only at two localities (Finale Ligure and Sanary-sur-Mer), always occupying shallower habitats than P. oceanica. The two seagrasses never grew intermingled. P. oceanica always occurred on the rocky bottom or in coarse-grained sand whereas C. nodosa occurred in fine sand partly mixed with silt.
Location of the sampling sites within the NW Mediterranean Sea. Posidonia oceanica roots were sampled at 11 localities in Croatia (HR), Italy (IT), France (FR) and Spain (ES). The numbering of the localities follows Table 1. Bar = 500 km
At each locality, roots of five different individuals per species (at least 3 m apart) were carefully excavated from the substrate by bare hands, separated from the shoots and delivered to the surface. We tried to sample the whole diversity of substrates present at each locality, including sand, rocks and the matte. Terminal fine roots (ca. 1–2 mm in diam.) were then separated and inserted into 50-ml plastic beakers. Roots from all five individuals per locality were pooled to produce compound samples. The beakers with the roots were filled with seawater and stored in the dark in a portable refrigerator, and the seawater was later substituted by 30 % ethanol. The roots were then transported to the laboratory and stored in a refrigerator at 8 °C until processing.
Microscopic observations
Fungal colonization of the roots from each locality was investigated using an Olympus BX60 microscope equipped with DIC and an Olympus DP70 camera at magnifications of ×200, ×400 and ×1000. Scanning electron microscopy (SEM) was performed using a FEI Quanta 200 scanning electron microscope in the Olympus ESEM mode at low temperatures (−6 to −3 °C). We mainly screened hand-made semi-thin longitudinal (ca. 3–5 mm in length) and transversal root sections. For each locality, ten longitudinal sections from each of ten randomly chosen roots and five transversal sections from each of five randomly chosen roots were screened for any fungal structure. When needed, the sections were stained with 0.05 % trypan blue in lactoglycerol. For transmission electron microscopy, P. oceanica root segments were fixed in 5 % glutaraldehyde solution in 0.1 M phosphate buffer (pH 7.2) and then in 2 % OsO4 in 0.1 M phosphate buffer. The samples were then dehydrated in increasing concentrations of ethanol, including a contrasting step with 1 % uranyl acetate. Infiltration was performed in increasing concentration series of propyleneoxide and Spurr Resin. Samples were embedded into Spurr Resin, and ultrathin sections (70 nm) were cut and contrasted with uranyl acetate and lead citrate. Photographs were taken with a digital TEM camera (Veleta, Olympus) using a JEOL 1011 microscope and modified for clarity in Paint.NET and Adobe Photoshop as needed.
Results
Roots of all sampled plants looked healthy, and the respective plants did not show any apparent signs of growth stress. There was no apparent extraradical mycelium in the form of rhizomorphs or sclerotia in the rhizosphere of the screened specimens. Seeking the identity of the respective mycobiont(s) forming the observed root–fungus association was beyond the scope of this work.
Light and scanning electron microscopy
The screened P. oceanica roots consisted of the stele surrounded by endodermis. The thick cortex frequently contained air lacunae. Its outer parts were formed by hypodermis with markedly thick-walled cells and rhizodermis without root hairs (Fig. 2a, b). Only one type of fungal colonization was found in the roots of P. oceanica, comprising characteristic melanized septate hyphae. Most of the fungal colonization occurred in the hypodermis, either intra- or intercellularly (Fig. 2c, d), and at the root surface (Fig. 2d, e). Rhizodermal cells were commonly (but not always) free of fungal colonization (Fig. 2c–e). The colonization in the hypodermis was formed either by intracellular microsclerotia (Figs. 2c, d and 3a) or narrow hyphae, occasionally reducing and expanding their diameter while exploring neighbouring longitudinally elongated hypodermal cells (Fig. 3a, b). On the root surface, the melanized hyphae produced sometimes extensive hyphal sheaths formed either by thick straight hyphae (Fig. 3c, d), parenchymatous labyrinthine nets of hyphae/hyphal sheaths (Fig. 3d–f) or a combination of both (Fig. 3d). Hyphae occurred in both living and dead cells (Fig. 3a, g), but it was unclear whether cell death resulted from the fungal colonization or whether the hyphae colonized already dead cells. This specific fungal colonization was regularly found in roots from all screened localities but varied in its extent. Infrequently, the whole hypodermis and outer parts of the root cortex were heavily colonized by melanized septate hyphae (Fig. 3g).
