Abstract
Background
Cystic fibrosis transmembrane conductance regulator (CFTR) was recently found in the enteric nervous system, where its role is unclear. We aimed to identify which enteric neuronal structures express CFTR, whether CFTR modulates enteric neurotransmission and if altered CFTR expression is associated with slow transit constipation (STC).
Methods
Immunofluorescence double labeling was performed to localize CFTR with various neuronal and glial cell markers in the human colon. The immunoreactivity (IR) of CFTR and choline acetyltransferase (ChAT) on myenteric plexus of control and STC colon was quantitatively analyzed. In control colonic muscle strips, electrical field stimulation (EFS) evoked contractile responses and the release of acetylcholine (ACh) was measured in the presence of the CFTR channel inhibitor, CFTR(inh)-172.
Results
CFTR-IR was densely localized to myenteric ganglia, where it was co-localized with neuronal markers HuC/D and β-tubulin, and glial marker S-100 but little with glial fibrillary acidic protein. Vesicular ACh transport was almost exclusively co-localized with CFTR, but neurons expressing nitric oxide synthase were CFTR negative. Significant reductions of CFTR-IR (P < 0.01) and ChAT-IR (P < 0.05) were observed on myenteric ganglia of STC compared to control. Pre-treatment of colonic muscle strips with CFTR(inh)-172 (10 µM) significantly inhibited EFS-evoked contractile responses (P < 0.01) and ACh release (P < 0.05).
Conclusions
Co-localization of CFTR-IR with cholinergic markers, inhibition of EFS-induced colonic muscle contractility and ACh release by CFTR(inh)-172 suggest that CFTR modulates enteric cholinergic neurotransmission. The downregulation of CFTR and ChAT in myenteric ganglia of STC correlated with the impaired contractile responses to EFS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic fibrosis transmembrane conductance regulator (CFTR) is a protein channel that is widely expressed in human bodies. CFTR is located not only in secretory cells associated with mucus, sweat and enzyme production [1,2,3], but also monocytes, lymphocytes, erythrocytes and ganglia [4,5,6,7]. CFTR belongs to the adenosine triphosphate (ATP)-binding cassette family and is known for its involvement in transporting anion, particularly chloride ions, across cell membranes [8]. Mutations of CFTR lead to chloride ion transport impairment causing cystic fibrosis, a pathological condition characterized by the accumulation of thick and sticky mucus in both digestive and respiratory systems. Some chronic inflammatory diseases such as idiopathic peritonitis and pancreatitis have been associated with CFTR recently [9, 10]. Furthermore, some studies have suggested that CFTR mediates the release of non-neuronal acetylcholine (ACh) and ATP from bladder urothelial cells, which might have a pathophysiological significance in multiple diseases [11, 12].
The enteric nervous system (ENS) is an intrinsic nervous system that regulates motility, water and electrolyte transport, blood flow and acid secretion throughout the gastrointestinal (GI) tract. The innervated enteric ganglia and bundle of nerve cells form two major plexuses, the submucosal plexus which is predominantly found between the mucosa and underlying smooth muscle layer, and the myenteric plexus which is located between the circular and longitudinal muscle layers throughout the GI tract and plays a critical role in regulating GI motility. The GI motility reflects a series of involuntary but rhythmical smooth muscle contraction and relaxation that aids the transport of food or fecal matter in the GI tract. Smooth muscle contraction and relaxation are regulated by different neurotransmitters. In contractions, neuronal activities are mainly governed by ACh that is released from enteric neurons. The synthesis of ACh requires choline acetyltransferase (ChAT) to transfer an acetyl group from acetyl coenzyme A to choline. Synthesized ACh is then taken into vesicles through vesicular ACh transferase (VAChT) and further transported to the synapse for release. Released ACh interacts with muscarinic receptors on smooth muscles which subsequently cause smooth muscle contraction. On the other hand, relaxation is mainly governed by nitric oxide (NO), ATP and vasoactive intestinal polypeptide via different pathways. Abnormal contraction or relaxation could disturb peristalsis and other types of movement, leading to multiple intestinal motility disorders.
Slow transit constipation (STC) is a chronic gut motility disorder which is characterized by prolonged colonic transit and slow stool movement. Common symptoms include abdominal pain, bloating, obstructed defecation, constipation, headaches and fatigue [13, 14]. STC patients are mostly young to mid-aged women [15]. Although STC has no known triggers, enteric neuropathy, impaired neurotransmission and abnormal hormonal influences are possible causes that have been identified [16,17,18,19,20]. Common therapies include laxatives, sacral nerve stimulation and biofeedback. The effectiveness of these treatments is often questioned. In many cases, subtotal colectomy could be employed to achieve the best long-term outcome [21, 22].
Since CFTR has a secretory function and is highly expressed in the colonic epithelial cells throughout the GI tract [23], previous studies concerning CFTR and constipation primarily focused on investigating the mucus secretory function of mucosal CFTR in an attempt to offer patients alternative management of constipation [24,25,26]. However, no comprehensive study had been carried out to investigate the pathophysiological role of CFTR in the muscularis propria region, until a recent study which suggested that dysfunctional CFTR in myenteric ganglia might explain the presence of abdominal discomforts suffered by cystic fibrosis patients [27]. We previously reported that the contractile responses of colonic circular muscle to electric field stimulation (EFS) were significantly altered in STC. We found that the contractility of circular muscle strips from patients with STC was significantly elevated upon lower-frequency stimulation but depressed at higher frequencies, suggesting that the dysfunction of inhibitory and/or excitatory enteric neurotransmission occur in STC [18]. This could possibly be associated with abnormal CFTR expression in the myenteric ganglia.
