Abstract
Anaphylactic shock is a potentially lethal complication during anesthesia and requires appropriate management to save the patient’s life. We report a 32-year-old man who developed anaphylaxis during induction of general anesthesia with remimazolam for hand surgery. He received general anesthesia with midazolam 4 weeks before. This time facial flushing followed by a decrease of peripheral oxygen saturation (SpO2) and blood pressure occurred 2 min after starting continuous remimazolam infusion at 6 mg/kg/h. Hypotension and SpO2 were recovered by repeated administration of adrenaline. Despite no increase of serum tryptase levels, intradermal allergy tests 4 weeks postoperatively revealed that remimazolam and midazolam were positive, suggesting remimazolam as a causative agent for anaphylaxis. In the previous surgery, midazolam, which has a similar structure to remimazolam, may have caused sensitization. This is probably the first case report of anaphylaxis caused by remimazolam.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaphylaxis is an acute, systemic allergic reaction that triggered by mediators released by mast cells and basophils activated via allergic or non-allergic mechanisms. Although rare, it can cause fatal shock [1,2,3]. During anesthesia induction, various drugs are administered over a short time period, often making it difficult to identify the causative agent of anaphylaxis occurring at this time. Currently, the known common causes of perioperative anaphylaxis are muscle relaxants and antimicrobial agents [4, 5].
Remimazolam besylate (Anerem®), which we use for induction of anesthesia, is an ultra-short-acting benzodiazepine intravenous anesthetic [6] that was newly launched in Japan on Aug 7, 2020, ahead of the rest of the world, by Mundipharma Co. (Sydney, Australia) [7, 8]. Its safety for use as a sedative has been previously evaluated [9]. So far (Feb 2021), no case of anaphylaxis caused by remimazolam has been reported.
In this study, we report our experience with a patient who suffered severe anaphylactic shock during induction of anesthesia with remimazolam. From these and postoperative allergen identification skin tests, we were able to identify the causative drug as remimazolam. Written consent for publication of this case report was obtained from the patient.
Case presentation
The patient was a 32-year-old man, 162 cm tall, weighing 60 kg. He had no significant previous medical history and no history of allergy. He underwent a surgery for the right wrist open injury on the day of admission, and autologous free composite tissue grafting on the eighth day under general anesthesia. The two surgeries were completed without any adverse events (Table 1).
Anaphylactic shock occurred during the third surgery, which involved right intraosseous insert removal on the 36th day. After entering the operating room, oxygenation was started at 6 L/min, followed by intravenous remifentanil 0.3 µg/kg/min and fentanyl 100 µg under standard monitoring. Then, an infusion of remimazolam was started at 6 mg/kg/h. Facial flushing occurred 2 min later, after infusion of remimazolam 12 mg. Desflurane inhalation was commenced, and rocuronium 50 mg was administered after confirming the loss of consciousness and stopping infusion of remimazolam. Following facial flushing, a decrease in SpO2 and systolic blood pressure was observed. The patient was manually ventilated using bag-mask ventilation, but since his SpO2 decreased further to 75% and he appeared cyanotic without typical wheezy sounds on auscultation, he was urgently intubated. Hypotension was treated with 12 mg ephedrine administered intravenously. However, there was no improvement in oxygenation and blood pressure, and SpO2 dropped to 68% and systolic blood pressure to 49 mmHg. Diagnosing anaphylactic shock, we administered 0.5 mg of adrenaline intravenously. Immediately after injection of adrenaline, improvement in oxygenation and increase in blood pressure were observed. An arterial catheter was inserted through the left radial artery. 20 min after the first dose of adrenaline, hypoxia and hypotension recurred, with a SpO2 of 77% and systolic blood pressure of 89 mmHg. Subsequently, adrenaline, 0.25 mg intravenously and 0.5 mg intramuscularly, was administered, which once again resulted in stabilization of his vital signs. Given the patient’s condition, the surgical procedure was changed to only reduction with debridement. After his vitals were stabilized, 20 mg of famotidine, 5 mg of d-chlorpheniramine, and 100 mg of hydrocortisone were administered. A total of 1600 ml of extracellular fluid were administered during surgery. At the end of surgery, dexmedetomidine was started at 40 μg/h, and adrenaline at 0.05 μg/kg/min to maintain systolic blood pressure above 90 mmHg, and the patient was discharged to the ICU with his trachea still intubated. The vital signs from the time of entry into the operating room to the time of their stabilization is shown in Fig. 1.