Anatomy of Posidonia oceanica roots possessing the dark septate endophytic colonization. a A transversal section through a typical P. oceanica root with the dark septate colonization. The stele (S) is surrounded by the endodermis (E). The multilayered cortex (C) contains several lacunae (L) and continues to the darkly brown hypodermis (H) formed by smaller cells with markedly thickened walls. The outer part of the root is the single-layer rhizodermis (R) with markedly inflated cells. Light microscopy with DIC, bar = 100 μm. b As in a but screened with scanning electron microscopy (SEM). Bar = 100 μm. c A transversal section through outer layers of a colonized root. The whole hypodermis is colonized by intracellular microsclerotia (arrows). The cortex and rhizodermis are free of any fungal colonization; an extraradical hyphal sheath is missing. SEM, bar = 50 μm. d Fungal intracellular colonization as in c but accompanied by a layered extraradical hyphal sheath (arrows). SEM, bar = 25 μm. e Intracellular fungal microsclerotia (arrowheads) accompanied by an extraradical hyphal sheath (arrows). Light microscopy with DIC, bar = 25 μm
Anatomy and morphology of the dark septate colonization in Posidonia oceanica roots. a The root hypodermis colonized by lightly to heavily melanized septate hyphae (Hy), exploring hyphae (arrows) and intracellular elongated microsclerotia (M). Bar = 20 μm. b A longitudinal section through the hypodermis colonized by narrow dark septate hyphae (Hy). Note the transversal hypha repeatedly reducing and expanding its diameter while exploring the longitudinally elongated hypodermal cells (arrows). Bar = 20 μm. c An extraradical hyphal sheath (HS) formed by narrow dark septate hyphae. Bar = 20 μm. d An upright view through a layered hyphal sheath (HS) towards the rhizodermis. At the root surface, fungal hyphae produce a finger-like parenchymatous net (arrows) resembling structures formed by dark septate endophytic/mycorrhizal fungi in roots of terrestrial plants. Bar = 50 μm. e, f Details of the hyphal sheath (arrows). Bars = 20 μm. g The whole hypodermis and outer parts of the root cortex are heavily colonized by melanized hyphae. Bar = 20 μm. All pictures light microscopy with DIC
In contrast to P. oceanica, C. nodosa roots lacked the thick-walled hypodermis (Fig. 4a, b), and its rhizodermis and root cortex were commonly free of any visible fungal colonization (Fig. 4c, d). Rhizodermal cells rarely contained single fungal hyphae, and single narrow hyphae were sometimes observed on the root surface. However, these significantly differed from those found in P. oceanica. All C. nodosa roots possessed numerous root hairs (Fig. 4e).