The purpose of this study is to investigate which enteric neuronal structures express CFTR, whether CFTR mediates enteric neurotransmission and whether abnormal expression of CFTR is associated with STC. We hypothesized that CFTR modulates enteric cholinergic activities and is abnormally expressed in the colon, particularly within the myenteric ganglia of STC.
Methods
Human colon specimens
For immunohistochemistry studies, frozen human colon specimens stored in -80 °C were used. All specimens, including control and STC, were from the sigmoid colon of female patients. All STC patients met Rome criteria for functional constipation with scintigraphically confirmed slow transit constipation, defined as colonic isotope retention ≥ 20% at 96 h. They did not respond to standard therapies including laxatives, dietary modification and biofeedback, and eventually underwent surgical intervention by colectomy with ileorectal anastomosis [14]. Region-matched and as much as possible age-matched control specimens obtained from female colorectal cancer patients were used for comparison.
In functional organ bath studies, either ascending or sigmoid colonic segments were freshly collected from patients having laparoscopic resection for colorectal carcinoma. In both immunohistochemistry and functional studies, all control samples were taken from a disease-free region that was at a maximal distance (10–15 cm) away from the tumor. Patients who had previously received chemotherapy or radiotherapy were excluded from this study.
The demographical and clinical information of patients with colorectal cancer or STC whose colorectal resection specimens were used in this study is described in Table S1. This project was approved by the Human Ethics Committees of the South Eastern Sydney and Illawarra Area Health Service. Each subject signed informed consent, and the study was conducted according to the criteria set by the Declaration of Helsinki.
Immunohistochemistry
Full-thickness sigmoid colon samples from female control and STC patients were preserved in Zamboni fixative and embedded in paraffin blocks. Samples were cut into 5 µm sections by microtome and mounted onto poly-l-lysine-coated microscope slides (Histology and Microscopy Unit, UNSW). Each slide contained one STC section and one age-matched control section. After deparaffinization, antigen retrieval was carried out using EnVision FLEX Target Retrieval Solution, Low pH (K8005 Concentrate; Dako, North Sydney, NSW, Australia).
Immunofluorescence
After antigen retrieval, the tissue sections were washed with phosphate-buffered solution (PBS) and incubated overnight at room temperature with corresponding primary antibodies diluted in Tris-buffered saline (TBS) with 0.05% Triton (TBS-TX). After incubating with the primary antibody, the slides were washed 3 times with PBS followed by 1 h incubation with the corresponding fluorescent-tagged secondary antibodies diluted in TBS-TX at room temperature (see Table S2). For negative controls, primary antibody incubation was omitted, and only secondary antibodies were used to ascertain non-specific signals.
The slides were then washed with PBS and subsequently mounted with DAPI (#P-36931; Life Technologies, Mulgrave, VIC, Australia). Images were captured with an Olympus BX51TF fluorescent microscope (Olympus, Tokyo, Japan) at 10×, 20×and 40×magnifications. Images from different color channels were merged together using ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, Maryland, USA).
3,3′-Diaminobenzidine staining
Primary antibodies targeting CFTR, ChAT, S-100 and β-tubulin and corresponding secondary antibodies were applied to different slides (Table S2). After overnight incubation, the avidin–biotin complex was applied and incubated for 60 min followed by the addition of 3,3′-diaminobenzidine (DAB) substrate reagent. The sections were counter-stained by hematoxylin. The slides were digitally scanned by ScanScope XT software (Aperio, Vista, CA, USA) and photographed at a 20× magnification.
For the immunoreactive quantification analysis, each image was color deconvoluted into DAB, hematoxylin and a complimentary image using IHC Profiler plugin on ImageJ (National Institute of Health) following the method described by Varghese et al. [28]. Each myenteric ganglion on the DAB image was then selected and thresholded, and the positive immunostaining signals were quantified and expressed as a percentage of the whole area of the ganglion.
Functional studies
The freshly dissected smooth muscle of human ascending and sigmoid colon samples was trimmed into 8 × 3 mm strips. The strips were strung up through 2 circular platinum electrode plates in a circular muscle orientation in glass organ baths containing 3.5 ml of Krebs–Henseleit (Krebs) solution at 37°C and aerated with 5% carbogen gas. The strips were suspended under 1 g of tension and rested for 30 min and then supplemented with the acetylcholinesterase inhibitor neostigmine (10 μM, Sigma-Aldrich, Castle Hill, NSW, Australia) to equilibrate for a further 30 min. EFS was conducted at 80 V, 10 Hz and 1 ms duration and the contraction was recorded as the initial response. The tissue was then washed with Krebs solution containing neostigmine and rested for 30 min. Tetrodotoxin (TTX, 10 μM) and the CFTR inhibitor CFTR(inh)-172 (10 μM) were added to respective baths. TTX and CFTR(inh)-172 (both from Sigma-Aldrich) were dissolved in H2O and DMSO, respectively, and stored in − 20 °C. They were diluted to the work concentration with Krebs on the day of the experiment. The strips were applied with EFS every 15 min at 0, 1, 5, 10 and 50 Hz, and bath fluid was collected 2 min after each EFS and stored at − 80 °C for later measurement of ACh and ATP release. EFS-induced smooth muscle contractility was registered using Grass FTO3C force transducers and recorded by Polygraph 3.0 computer program (Mr E. Crawford, University of New South Wales, NSW, Australia). The contractile response was measured in gram and presented as a percentage of the initial response to 10 Hz.