On admission of ICU, his serum lactate level was 98.6 mg/dl and Troponin T level was 0.214 ng/ml. Echocardiography showed that his left-ventricular wall motion was normal. Since laryngoscopy showed marked laryngeal edema, we judged that extubation was not possible until the laryngeal edema improved. Intravenous hydrocortisone (100 mg) was administered to prevent a bimodal anaphylaxis reaction and to treat laryngeal edema. While in the ICU, his blood pressure and heart rate remained stable at 90/40–120/60 mmHg and 80–110 beats/min, respectively. Fortunately, there was no reappearance of anaphylaxis. We extubated the patient the next day, after confirming that there were no airway or respiratory issues. Since evaluation revealed that there were no persistent circulatory or central nervous system abnormalities, the patient was transferred to the general ward.
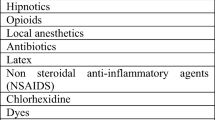
Serum tryptase and histamine were 5.8 µg/ml and 1.5 ng/ml, respectively, immediately after the onset of anaphylaxis. Tryptase levels were 5.6 µg/ml and 4.7 µg/ml at 3 and 6 h after the onset of anaphylaxis, respectively. About 4 weeks after the occurrence of anaphylaxis, skin tests for allergen identification were performed (Fig. 2). A total of three drugs were tested: rocuronium and remimazolam, which were suspect drugs, and midazolam, which is a benzodiazepine sedative drug like remimazolam. The results indicated positive intradermal tests with remimazolam and midazolam (Table 2).
Discussion
The patient developed anaphylaxis to remimazolam, and probably, this is the first report. Skin tests performed about 4 weeks later evidenced a clear relationship between remimazolam and anaphylaxis. It should be noted that midazolam also showed positive results, suggesting that midazolam used in the second surgery might have caused a sensitization reaction, and remimazolam, which has similar chemical structure to midazolam, subsequently caused anaphylaxis. Both remimazolam and midazolam have an imidazobenzodiazepine structure [6]. Allergic reaction due to cross-reactivity of drugs with similar chemical structures has been reported previously [10].
Although rare, some cases of anaphylaxis due to midazolam have been reported [11,12,13]. In these previous reports, anaphylaxis due to midazolam was diagnosed when severe hypotension, decreased oxygen saturation, and skin symptoms suddenly appeared 1–2 min after intravenous midazolam. Although there are few reports of anaphylaxis due to midazolam allergy itself, most cases occurred within a few minutes after its administration, which is consistent with the fact that allergy to remimazolam, which is structurally similar to midazolam, would also cause anaphylaxis within a few minutes after administration. The high levels of histamine in blood samples obtained after the onset of symptoms in this case also suggest an immediate allergic reaction. Serum tryptase is known to be elevated during anaphylaxis, but there was no significant change in this case. Jeon et al. [11] also reported midazolam anaphylaxis without elevation of serum tryptase levels, which should be carefully evaluated in the future [14].
The first-line drug for the treatment of anaphylaxis is adrenaline. However, appropriate use of adrenaline in terms of dosage and rate of administration requires careful monitoring, because excessive amounts can cause ventricular tachycardia, excessive hypertension, myocardial damage, coronary artery spasm, and pulmonary edema [15]. In our case, adrenaline was administered intravenously at an initial dose of 0.5 mg, followed by 0.25 mg intravenously. The adrenaline doses recommended by the guidelines of different countries vary [16,17,18,19]. We believe that the intravenous dose of 0.5 mg of adrenaline was acceptable, even though his systolic blood pressure increased to more than 180 mmHg after the administration of adrenaline, because our patient seemed about to go into cardiac arrest.
In anaphylactic shock, which requires high doses of adrenaline for treatment, it is necessary to be aware of subsequent complications. A study by Cha [20] assessing troponin levels and echocardiography confirmed the occurrence of myocardial damage in 7.3% of 300 patients with anaphylaxis. In the present case, troponin was positive in blood samples collected at the time of ICU admission. During intensive care management, occurrence of a second wave of anaphylaxis and cardiac disorders that can occur after anaphylaxis, such as Kounis syndrome [21] and Takotsubo cardiomyopathy [22], was ruled out by continued echocardiography monitoring. Fortunately, the patient recovered without major complications and could be extubated the day after the event.
This case report shows that remimazolam, although a useful drug, can cause anaphylaxis. Cross-reactivity with midazolam is also possible. Therefore, it is important to observe the patient during induction of anesthesia and to treat anaphylaxis-induced hypotension with adrenaline as soon as possible.
References
LoVerde D, Iweala OI, Eginli A, Krishnaswamy G. Anaphylaxis. Chest. 2018;153:528–43.
Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140:321–33.
Moore LE, Kemp AM, Kemp SF. Recognition, treatment, and prevention of anaphylaxis. Immunol Allergy Clin North Am. 2015;35:363–74.
Horiuchi T, Takazawa T, Orihara M, Sakamoto S, Nagumo K, Saito S. Drug-induced anaphylaxis during general anesthesia in 14 tertiary hospitals in Japan: a retrospective, multicenter, observational study. J Anesth. 2021;35:154–60.