Anatomy and morphology of Cymodocea nodosa roots lacking any typical fungal colonization. a A transversal section through a typical C. nodosa root with no apparent fungal colonization. Similarly to P. oceanica, the stele (S) is surrounded by the endodermis (E), the multilayered cortex (C) contains several lacunae (L) and the outer part of the root is formed by the single-layer rhizodermis (R) which cells are however not inflated as in P. oceanica. The hypodermis with markedly thick-walled cells is missing. Light microscopy with DIC, bar = 100 μm. b As in a but visualized with SEM. Bar = 50 μm. c A detail of the rhizodermis (R) and the cortex (C) free of any apparent fungal colonization. SEM, bar = 50 μm. d As in c but visualized with light microscopy with DIC. Bar = 50 μm. e An SEM photograph of the C. nodosa root surface with numerous root hairs (arrows). Bar = 50 μm
Transmission electron microscopy
Inflated rhizodermal cells of P. oceanica had approximately twice the diameter of hypodermal cells with markedly thickened outer cell walls. The cell walls of hypodermal cells were of a similar size as the rhizodermal outer cell walls, but much thicker than the inner rhizodermal cell walls (Fig. 5a). The spaces between neighbouring rhizodermal cells were usually occupied by prolonged, pointed, thick-walled, pentagonal to hexagonal cells which seemed to serve as entry points for fungal colonization (hence “entry cells”); the entering hyphae never seemed to penetrate directly through the thick-walled outer rhizodermal cell walls (Fig. 5b, c). In heavily colonized roots, the intraradical fungal colonization was mostly present throughout the hypodermis (Fig. 5b). However, in roots with low colonization intensity, intraradical fungal hyphae were often limited only to entry cells (Fig. 5c). When entering the root symplast or spreading from one cell to another, the hyphae first degraded the cell wall components and then proliferated through the resulting spaces (Fig. 5c, d). Occasionally, intraradical hyphae grew through intercellular spaces (Fig. 5e). It was not possible to unambiguously decide whether the intracellular hyphae were always surrounded by the host’s cytoplasmic membrane (Fig. 5e). Rather, it seemed that sometimes they were and sometimes they were not. Although most colonized and non-colonized hypodermal cells seemed to be alive, some microsclerotia and intracellular hyphae were evidently formed in already dead cells (also compare with Figs. 2e and 3b, g). We have not found any structures which would resemble arbuscules and vesicles typical of AM, fine intracellular hyphal coils typical of ericoid mycorrhiza or hyphal pelotons typical of orchid mycorrhiza.
Anatomy of P. oceanica roots with the fungal colonization visualized by transmission electron microscopy. a A transversal section through the rhizodermis and the hypodermis of a P. oceanica root. The inflated rhizodermal cells (RC) have thickened outer cell walls whereas their inner cell walls are very thin (arrowheads). The layers below the rhizodermis are formed by hypodermal cells (HC) with thickened cell walls. Each two rhizodermal cells is accompanied by a prolonged pointed cell which is the entry point for fungal colonization (hence “entry cells”, EC). Note several fungal hyphae occurring on the root surface in the sulci between the rhizodermal cells, right against the entry cells (arrows). Bar = 10 μm. b A transversal section through the upper layers of a colonized root. The fungal colonization comprises extraradical hyphal net/sheath (HN) which hyphae penetrate the spaces between the rhizodermal cells (arrow), and numerous intracellular hyphae formed first in the entry cells (EC) and then spreading to the hypodermal cells (HC). The inflated rhizodermal cells (RC) are usually free of fungal colonization. Bar = 10 μm. c A detail of an extraradical hypha (arrow) penetrating the space between two rhizodermal cells (RC) and consequently forming vigorous fungal colonization in an entry cell (EC). The remaining host cells are free of fungal colonization. Bar = 10 μm. d A rare example of a fungal colonization in the rhizodermis: an intracellular fungal hypha (IH) degrades components of the cell wall (CW) resulting in a cell wall lysis (CWL) while passing from one cell to another. Bar = 2 μm. e The typical colonization pattern in a thick-walled hypodermal cell possessing both primary cell wall (PCW) and secondary cell wall (SCW). Note several intracellular hyphae (IH) as well as one intercellular hypha (arrow). Bar = 2 μm
Discussion
First reports on interactions between seagrasses and root-inhabiting fungi date back to the 1980s. With respect to the genus Posidonia, Kuo et al. (1981) provided microscopic evidence for an association between certain fungi and roots of two Australian species, Posidonia australis and Posidonia sinuosa. Similarly to our observations in P. oceanica, the fungal colonization was mostly restricted to the thick-walled hypodermis. The fungi often lysed cell walls and middle lamellae of colonized hypodermal cells, and although the authors speculated that the fungi might play a role in the nutrient uptake of the seagrasses, the reported association resembled rather a parasitic/pathogenic than a mutualistic interaction. More recently, García-Martínez et al. (2005) noticed some fungal hyphae growing on the root surface while examining microbial colonization of P. oceanica roots by electron microscopy, but did not report any intraradical fungal colonization. The mycoflora of P. oceanica leaves, rhizomes, roots and matte has been studied by Panno et al. (2013); however, the authors did not report any specific fungal colonization pattern in the screened tissues. Most recently, Torta et al. (2014) reported fungal colonization of P. oceanica roots at two localities in NW Sicily. The observed septate hyphae and microsclerotia, however, seemed to lack the dark pigmentation typical of DSE. Although it was evident that seagrass roots harbour fungal endophytes, the reported colonization seemed to be rather random, i.e. resulting from common non-specific contacts with marine fungi, as the authors did not report any typical fungal structures attributed to so far recognized root–fungus associations. In contrast, here, for the first time, we describe a dark septate association in the roots of a seagrass exhibiting all typical structures, i.e. extraradical and intraradical dark septate hyphae, dense melanized parenchymatous nets/hyphal sheaths on the root surface and melanized intracellular microsclerotia.