The quantities of ACh and ATP released from human colonic muscle strips into the bath fluid were measured, respectively, by Amplex® Acetylcholine Assay Kit (cat# A12217; Thermo Fisher Scientific, MA, USA) and ATP Bioluminescent Assay Kit (cat# FLAA-1KT, Sigma-Aldrich).
Statistical analysis
For quantitative immunohistochemistry analysis, the percentage coverage of the highlighted myenteric plexus areas of both STC and control sections was paired and compared using GraphPad Prism Version 7.01 (GraphPad Software Inc, San Diego, CA). The data normality test was performed using Prism. If non-parametric, the data were expressed as median and interquartile range (IQR), and the differences between STC and control were analyzed by the Mann–Whitney test. Paired t test was performed to compare the matched pairs of STC and control.
For functional studies, EFS-induced contractile response and ACh and ATP release were analyzed by two-way ANOVA, followed by the Bonferroni test.
Results
Localization of CFTR in control human colon by immunofluorescence
The immunoreactivity (IR) of CFTR was detected from various structures of the human colon. In the mucosa, CFTR-IR was densely localized to surface epithelial cells and crypt goblet and columnar cells, as well as to cells in the lamina propria which appeared to be leukocytes. In these stained cells, CFTR-IR was predominately located on cell membranes and in the cytoplasm, and no staining was found within the nuclei. In the submucosa layer, CFTR-IR was detected on blood vessels and submucosal ganglia. As for the muscularis layer, CFTR-IR was densely localized at the myenteric ganglia, and weak to moderate CFTR-IR was seen on smooth muscle (Fig. 1).
Localization of CFTR immunoreactivity (IR) in control human colonic mucosa and smooth muscle. a–c, in the mucosa region, CFTR-IR (red) was localized to surface epithelial cells, crypt goblet, columnar cells and leukocytes in the lamina propria, and on submucosal ganglia (indicated by arrows). d–f, In the muscle layer, dense CFTR-IR was seen on myenteric ganglia, and weak to moderate CFTR-IR was seen on the longitudinal and circular muscle. 4′,6-Diamidino-2-phenylindole (DAPI) represents the nuclei (blue). Circular muscle (CM), crypts (C), longitudinal muscle (LM), lumen (L), muscularis mucosa (MM), myenteric ganglion (MG), submucosa (S). Scale bar = 100 µm
Co-localization of CFTR with neuronal markers
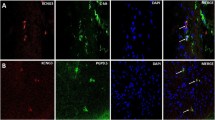
Strong HuC/D-IR, the neuronal cell body marker, was detected in most of the myenteric neurons. Approximately half of the HuC/D-expressing neurons had denser staining at nuclei compared to the cytoplasm. For the other half, HuC/D-IR was more evenly distributed within the cytoplasm and nuclei. HuC/D was co-localized with CFTR-IR in the cytoplasm of the myenteric neurons (Fig. 2a, b, c).
Double labeling of CFTR-IR with neuronal and glial cell markers in control human colonic smooth muscle. a–c Dense CFTR-IR was seen on myenteric ganglia. HuC/D-IR was localized to the cytoplasm and nuclei of myenteric neurons. The merged image indicates that CFTR-IR was co-localized with HuC/D-IR in the cytoplasm of the myenteric neurons (arrows). a–f β-Tubulin-IR was localized to the nerve fibers within the myenteric ganglia and nerve bundles running in the smooth muscle. CFTR-IR was seen to co-localize with β-tubulin on the nerve fibers within the myenteric ganglia and the smooth muscle (arrows). g–i S-100-IR was localized to the nuclei and processes of myenteric glial cells. CFTR-IR was seen to co-localize with S-100-IR at glial cell processes, especially those surrounding the membranes of myenteric neurons (arrows). Stars and triangles indicate, respectively, CFTR-positive and -negative myenteric neurons where S-100-IR was absent. j–l, GFAP-IR was localized to the processes of myenteric glial cells. CFTR was largely not co-localized with GFAP except on some of the glial cell processes indicated by arrows. Experiments performed on n = 6–11 control and STC specimens showed no observable differences in the pattern of co-localization. Images displayed are from control colonic specimens. Circular muscle (CM), longitudinal muscle (LM), myenteric ganglia (MG). DAPI nuclear marker represents the nuclei (blue). Scale bar = 50 µm
The neuronal marker, β-tubulin class III displayed the strongest immunoreactive signal in the varicose nerve fibers around the myenteric neurons. Weak to moderate β-tubulin-IR was detected within the cytoplasm of the myenteric neurons and on nerve fibers in the smooth muscle layer. β-Tubulin-IR was partially co-localized with CFTR-IR on these structures. (Fig. 2d, e, f).
The staining of glial cell marker S-100 was detected within the myenteric ganglia but primarily in the nuclei and processes of glial cells. CFTR was seen to co-localize with S-100 at glial cell processes, especially those surrounding the membranes of myenteric neurons (Fig. 2g, h, i).
The staining of glial fibrillary acidic protein (GFAP) was detected on glial cells and their processes within the myenteric plexus. Strong GFAP-IR was located at the myenteric glial cell processes, but only a minority of GFAP was co-localized with CFTR (Fig. 2j, k, l).
VAChT and neuronal nitric oxide synthase (nNOS), the representative excitatory and inhibitory motor neurons, respectively, were then examined to further define the types of neuronal cells expressing CFTR. VAChT-IR was densely presented on neurons and varicose fibers of myenteric ganglia. In addition, positive VAChT nerve fibers ran between smooth muscle bundles. The majority of VAChT were co-localized with CFTR (Fig. 3a, b, c). VAChT-IR was also seen on submucosal ganglia where it was co-localized with CFTR (data not shown). On the other hand, nNOS-positive myenteric neurons, varicose fibers and nerve trunk in the myenteric plexus showed no or very limited co-localization with CFTR (Fig. 3d, e, f).