Harper NJN, Cook TM, Garcez T, Farmer L, Floss K, Marinho S, Torevell H, Warner A, Ferguson K, Hitchman J, Egner W, Kemp H, Thomas M, Lucas DN, Nasser S, Karanam S, Kong KL, Farooque S, Bellamy M, McGuire N. Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6). Br J Anaesth. 2018;121:159–71.
Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107:60–6.
Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA class III): results of a multicentre, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34:491–501.
Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicentre, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543–53.
Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, Wahidi M, Shojaee S, Tanner NT, Callahan SP, Feldman G, Lorch DG Jr, Ndukwu I, Pritchett MA, Silvestri GA. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155:137–46.
Patton K, Borshoff DC. Adverse drug reactions. Anaesthesia. 2018;73(Suppl 1):76–84.
Jeon YS, Shim JW, Jun EH, Choi ST, Jung HS. Midazolam anaphylaxis during general anesthesia: a case report. Medicine (Baltimore). 2019;98:e17405.
George C, Williams A. Anaphylaxis with midazolam—our experience. Indian J Anaesth. 2011;55:630–1.
Landsem LM, Ross FJ, Eisses MJ. A case of midazolam anaphylaxis during a pediatric patient’s first anesthetic. J Clin Anesth. 2017;43:75–6.
Buka RJ, Knibb RC, Crossman RJ, Melchior CL, Huissoon AP, Hackett S, Dorrian S, Cooke MW, Krishna MT. Anaphylaxis and clinical utility of real-world measurement of acute serum tryptase in UK emergency departments. J Allergy Clin Immunol Pract. 2017;5:1280–7.
Simons ER, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8:32.
Harper NJN, Dixon T, Dugue P, Edgar DM, Fay A, Gooi HC, Herriot R, Hopkins P, Hunter JM, Mirakian R, Pumphrey RSH, Seneviratne SL, Walls AF, Williams P, Wildsmith JA, Wood P, Nasser AS, Powell RK, Mirakhur R, Soar J, AAGBI. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009;64:199–211.
Garvey LH, Dewachter P, Hepner DL, Mertes PM, Voltolini S, Clarke R, Cooke P, Garcez T, Guttormsen AB, Ebo DG, Hopkins PM, Khan DA, Kopac P, Krøigaard M, Laguna JJ, Marshall S, Platt P, Rose M, Sabato V, Sadleir P, Savic L, Savic S, Scherer K, Takazawa T, Volcheck GW, Kolawole H. Management of suspected immediate perioperative allergic reactions: an international overview and consensus recommendations. Br J Anaesth. 2019;123:e50–64.
Kroigaard M, Garvey LH, Gillberg L, Johansson SGO, Mosbech H, Florvaag E, Harboe T, Eriksson LI, Dahlgren G, Seeman-Lodding H, Takala R, Wattwil M, Hirlekar G, Dahlén B, Guttormsen AB. Scandinavian clinical practice guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007;51:655–70.
Mertes PM, Malinovsky JM, Jouffroy L, Aberer W, Terreehorst I, Brockow K, Demoly P, The Working Group of the SFAR, SFA, for ENDA, the EAACI Interest Group on Drug Allergy. Reducing the risk of anaphylaxis during anaesthesia: 2011 updated guidelines for clinical practice. J Investig Allergol Clin Immunol. 2011;21:442–53.
Cha YS, Kim H, Bang MH, Kim OH, Kim HI, Cha K. Evaluation of myocardial injury through serum troponin I and echography in anaphylaxis. Am J Emerg med. 2016;34:140–4.
Takenaka I, Okada E, Aoyama K, Iwagaki T, Kadoya T. Kounis syndrome during general anesthesia and administration of adrenaline. Int J Cardiol. 2012;154:e34–5.
Margonato D, Abete R, Giovine GD, Delfino P, Grillo M, Mazzetti S, Poggio D, Rossi J, Khouri T, Mortara A. Takotsubo cardiomyopathy associated with Kounis syndrome: a clinical case of the “ATAK” complex. J Cardiol Cases. 2019;20:52–6.
Acknowledgements
The authors would like to thank Forte (www.forte-science.co.jp) for English language editing.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
KT acquired the data and prepared the article. ST acquired the data, prepared the article, and designed this report. YH acquired the data. KN acquired and analyzed the skin test. TT acquired the data and reviewed the article. YK reviewed the article. The article has been read and approved by all the authors.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tsurumi, K., Takahashi, S., Hiramoto, Y. et al. Remimazolam anaphylaxis during anesthesia induction. J Anesth 35, 571–575 (2021). https://doi.org/10.1007/s00540-021-02934-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-021-02934-8