DSE associations typically lack fungal structures designed for nutrient transport and communication with the host plant (Peterson et al. 2008). On the other hand, certain DSE are able to form the Hartig net, i.e. the hyphal structure typical of ectomycorrhizal symbiosis (Danielson and Visser 1990; O’Dell et al. 1993; Fernando and Currah 1996; Münzenberger et al. 2009; Lukešová et al. 2015). Moreover, some authors hypothesized that the dense parenchymatous net on the root surface (also known as DSE labyrinthine tissue), detected here for the first time in a seagrass, may functionally parallel the Hartig net (O’Dell et al. 1993; Fernando and Currah 1996; Wurzburger and Bledsoe 2001; Vohník and Albrechtová 2011). Due to its frequency in some of the P. oceanica roots screened, the functioning of this fungal structure apparently deserves further investigation, as it might play a role in nutrient uptake from the matte or the mineral substrate. On the other hand, DSE microsclerotia, here commonly found in P. oceanica hypodermis, serve as storage and propagation organs of the mycobiont and most likely do not play any role in the host’s nutrient uptake (Yu et al. 2001). In endomycorrhizal symbioses, the intracellular hyphal phase is always separated from the cell cytoplasm by a plant-derived membrane (Peterson and Massicotte 2004). As was the case with other studies on DSE (Peterson et al. 2008), we were unable to unambiguously decide whether this was always true for the screened P. oceanica roots. Some intracellular hyphae evidently occurred in dead host cells, which is not typical of mycorrhizal symbioses (Peterson and Massicotte 2004); in some cases, however, DSE may cause host cell breakdown (Peterson et al. 2008), and host cell death is required for the proliferation of the mutualistic endophyte Piriformospora indica within host roots (Deshmukh et al. 2006).
Intriguingly, most of the intraradical colonization appeared within the hypodermis (cf. Kuo et al. 1981) whereas the rest of the root cortex usually remained free of fungal colonization. Hypodermis is commonly defined as a uni- or multiseriate layer of cells morphologically distinct from those of the neighbouring cortex formed just below the rhizodermis, and thus forming the outer layer of the root cortex (Peterson 1989). Its cell walls usually contain varying amounts of suberin, lignin, carbohydrates and structural cell wall proteins forming an important apoplastic barrier as well as a barrier against pathogenic microorganisms (Schreiber et al. 1999). Kuo et al. (1981) hypothesized that it may restrict the free movement of water and ions from sediments to the stele and the diffusion of root exudates into the rhizosphere in the two Australian Posidonia species. Given the apparent ability of mycobionts associated with P. oceanica to lyse even hypodermal cell walls (cf. Kuo et al. 1981), it is unclear why they do not progress to the cortex. On the other hand, frequent lysis of hypodermal cell walls may eliminate the hypodermal apoplastic barrier with as yet unknown consequences for P. oceanica.