Double labeling of CFTR-IR with VAChT-IR and nNOS-IR in control human colonic smooth muscle. CFTR (a) and VAChT (b) were seen on neurons and nerve fibers of myenteric plexus as well as nerve bundles running between smooth muscle fibers, where they were largely co-localized as shown in yellow or orange color (c). CFTR (red) was densely present on the nerve fibers and the cytoplasm of neurons within the myenteric ganglia (d) whereas nNOS was expressed on some of the neurons, varicose nerve fibers and nerve trunks of the myenteric plexus (e). There was virtually no co-localization between CFTR with nNOS in all structures. Arrows indicate that CFTR-positive neurons are free of nNOS-IR. Triangles indicate that nNOS-positive neurons are free of CFTR-IR (f). Circular muscle (CM), longitudinal muscle (LM), myenteric ganglia (MG). DAPI nuclear marker represents the nuclei (blue). Scale bar = 50 µm
Changes within the myenteric ganglia
The myenteric ganglia of colon specimens from STC patients showed weaker CFTR-IR compared to their paired control specimens (Fig. 4a). The median value of the percentage area of CFTR-IR from individual myenteric ganglion in STC tissues was 27% (IQR 15–41%, 113 ganglia from 15 STC specimens), which was significantly lower than that of control (median 58%, IQR 41–71%, 134 ganglia from 15 control specimens, P < 0.0001, Mann–Whitney test, Fig. 4b). When the CFTR-IR of individual myenteric ganglion from the same specimen was combined and averaged, a reduced CFTR-IR was seen in 13 of the 15 STC specimens examined compared to their matched control specimens (P < 0.01, a paired analysis by Wilcoxon test, Fig. 4c). CFTR-IR was not correlated with age in either control or STC specimens (the Pearson correlation coefficient r2 values were 0.069 for control and 0.139 for STC, both P > 0.05, n = 14).
Comparison of the expression of CFTR-IR within the myenteric ganglia between control and STC. a Immunoreactivity of CFTR (brown) in myenteric ganglia (MG) of two control colon specimens (a, c) and two STC colon specimens (b, d). Weaker CFTR-IR was seen in STC compared to control. Scale bar = 25 µm. b Scatter plot showing percentage area of immunostaining of CFTR in individual myenteric ganglion of human colon smooth muscle from 124 ganglia of 14 control patients and 107 ganglia of 14 STC patients. Bars show the median values. ****P < 0.0001, Mann–Whitney test. c Scatter plot showing the averaged percentage area of CFTR-IR within the same specimens from control colon (n = 14) compared with age-matched STC colon (n = 14). The solid line indicates the paired specimens on the same slide. **P < 0.01 by paired t test
Likewise, the myenteric ganglia of STC showed weaker ChAT staining compared to the control (Fig. 5a). The median value of the percentage area of ChAT-IR of individual myenteric ganglion in STC tissues was 12% (IQR 5.5–22%, 57 ganglia of 6 STC specimens), which was significantly lower than the control (median 23, IQR 8.3–32%, 52 ganglia of 6 control specimens, P < 0.01, Fig. 5b). A significant reduction in the average percentage area of ChAT-IR was also observed in the paired study (P < 0.05, Fig. 5c).
Comparison of the expression of ChAT-IR within the myenteric ganglia between control and STC. a Immunoreactivity of CHAT (brown) in myenteric ganglia (MG) of two control colon specimens (a, c) and two STC colon specimens (b, d). Weaker ChAT-IR was seen in STC compared to control. Scale bar = 25 µm. b Scatter plot showing percentage area of immunostaining of ChAT in individual myenteric ganglion of human colon smooth muscle from 52 ganglia of six control patients and 57 ganglia of 6 STC patients. Bars show the median values. **P < 0.01, Mann–Whitney test. c Scatter plot showing the averaged percentage area of ChAT-IR within the same specimens from control colon (n = 6) compared with age-matched STC colon (n = 6). The solid line indicates the paired specimens on the same slide. *P < 0.05 by paired t test
Dense β-tubulin-IR was localized primarily to myenteric ganglia (Fig. 6a). Compared to the control, STC colon tissues displayed a significant elevation of β-tubulin-IR in both individual myenteric ganglion (P < 0.0001, Fig. 6b) and data within the same specimens averaged (P < 0.05, Fig. 6c). S-100-IR in myenteric ganglia (Fig. 6 d) showed no significant difference in the percentage area of staining in either individual or averaged data from STC tissues compared to control (Fig. 6e, f). The mean number of HuC/D-positive myenteric neurons per ganglion in STC tissues was 8 ± 0.78 (n = 14), which had no significant difference compared to the control (mean 7 ± 0.57, n = 11).
