Abstract
Background
A novel morphological classification using resected specimens predicted malignant potential and prognosis in patients with pancreatic neuroendocrine tumors (P-NETs). The aim of this study was to examine the predictive ability of morphological diagnoses made using non-invasive multi-detector computed tomography (MDCT) in P-NETs.
Methods
Between 2002 and 2015, 154 patients were diagnosed with P-NETs at the Tokyo Medical and Dental University, and 82 patients who underwent surgical treatment were enrolled. The primary tumors were classified by MDCT into three types: Type I, simple nodular tumor; Type II, simple nodular tumor with extra-nodular growth; and Type III, confluent multinodular tumor. Patients were stratified by 15 clinical specialists according to classification and without any other clinical or pathological information. Clinicopathological features and patient survival were reviewed retrospectively.
Results
The mean observation time was 1004 days. Forty-six, 22, and 14 patients had Type I, II, and III tumors, respectively. Morphological classification was significantly correlated with advanced features such as tumor size, Ki-67 index, and synchronous liver metastasis (p < 0.001 for all). There were significant differences between all three tumor types as judged by ENETS TNM classification (p < 0.001), AJCC TNM classification (p = 0.046), WHO 2004 classification (p < 0.001), and WHO 2010 classification (p < 0.001). Five-year progression-free survival (PFS) rates for patients with Type I, II, and III tumors were 97, 43, and 31%, respectively (I vs. II, p < 0.001; I vs. III, p < 0.001; II vs. III, p = 0.017). Multivariate analysis revealed Type II/III tumors and synchronous liver metastasis to be independent risk factors for poor PFS.
Conclusion
A novel simple morphological classification system would predict Type II and III tumors that may have higher malignant potential than Type I tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumors (P-NETs) [1] are rare; however, P-NET diagnoses have remarkably increased in recent years, increasing 4.8-fold from 1997 (1.09/100,000) to 2004 (5.25/100,000) in the United States and 1.2-fold from 2005 (2.23/100,000) to 2010 (2.69/100,000) in Japan [2, 3]. The increase seems to be due in part to advanced diagnostic technology [4].
The P-NETs classification has changed substantially in the last decade [5, 6]. In 2010, the World Health Organization (WHO) classified P-NETs into classes G1, G2, and neuroendocrine carcinoma (NEC), based on mitotic count and/or Ki-67 index [7]. This pathological classification may define P-NETs by their proliferative ability. However, it remains unclear whether a single proliferative feature can properly evaluate malignant potential, including the ability to metastasize to distant organs.
To examine the clinical significance of morphological appearance in P-NETs, we previously established a pathological classification and identified an important link between macroscopic morphology and malignant potential [8]. Resected primary P-NETs can have a pathological classification of simple nodular type (Type I), simple nodular type with extra-nodular growth (Type II), or confluent multinodular type (Type III). In previous studies, patients with simple nodular tumors had significantly better survival than patients with tumors of the other macroscopic types [8]. There is no other report describing the link between morphological appearance and prognosis of patients with P-NETs. However, many previous studies have determined that macroscopic morphology is one of the best prognostic factors in other tumor types, e.g., hepatocellular carcinoma [9,10,11,12], and that morphological classification is an important determinant of a malignant gene signature [13].
Recent studies have found that computed tomography (CT) plays an important role in early diagnosis by detecting small tumors, thus allowing for more precise staging of P-NETs [14]. Several studies have reported on the relevance of preoperative CT findings in predicting P-NET characteristics and prognoses [15,16,17]. However, there have been no studies evaluating the morphological appearance of P-NETs using CT.
In this study, we examined whether preoperative morphological diagnoses made using multi-detector computed tomography (MDCT) could predict long-term prognoses of patients with P-NETs. We stratified the patients by morphological type prior to surgical treatment and compared the clinicopathological features between the groups. This study identifies a simple and non-invasive morphological classification system that can be used to predict the malignant potential of P-NETs.
Methods
Patients and methods
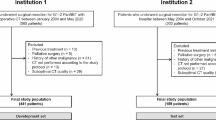
Between 2002 and 2015, 154 patients with P-NETs received treatment at the Tokyo Medical and Dental University. Of these, 108 underwent surgical treatment. Eighty-two patients who underwent arterial, portal, and equilibrium phase imaging by MDCT before surgical treatment were enrolled in this study (Fig. 1). Written informed consent was obtained from each subject, and all study procedures were approved by an institutional review board (The Human Research Ethics Committee, Tokyo Medical and Dental University ID:1080).