The fungal association reported here was absent in the roots of the neighbouring seagrass C. nodosa, which points at its host/substrate specificity. Similarly, co-occurring terrestrial plants may host different spectra of root endophytes (Tejesvi et al. 2013). The reason for this specificity remains unknown. It may involve complex molecular mechanisms or simply reflect the absence of a root tissue suitable for colonization by mycobionts, as C. nodosa lacked the thick-walled hypodermis found in all P. oceanica individuals screened in the present study. Moreover, in contrast to P. oceanica, roots of C. nodosa (at least those sampled) produced abundant root hairs, which aid nutrient uptake in many terrestrial vascular plants (Gilroy and Jones 2000) and therefore reduce the need for symbiosis with fungi facilitating nutrient uptake in exchange for photosynthates. As root hairs are usually reduced to absent in mycorrhizal plants, the absence of root hairs in P. oceanica might also indicate the involvement of the root–fungus symbiosis in the acquisition of mineral nutrients. This, however, needs to be addressed in future studies.
Conclusions
Our survey of seagrass roots from 11 localities in the NW Mediterranean Sea resulted in the discovery of a specific fungal symbiosis in P. oceanica. Its anatomy and morphology are unique among seagrasses and displays a high degree of similarity to the ubiquitous DSE association occurring in the roots of most terrestrial vascular plants. Despite its ubiquity and abundance in healthy looking populations of P. oceanica, it remains unclear whether the mycobionts engage in nutrient capture and transport to the host (as suggested by the presence of parenchymatous labyrinthine hyphal nets/hyphal sheaths on the root surface and the absence of root hairs) and, more generally, whether it is of any benefit to the dominant Mediterranean seagrass.
References
Addy HD, Piercey MM, Currah RS (2005) Microfungal endophytes in roots. Can J Bot 83:1–13
Arnaud-Haond S, Duarte CM, Diaz-Almela E, Marbá N, Sintes T et al (2012) Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLoS ONE 7, e30454. doi:10.1371/journal.pone.0030454
Beck-Nielsen D, Madsen TV (2001) Occurrence of vesicular-arbuscular mycorrhiza in aquatic macrophytes from lakes and streams. Aquat Bot 71:141–148
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304
Brundrett MC (2004) Diversity and classification of mycorrhizal associations. Biol Rev 79:473–495
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Cuomo V, Vanzanella F, Fresi E, Cinelli F, Mazzella L (1985) Fungal flora of Posidonia oceanica and its ecological significance. Trans Br Mycol Soc 84:35–40
Danielson RM, Visser S (1990) The mycorrhizal and nodulation status of container-grown trees and shrubs reared in commercial nurseries. Can J For Res 20:609–614
den Hartog C (1970) Seagrasses of the world. North-Holland Publishing, Amsterdam
den Hartog C, Kuo J (2007) Taxonomy and biogeography of seagrasses. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht
Deshmukh S, Hückelhoven R, Schäfer P, Imani J, Sharma M, Weiss M, Waller F, Kogel K-H (2006) The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc Natl Acad Sci U S A 103:18450–18457
Eberl R (2011) Mycorrhizal association with native and invasive cordgrass Spartina spp. in San Francisco Bay, California. Aquat Biol 14:1–7
Fernando AA, Currah RS (1996) A comparative study of the effects of the root endophytes Leptodontidium orchidicola and Phialocephala fortinii (Fungi Imperfecti) on the growth of some subalpine plants in culture. Can J Bot 74:1071–1078
Fourqurean JW, Duarte CM, Kennedy H, Marba N, Holmer M et al (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:505–509