Comparison of the expression of β-tubulin and S-100 within the myenteric ganglia between control and STC. a Immunoreactivity of β-tubulin (brown) in myenteric ganglia (MG) of one control colon specimen (a) and one STC colon specimen (b). Denser β-tubulin-IR was seen in STC compared to control. Scale bar = 25 µm. b Scatter plot showing percentage area of immunostaining of β-tubulin in individual myenteric ganglion of human colon smooth muscle from 60 ganglia of six control patients and 57 ganglia of 6 STC patients. Bars show the median values. ***P < 0.001, Mann–Whitney test. c Scatter plot showing the averaged percentage area of β-tubulin-IR within the same specimens from control colon (n = 6) compared with age-matched STC colon (n = 6). The solid line indicates the paired specimens on the same slide. *P < 0.05 by paired t test. d Immunoreactivity of S-100 (brown) in myenteric ganglia of one control colon specimen (a) and one STC colon specimen (b). Control and STC showed similar S-100-IR. Scale bar = 25 µm. e Scatter plot showing no significant changes of percentage area of immunostaining of S-100 in individual myenteric ganglion of human colon smooth muscle from 38 ganglia of 6 control patients and 52 ganglia of 6 STC patients. Bars show the median values. f Scatter plot showing no significant changes in the averaged percentage area of S-100-IR within the same specimens from control colon (n = 6) compared with age-matched STC colon (n = 6). The solid line indicates the paired specimens on the same slide
CFTR-mediated contractile response and ACh and ATP release
The contractility of smooth muscle strips from either ascending or sigmoid colon of control specimens in response to EFS was increased in a frequency-dependent manner (Fig. 7a). It is interesting to note that in the strips obtained from seven patients that were treated with CFTR(inh)-172, four of them showed spontaneous contractions during the equilibrium period, and these spontaneous activities were inhibited or abolished by the addition of CFTR(inh)-172 (10 μM). Furthermore, in the presence of CFTR(inh)-172 (10 μM), the contractile responses induced by EFS were diminished (Fig. 7B), and significant reductions were recorded at 10 Hz (P < 0.05) and 50 Hz (P < 0.01, n = 7) stimulation compared to the control (Fig. 7c). In the presence of tetrodotoxin (TTX, 10 μM), a significant reduction of contractility was found at all frequencies (P < 0.01–P < 0.0001, n = 7) except 1 Hz. The results with the addition of both TTX and CFTR(inh)-172 did not display any further reduction (data not shown).
EFS-induced contraction of human colonic smooth muscle strips and ACh and ATP release. a, b, Representative contractile traces of EFS-induced colonic contraction of human colonic smooth muscle strips in the absence and presence of CFTR inhibitor, CFTR(inh)-172. CFTR(inh)-172 was added 30 min before the EFS (indicated by the arrow). All experiments were carried out in the presence of cholinesterase inhibitor neostigmine (10 μM). c EFS-induced frequency-dependent contractile responses which were significantly inhibited by CFTR(inh)-172 and TTX. d In parallel, EFS-induced frequency-dependent ACh release was inhibited by both CFTR(inh)-172 and TTX. E, EFS-induced ATP release was not affected by the presence of CFTR(inh)-172 and TTX. Data are expressed as the percentage of the initial value of 10 Hz stimulation (n = 5–7). *P < 0.05, **P < 0.01, ****P < 0.0001. Two-way ANOVA followed by Bonferroni test
The amount of ACh released from human colonic muscle strips was also measured. The basal level of ACh was 79 \(\pm\) 39 nmol/g strip weight (n = 7). The two-way ANOVA analysis showed that both CFTR(inh)-172 (P < 0.05) and TTX (P < 0.01) significantly inhibited ACh release. Although there were trend reductions at 5 and 10 Hz stimulations, the Bonferroni post-test showed significant reductions only at 50 Hz for both CFTR(inh)-172 (P < 0.05) and TTX (P < 0.01) (Fig. 7d). The basal ATP release was 0.19 \(\pm\) 0.025 (nmol/g strip weight). There were no differences for EFS-induced ATP among the three groups (Fig. 7e).
Discussion
CFTR has long been seen as an epithelial ion channel that regulates the transport of various ions across the epithelium. To our knowledge, our study is the first to report the functional role of CFTR in mediating neuronal ACh release and its pathophysiological significance in STC. The present study provides strong evidence that CFTR is localized on various structures in the myenteric ganglia and plays a critical role in regulating neurotransmission and colonic smooth muscle contractility. STC is an idiopathic disease that has no known causes. Our study provides a novel finding that there is a change of composition within the myenteric ganglia in STC which may explain why a prolonged colonic transit time is observed in STC. Overall, the present study has largely extended the understanding of CFTR and its pathophysiological association with STC.
The immunofluorescence result agrees with the previous finding that CFTR is present in the colon and predominately located at the mucosa, particularly on epithelial cells of the colon crypts [23, 29, 30]. Dense CFTR-IR seen on lymphocytes in the lamina propria, submucosal blood vessels, enteric ganglia and nearby nerve fibers suggests the involvement of CFTR in multiple cellular activities such as inflammation, mucus production and gut motility. It is, therefore, possible that several colonic diseases that are characterized by inflammation, abnormal mucus production and gut motility may be associated with abnormal CFTR functions.
The co-localization of CFTR with HuC/D and β-tubulin suggests that CFTR may play a role in mediating nerve signals. The fact of VAChT-IR being largely if not entirely co-localized with CFTR in the myenteric plexus indicates a possible role of CFTR in regulating cholinergic neuron activities. This was confirmed by our functional study showing that EFS-induced contractile responses and ACh release from colonic muscle strips were reduced in the presence of CFTR(inh)-172, an inhibitor of CFTR transport activities [31]. Furthermore, CFTR(inh)-172 also inhibited or abolished spontaneous contractions of the muscle. Our previously published study has shown that EFS-evoked human colonic muscle contraction is TTX- and atropine-dependent, suggesting that the effect is primarily via the release of ACh from myenteric neurons [18]. The spontaneous muscle contractions under the rest condition were also likely to be caused by the release of Ach, since the contractions were enhanced by the addition of acetylcholinesterase inhibitor neostigmine.