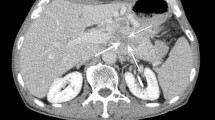
We established a novel simple morphological classification system for P-NETs as shown in Fig. 2. The shape of the primary tumor was classified by CT imaging into 3 types: Type I, simple nodular (the round shape with clear demarcation); Type II, simple nodular with extra-nodular growth (a tumor similar to Type I, with extra-nodular growth); and Type III, confluent multinodular (a tumor formed by a cluster of small and contiguous nodules). The largest area of the lesion was evaluated, and 15 clinical specialists (1 radiologist and 14 pancreatic surgeons) independently determined the morphological type without any other clinical or pathological information. The majority decision was taken as the final diagnosis. The 15 specialists had to decide only one morphological type and the majority opinion was selected as the final diagnosis. In 81 patients, more than half of the specialists (=8 specialists) were in agreement. With 1 patient, 6, 5, and 4 specialists selected Type I, II and III, respectively. The case turned out to be “Type I”.
The morphological classification system for pancreatic neuroendocrine tumors. Type I, simple nodular tumor: the round shape with clear demarcation; Type II, simple nodular tumor with extra-nodular growth (similar to Type I but showing extra-nodular growth); Type III, contiguous multinodular tumor, formed by a cluster of small and contiguous nodules
Patient background characteristics and other pathological findings were examined. Background characteristics included age, gender, genetic syndrome such as multiple endocrine neoplasia Type I, tumor functionality, tumor location, synchronous lymph node and liver metastases, and surgical procedure. Pathological findings included tumor size, ENETS classification, AJCC classification, and immunohistochemical findings such as Ki-67 index and hormone production.
According to the WHO 2010 Classification of Tumors of the Digestive System, P-NETs can be classified into three grades on the basis of mitotic count and Ki-67 proliferative index: G1, mitotic count of <2 per 10 high-power fields (HPF) or <3% Ki-67 index; G2, mitotic count of 2–20/10 HPF or 3–20% Ki-67 index; and NEC: mitotic count of >20/10 HPF or >20% Ki-67 index. The higher grade was assigned, per WHO recommendation, if there was a discrepancy between Ki-67 index and mitotic count [7]. We quantified the Ki-67 proliferative index and mitotic count by counting at least 500 cells in “hot spots.”
All patients were followed up regularly with laboratory tests and MDCT with a bolus injection of contrast medium at least every 3–6 months. Evaluation of progression/relapse was performed by at least two radiologists. Progression-free survival (PFS) was defined as the time from the surgical treatment to the date of the first observation of progression/relapse or death due to any cause. Information on outcomes more than 5 years after surgery was collected by personal interview, if patients had been observed in other hospitals. Total survival was examined in July 2015.
Statistical analysis
Statistical comparisons for significance of clinicopathological features were performed using Chi-square test or Fisher’s exact test with a single degree of freedom. Continuous data are expressed as the median (range). Continuous variables were compared among the groups using the Kruskal–Wallis test, and categorical variables were analyzed by Student’s t test. Survival curves were illustrated using the Kaplan–Meier method and compared with log-rank tests. Significant variables were subjected to univariate analysis using a Cox proportional hazards model. p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 23.0 (SPSS, Chicago, IL).
Results
The mean observation time was 1004 days. Of 82 patients, 46, 22, and 14 had Type I, II, and III tumors, respectively. The clinicopathological features of the three groups are listed in Table 1. Five patients had a genetic syndrome, such as multiple endocrine neoplasia Type I or von Hippel-Lindau disease. Functional tumors were found in 16 patients. Hyperglycemia, ulcer of the upper gastro-intestinal tract, and hypoglycemia that diminished after pancreatectomy were observed in 1, 2, and 10 patients, respectively. On pathological examination, gastrin-producing tumors, insulin-producing tumors, and glucagon-producing tumors were identified in 7, 16, and 27 patients, respectively. Twenty-nine patients had tumors in the pancreatic head, and 53 in the pancreatic body/tail. There were no significant differences among the three groups in age, gender, genetic syndrome, tumor functionality, or tumor location (head vs. body/tail). Morphological classification (Type I vs. II vs. III) was significantly correlated with the features of advanced P-NETs, in particular, large tumor size, high Ki-67 index, synchronous lymph node metastasis, and synchronous liver metastasis (p < 0.001 for all). Curative operation (R0 vs. R2) and surgical procedure (enucleation, pancreatoduodenectomy, distal pancreatectomy, total pancreatoduodenectomy, and exploratory laparotomy) were significantly different between each classification (p < 0.001 for all).