García-Martínez M, Kuo J, Kilminster K, Walker D, Rossello-Mora R et al (2005) Microbial colonization in the seagrass Posidonia spp. roots. Mar Biol Res 1:388–395
Gilroy S, Jones DL (2000) Through form to function: root hair development and nutrient uptake. Trends Plant Sci 5:56–60
Green EP, Short FT (2003) World atlas of seagrasses. University of California Press, Berkeley
Grünig C, Queloz V, Sieber T, Holdenrieder O (2008) Dark septate endophytes (DSE) of the Phialocephala fortinii s. l.—Acephala applanata species complex in tree roots: classification, population biology, and ecology. Botany 86:1355–1369
Hemminga MA, Duarte CM (2000) Seagrass ecology. Cambridge University Press, Cambridge
Khan AG, Belik M (1995) Occurrence and ecological significance of mycorrhizal symbiosis in aquatic plants. In: Varma A, Hock B (eds) Mycorrhiza. Springer, Berlin
Kohout P, Sýkorová Z, Čtvrtlíková M, Rydlová J, Suda J et al (2012) Surprising spectra of root-associated fungi in submerged aquatic plants. FEMS Microbiol Ecol 80:216–235
Kothamasi D, Kothamasi S, Bhattacharyya A, Kuhad RC, Babu CR (2006) Arbuscular mycorrhizae and phosphate solubilising bacteria of the rhizosphere of the mangrove ecosystem of Great Nicobar island, India. Biol Fertil Soils 42:358–361
Kuo J, den Hartog C (2007) Seagrass morphology, anatomy and ultrastructure. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht
Kuo J, McComb AJ (1989) Seagrass taxonomy, structure and development. In: Larkum AWD, McComb AJ, Shepherd SA (eds) Biology of seagrasses. Elsevier, Amsterdam
Kuo J, McComb AJ (1998) Cymodoceaceae. In: Kubitzki K (ed) The families and genera of vascular plants: Flowering Plants - Monocotyledons. Springer, Berlin, Heidelberg
Kuo J, McComb AJ, Cambridge ML (1981) Ultrastructure of the seagrass rhizosphere. New Phytol 89:139–143
Les DH, Cleland MA, Waycott M (1997) Phylogenetic studies in Alismatidae, II: evolution of marine angiosperms (seagrasses) and hydrophily. Syst Bot 22:443–463
Lukešová T, Kohout P, Větrovský T, Vohník M (2015) The potential of Dark Septate Endophytes to form root symbioses with ectomycorrhizal and ericoid mycorrhizal middle European forest plants. PLoS ONE 10, e0124752. doi:10.1371/journal.pone.0124752
Münzenberger B, Bubner B, Wöllecke J, Sieber TN, Bauer R et al (2009) The ectomycorrhizal morphotype Pinirhiza sclerotia is formed by Acephala macrosclerotiorum sp nov., a close relative of Phialocephala fortinii. Mycorrhiza 19:481–492
Nielsen SL, Thingstrup I, Wigand C (1999) Apparent lack of vesicular-arbuscular mycorrhiza (VAM) in the seagrasses Zostera marina L. and Thalassia testudinum Banks ex Konig. Aquat Bot 63:261–266
O’Dell TE, Massicotte HB, Trappe JM (1993) Root colonization of Lupinus latifolius Agardh. and Pinus contorta Dougl. by Phialocephala fortinii Wang & Wilcox. New Phytol 124:93–100
Panno L, Bruno M, Voyron S, Anastasi A, Gnavi G et al (2013) Diversity, ecological role and potential biotechnological applications of marine fungi associated to the seagrass Posidonia oceanica. N Biotechnol 30:685–694
Peterson CA (1989) Significance of the exodermis in root function. In: Loughman BC, Gašparíková O, Kolek J (eds) Structural and functional aspects of transport in roots. Developments in Plant and Soil Sciences 36:35–40. Springer
Peterson RL, Massicotte HB (2004) Exploring structural definitions of mycorrhizas, with emphasis on nutrient-exchange interfaces. Can J Bot 82:1074–1088
Peterson RL, Wagg C, Pautler M (2008) Associations between microfungal endophytes and roots: do structural features indicate function? Botany 86:445–456
Radhika KP, Rodrigues BF (2007) Arbuscular mycorrhizae in association with aquatic and marshy plant species in Goa, India. Aquat Bot 86:291–294