Although the mechanisms of action of CFTR in regulating gut motility remained unclear, the mechanism could involve Ca2+ channels. It is well accepted that the cAMP and protein kinase A signaling pathway is the primary controlling mechanism for CFTR channel function. However, there is growing evidence that CFTR also regulates Ca2+ influx and contributes to cholinergic and purinergic receptor activities in tissues, CFTR-expressing human bronchial epithelial cell line and baby hamster kidney cells co-expressing CFTR and muscarinic M3 receptors [32,33,34]. Interestingly, a study by Thiagarajah et al. has shown that CFTR(inh)-172 inhibited pilocarpine-stimulated submucosal gland fluid secretion in normal pig and human airways [35]. Taken together, these studies have pointed to the close relationship between CFTR and cholinergic activities. We, therefore, postulate that the effect of CFTR on modulating ACh-mediated colonic contractility may involve the action of CFTR on regulating Ca2+ influx (see Figure S1). The addition of CFTR(inh)-172 blocks the function of CFTR on smooth muscle cells which could pose an inhibitory effect on Ca2+ influx, therefore leading to decreased contractility. Furthermore, the addition of CFTR(inh)-172 inhibits the function of CFTR on enteric neurons, resulting in a diminished Ca2+ influx into the nerve end, thereby a reduction of ACh release. Since the M3 receptor coupled diacylglycerol (DAG) and 1,4,5-trisphosphate (IP3) signaling pathways activate CFTR [34], with less ACh being released, the activities of CFTR on the smooth muscle would be subsequently attenuated. This is in line with the previous findings which showed that CFTR inhibition significantly decreased Ca2+ influx via the transient receptor potential cation channels [32, 36].
CFTR-IR was more abundant than ChAT-IR and VChAT-IR within the myenteric ganglia, and thus it cannot be ruled out that CFTR plays other functional roles in the GI tract in addition to modulating ACh release and contractility. In contrast, CFTR-IR was barely co-localized with nNOS, suggesting a minimal effect of CFTR on regulating NO release and possibly NO-mediated descending relaxation. Overall, the co-localization assay suggests a more important role of CFTR in regulating colonic contraction than relaxation.
CFTR is a member of the ATP-binding cassette superfamily that mediates ATP efflux. However, we have shown that EFS-induced ATP release from human colonic muscle strips remained unchanged compared to the basal level, and inhibition of CFTR by CFTR(inh)-172 had no influence on ATP release. Our result was consistent with a previous study which showed that CFTR(inh)-172 failed to suppress bradykinin-inducible export of ATP from cultured guinea pig taenia coli smooth muscle cells [37] On the other hand, although it was reported that CFTR channel blockers gadolinium chloride and 4,4′-diisothiocyanatostilbene-2,2′disulfonic acid attenuated the basal and hypotonic stress-induced ATP release from CFTR-expressing human bronchial epithelial cells, the action appeared to be mediated by a CFTR-dependent ATP-releasing channel rather than directly by CFTR [38].
Although the increase in the ratio of glial cells to myenteric neurons in the rectosigmoid colon of STC has been reported [39], our result was in line with a previous study showing no changes in S-100-IR in the sigmoid colon of STC compared to control [40]. A recent study has shown that glial cells may play a role in regulating gut motility by responding to neurotransmitters, primarily ATP and ADP [41]. The marginal co-localization of CFTR with GFAP-positive glial cells suggests that these cells are unlikely to influence gut motility. However, the observation that CFTR was co-localized with S-100 has pointed to the significance of CFTR to the S-100-positive glial cells. Previous research has identified four morphologically distinct subpopulations of glial cells in the ENS of mice. Type-I, Type-II and Type-III of the enteric glia were found to express GFAP, S-100 and Sox10 differently [42]. This is consistent with our present study, which shows differential expression of glial markers on the myenteric ganglia. Type-I glial cells co-express GFAP and S-100, while the majority of Type-II and Type-III glial cells are GFAP negative and S-100 positive [42], CFTR is likely to be present on Type-II and Type-III glial cells in the human enteric nervous system. Although the precise function of enteric glial cells in the GI tract remains elusive, studies in recent years have highlighted the role of enteric glia in regulating gut motility, synaptic transmission, mucosal secretion and absorption and host defense against pathogens [41,42,43,44,45,46]. Under certain circumstances, enteric glial cells can even become enteric neurons [45].
One of the interesting and novel findings of our study is CFTR and ChAT proteins are significantly downregulated within the myenteric ganglia in STC, which was consistent with the previous finding that STC has impaired colonic motor activities upon cholinergic stimulation [47]. It further agrees with our results of the co-localization assay and the functional study suggesting that CFTR plays a role in regulating ACh release and ACh-mediated colonic muscle contraction. We have previously reported abnormal contractile responses to EFS in the colon smooth muscle of STP patients, showing that EFS-evoked contractions were unrelated to stimulation frequency: the contractile amplitudes were higher to lower frequency stimulations (0.5–5 Hz), but lower to higher frequency stimulations (10–40 Hz) in STC compared to control [18]. Our current and previous findings have revealed an association between abnormal CFTR expression/cholinergic activities and dysmotility of STC. Such a downregulation may not be due to a loss of myenteric neurons as reported in previous findings [39, 48], since our study has shown that the number of HuC/D-positive neurons per myenteric ganglion in STC has no significant changes compared to control. In fact, a slight but significant upregulation of β-tubulin was observed, which indicates an increase of nerve fibers within the myenteric plexus. Whether the upregulation of β-tubulin was a compensatory consequence of the downregulation of CFTR and ChAT is unknown and is outside the scope of this study.