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Read DJ, Leake JR, Perez-Moreno J (2004) Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can J Bot 82:1243–1263
Redecker D, Kodner R, Graham LE (2000) Glomalean fungi from the Ordovician. Science 289:1920–1921
Schreiber L, Hartmann K, Skrabs M, Zeier J (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot 50:1267–1280
Selosse MA, Le Tacon F (1998) The land flora: a phototroph-fungus partnership? Trends Ecol Evol 13:15–20
Sengupta A, Chaudhuri S (2002) Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza 12:169–174
Sieber TN (2002) Fungal root endophytes. In: Waisel Y, Eshel A, Kafkafi I (eds) Plant roots—the hidden half, 3rd edn. Marcel Dekker, New York
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, London
Søndergaard M, Laegaard S (1977) Vesicular-arbuscular mycorrhiza in some aquatic vascular plants. Nature 268:232–233
Sudová R, Rydlová J, Čtvrtlíková M, Havránek P, Adamec L (2011) The incidence of arbuscular mycorrhiza in two submerged Isoetes species. Aquat Bot 94:183–187
Tejesvi MV, Sauvola T, Pirttilä AM, Ruotsalainen AL (2013) Neighboring Deschampsia flexuosa and Trientalis europaea harbor contrasting root fungal endophytic communities. Mycorrhiza 23:1–10
Torta L, Lo Piccolo S, Piazza G, Burruano S, Colombo P et al (2014) Lulwoana sp., a dark septate endophyte in roots of Posidonia oceanica (L.) Delile seagrass. Plant Biol (Stuttg) 17:505–511
Usuki F, Narisawa K (2007) A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia 99:175–184
Vohník M, Albrechtová J (2011) The co-occurrence and morphological continuum between ericoid mycorrhiza and dark septate endophytes in roots of six European Rhododendron species. Folia Geobot 46:373–386
Vohník M, Lukančič S, Bahor E, Regvar M, Vosátka M et al (2003) Inoculation of Rhododendron cv. Belle-Heller with two strains of Phialocephala fortinii in two different substrates. Folia Geobot 38:191–200
Welsh AK, Burke DJ, Hamerlynck EP, Hahn D (2010) Seasonal analyses of arbuscular mycorrhizae, nitrogen-fixing bacteria and growth performance of the salt marsh grass Spartina patens. Plant Soil 330:251–266
Wurzburger N, Bledsoe CS (2001) Comparison of ericoid and ectomycorrhizal colonization and ectomycorrhizal morphotypes in mixed conifer and pygmy forests on the northern California coast. Can J Bot 79:1202–1210
Yu T, Nassuth A, Peterson RL (2001) Characterization of the interaction between the dark septate fungus Phialocephala fortinii and Asparagus officinalis roots. Can J Microbiol 47:741–753
Acknowledgments
Financial support was provided by the Czech Science Foundation (GACR P504/10/0781) and the Grant Agency of Charles University in Prague (GAUK 68313/PrF/B-BIO). This study is a part of the long-term research projects of the Institute of Botany ASCR (RVO 67985939) and Faculty of Science, Charles University in Prague (MŠMT LO1417). Lukáš Kalous organized a workshop on marine biology in Borak, Croatia which substantially stimulated our research at its beginning, Kateřina Rylková helped with collection and processing of some Croatian root samples and Jiří Machač took SEM microphotographs and assembled the figures; all these contributions are greatly acknowledged. In Croatia, root samples were collected in accordance with a permit issued by the Croatian Ministry of Environmental and Nature Protection (Klasa UP/I-612-07/13-48/48, Urbroj 517-07-1-1-1-13-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vohník, M., Borovec, O., Župan, I. et al. Anatomically and morphologically unique dark septate endophytic association in the roots of the Mediterranean endemic seagrass Posidonia oceanica . Mycorrhiza 25, 663–672 (2015). https://doi.org/10.1007/s00572-015-0642-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0642-7