It is essential to point out that the reduction in ACh synthesis and CFTR downregulation may have some causal relationship, but this has yet been investigated in the present study. Overall, the change of composition within the myenteric ganglia suggests a pathophysiological role of CFTR in idiopathic STC. Future investigations are necessary to reveal the causes of the changes and whether there are changes of other components within the myenteric ganglia in STC.
GI motility disorder has been associated with cystic fibrosis previously. A significant reduction of gut motility as characterized by prolonged gastric emptying and transit has been reported in cystic fibrosis mice [49]. However, the disturbed motility in cystic fibrosis was believed to be secondary to the inflammation induced by impaired mucus clearance [50,51,52,53]. Our study shows that disturbed motility may be more related to abnormal CFTR expression within the myenteric ganglia rather than to inflammation, at least in non-inflammatory STC. CFTR is conventionally viewed as predominantly affecting the mucosa owing to its secretory functions, but the result of this study extends this view by suggesting its pathophysiological role in deeper smooth muscle layers. In a chronically constipated mouse strain (C3H/HeJ) with impaired interactions between gut microbiota and enteric neurons, CFTRact-J027, a CFTR activator, when orally given, significantly increased pellet number, stool weight and water content to the level of its wild-type control [54]. Therefore, the benefits of CFTR as a target for the treatment of STC may be twofold, improving both motility and secretion.
Conclusions
In conclusion, the co-localization of CFTR with cholinergic markers, the inhibition of EFS-induced colonic muscle contractility and ACh release by CFTR(inh)-172, as well as the downregulation of CFTR within the myenteric ganglia in STC have not been reported previously. These novel findings have greatly extended the current understanding of the functional role of CFTR in regulating the neuronal release of ACh in neurotransmission and its pathophysiological association with STC. Future studies are essential to investigate the causal relationship and the mechanism of CFTR in mediating neuronal activities in the GI tract.
References
Gustafsson JK, Ermund A, Ambort D, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–72.
Char JE, Wolfe MH, Cho HJ, et al. A little CFTR goes a long way: CFTR-dependent sweat secretion from G551D and R117H–5T cystic fibrosis subjects taking ivacaftor. PLoS ONE. 2014;9:e88564.
Oca F, Dreux S, Gerard B, et al. Amniotic Fluid Digestive Enzyme Analysis Is Useful for Identifying CFTR Gene Mutations of Unclear Significance. Clin Chem. 2009;55:2214–7.
Sorio C, Buffelli M, Angiari C, et al. Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS ONE. 2011;6:e22212.
Johansson J, Vezzalini M, Verze G, et al. Detection of CFTR protein in human leukocytes by flow cytometry. Cytometry A. 2014;85:611–20.
Verloo P, Kocken CH, Van der Wel A, et al. Plasmodium falciparum-activated chloride channels are defective in erythrocytes from cystic fibrosis patients. J Biol Chem. 2004;279:10316–22.
Su M, Guo Y, Zhao Y, et al. Expression of cystic fibrosis transmembrane conductance regulator in paracervical ganglia this paper is one of a selection of papers published in this special issue entitled “Second International Symposium on Recent Advances in Basic, Clinical, and Social Medicine” and has undergone the journal's usual peer review process. Biochem Cell Biol. 2010;88:747–55.
Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73.
Dalli J, Rosignoli G, Hayhoe RP, et al. CFTR inhibition provokes an inflammatory response associated with an imbalance of the annexin A1 pathway. Am J Pathol. 2010;177:176–86.
Malats N, Casals T, Porta M, et al. Cystic fibrosis transmembrane regulator (CFTR) ΔF508 mutation and 5T allele in patients with chronic pancreatitis and exocrine pancreatic cancer. Gut. 2001;48:70–4.
McLatchie LM, Young JS, Fry CH. Regulation of ACH release from guinea pig bladder urothelial cells: potential role in bladder filling sensations. Br J Pharmacol. 2014;171:3394–403.
Ochodnicky P, Michel MB, Butter JJ, et al. Bradykinin modulates spontaneous nerve growth factor production and stretch-induced ATP release in human urothelium. Pharmacol Res. 2013;70:147–54.
Lubowski DZ, Chen FC, Kennedy ML, et al. Results of colectomy for severe slow transit constipation. Dis Colon Rectum. 1996;39:23–9.
Wong SW, Lubowski DZ. Slow-transit constipation: evaluation and treatment. ANZ J Surg. 2007;77:320–8.
Preston DM, Lennardjones JE. Severe chronic constipation of young-women - idiopathic slow transit constipation. Gut. 1986;27:41–8.
Bassotti G, Villanacci V, Maurer CA, et al. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41–6.
Tomita R, Tanjoh K, Fujisaki S, et al. Regulation of the enteric nervous system in the colon of patients with slow transit constipation. Hepatogastroenterology. 2002;49:1540–4.
Liu L, Shang F, Morgan MJ, et al. Cyclooxygenase-dependent alterations in substance P-mediated contractility and tachykinin NK1 receptor expression in the colonic circular muscle of patients with slow transit constipation. J Pharmacol Exp Ther. 2009;329:282–9.
Jung HK, Kim DY, Moon IH. Effects of gender and menstrual cycle on colonic transit time in healthy subjects. Korean J Intern Med. 2003;18:181.
Björnsson ES, Chey WD, Hooper F, et al. Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT3 pathways. Am J Physiol Gastrointest Liver Physiol. 2002;283:G400–G407407.
Frattini JC, Nogueras JJ. Slow transit constipation: a review of a colonic functional disorder. Clin Colon Rectal Surg. 2008;21:146–52.
Wong SW, Lubowski DZJ. Slow‐transit constipation: evaluation and treatment. ANZ J Surg. 2007;77:320–8.
Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994;93:347–54.
Beyder A, Farrugia G. Ion channelopathies in functional GI disorders. Am J Physiol Gastrointest Liver Physiol. 2016;311:G581–G586586.
Cil O, Phuan PW, Lee S, et al. CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell Mol Gastroenterol Hepatol. 2016;2:317–27.
Sharma S, Sharma T, Dhingra R, et al. Linaclotide-a novel secretagogue in the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. Mini Rev Med Chem. 2013;13:1685–90.
Xue R, Gu H, Qiu Y, et al. Expression of cystic fibrosis transmembrane conductance regulator in ganglia of human gastrointestinal tract. Sci Rep. 2016;6:30926.
Varghese F, Bukhari AB, Malhotra R, et al. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE. 2014;9:e96801.
Dhooghe B, Noel S, Bouzin C, et al. Correction of chloride transport and mislocalization of CFTR protein by vardenafil in the gastrointestinal tract of cystic fibrosis mice. PLoS ONE. 2013;8:e77314.
Doucet L, Mendes F, Montier T, et al. Applicability of different antibodies for the immunohistochemical localization of CFTR in respiratory and intestinal tissues of human and murine origin. J Histochem Cytochem. 2003;51:1191–9.
Valdivieso AG, Marin MC, Clauzure M, et al. Measurement of cystic fibrosis transmembrane conductance regulator activity using fluorescence spectrophotometry. Anal Biochem. 2011;418:231–7.
Walsh DE, Harvey BJ, Urbach V. CFTR regulation of intracellular calcium in normal and cystic fibrosis human airway epithelia. J Membr Biol. 2000;177:209–19.
Billet A, Luo Y, Balghi H, et al. Role of tyrosine phosphorylation in the muscarinic activation of the cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem. 2013;288:21815–233.
Billet A, Hanrahan JW. The secret life of CFTR as a calcium-activated chloride channel. J Physiol. 2013;591:5273–8.
Thiagarajah JR, Song Y, Haggie PM, et al. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J. 2004;18:875–7.
Leveque M, Penna A, Le Trionnaire S, et al. Phagocytosis depends on TRPV2-mediated calcium influx and requires TRPV2 in lipids rafts: alteration in macrophages from patients with cystic fibrosis. Sci Rep-Uk. 2018;8:4310.
Zhao YM, Migita K, Sun J, et al. MRP transporters as membrane machinery in the bradykinin-inducible export of ATP. N-S Arch Pharmacol. 2010;381:315–20.
Braunstein GM, Roman RM, Clancy JP, et al. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem. 2001;276:6621–30.
Wedel T, Roblick UJ, Ott V, et al. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum. 2002;45:54–62.
Park HJ, Kamm MA, Abbasi AM, et al. Immunohistochemical study of the colonic muscle and innervation in idiopathic chronic constipation. Dis Colon Rectum. 1995;38:509–13.
Broadhead MJ, Bayguinov PO, Okamoto T, et al. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol. 2012;590:335–50.
Boesmans W, Lasrado R, Vanden Berghe P, et al. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. GLIA. 2015;63:229–41.
MacEachern SJ, Patel BA, McKay DM, et al. Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J Physiol. 2011;589:3333–48.
De Giorgio R, Giancola F, Boschetti E, et al. Enteric glia and neuroprotection: basic and clinical aspects. Am J Physiol Gastrointest Liver Physiol. 2012;303:G887–G89393.
Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–32.
Neunlist M, Van Landeghem L, Mahé MM, et al. The digestive neuronal–glial–epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:90–100.
Bassotti G, Chiarioni G, Imbimbo BP, et al. Impaired colonic motor response to cholinergic stimulation in patients with severe chronic idiopathic (slow transit type) constipation. Dig Dis Sci. 1993;38:1040–5.
Wedel T, Spiegler J, Soellner S, et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–67.
De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G104–G111111.
Lynch SV, Goldfarb KC, Wild YK, et al. Cystic fibrosis transmembrane conductance regulator knockout mice exhibit aberrant gastrointestinal microbiota. Gut Microbes. 2013;4:41–7.
De Lisle RC, Meldi L, Flynn M, et al. Altered eicosanoid metabolism in the cystic fibrosis mouse small intestine. J Pediatr Gastroenterol Nutr. 2008;47:406–16.
De Lisle RC, Sewell R, Meldi L. Enteric circular muscle dysfunction in the cystic fibrosis mouse small intestine. Neurogastroenterol Motil. 2010;22:341–e87.
De Lisle RC, Borowitz D. The cystic fibrosis intestine. Cold Spring Harb Perspect Med. 2013;3:a009753.
Cil O, Phuan PW, Son JH, et al. Phenylquinoxalinone CFTR activator as potential prosecretory therapy for constipation. Transl Res. 2017;182(14–26):e4.
Acknowledgments
This study was supported by a project Grant from the National Health and Medical Research Council of Australia (APP1048885), and by the UNSW and Shanghai Jiao Tong University Collaborative Seed Grant. We would like to offer our special thanks to Stelina Drimousis and Erica Diezmos in specimen collection and preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yeh, K.M., Johansson, O., Le, H. et al. Cystic fibrosis transmembrane conductance regulator modulates enteric cholinergic activities and is abnormally expressed in the enteric ganglia of patients with slow transit constipation. J Gastroenterol 54, 994–1006 (2019). https://doi.org/10.1007/s00535-019-01610-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01610-9