Table 2 shows the relationship between morphological classification and tumor malignancy using various staging systems. The morphological classification (Type I, II, III) was significantly correlated with advanced grade and/or stage as judged by ENETS TNM classification (p < 0.001), AJCC TNM classification (p = 0.046), WHO 2004 classification (p < 0.001), and WHO 2010 classification (p < 0.001).
Figure 3 shows the 5-year PFS rates after surgical treatment of patients with Type I, II, and III tumors (97, 43, and 31%, respectively). The differences between the three types were significant (Type I vs. II, p < 0.001; Type I vs. III, p < 0.001; Type II vs. III, p = 0.017).
Table 3 summarizes the results of univariate analysis of various risk factors for PFS. Large tumor size (p = 0.001), synchronous liver metastasis (p < 0.001), high Ki-67 index (p < 0.001), non-curative resection (p < 0.001), and Type II/III (vs. Type I, p = 0.001) were significant risk factors in univariate analyses, though the early strong enhancement, cystic change, and calcification did not increase the risk of tumor progression. Multivariate analysis revealed that synchronous liver metastasis [hazard ratio (HR), 4.49; 95% confidence interval (CI), 1.63–12.38; p = 0.004] and Type II/III tumors (HR, 18.95; 95% CI, 2.40–149.7; p = 0.005) were independent risk factors for poor PFS. Moreover, these risk factors were also the independent factors in patients with non-functional tumors (supplemental Table 1).
As shown in the supplemental figure, the 14 Type III patients had relatively poor overall survival in the 46 patients with no surgical treatment. These patients included 11 unresectable liver metastases, 1 locally advanced unresectable case, 1 advanced tumor thrombus into major portal veins, and 1 observed case. On the other hand, the 21 Type I patients had relatively better overall survival. All Type I patients were observed because they might be estimated as low malignant P-NETs. In 11 Type II patients, 5 were observed, 1 was unresectable for locally advanced tumor, and 5 were unresectable for advanced liver metastases.
Discussion
Despite substantial progress over the past few years, predicting prognosis in P-NETs has been a major problem, owing to their low prevalence. We previously reported a novel pathological classification system for P-NETs based on morphology and identified an important link between macroscopic morphology and malignant potential. The current series involved 82 patients with P-NETs treated at a single center.
To our knowledge, this is the first report to classify macroscopic morphological type as judged by MDCT in patients with P-NETs. As shown in Table 1, the non-simple nodular type P-NETs had synchronous lymph node and liver metastases, micro-invasion of adjacent organs, microvascular invasion, and neural invasion, which we did not observe in the simple nodular type. Moreover, all simple nodular type tumors except for two advanced cases were classified as early stage (stage I or II) by both the AJCC and ENETS TNM classification systems (Table 2). The two advanced cases had simultaneous liver metastases, microvascular invasion, neural invasion, positive chromogranin A, positive synaptophysin, positive CD56 and 1/10HPF mitotic indices. One had lymph node metastases and the other did not. Tumor size of the two cases were 11 and 13 mm. Ki67 indices were 0.4 and 1%.
This study identified a novel imaging classification system that can be used to predict the malignant potential of P-NETs. The morphological classification shows a relationship between the advanced features of P-NETs and higher stage tumors, including tumor size, Ki-67 index, and synchronous liver metastasis (p < 0.001 for all). This new classification system predicted malignant potential by ENETS TNM classification (p < 0.001), AJCC TNM classification (p = 0.046), WHO 2004 classification (p < 0.001), and WHO 2010 classification (p < 0.001). Moreover, multivariate analysis revealed Type II/III tumors (the non-simple nodular type) and synchronous liver metastases to be independent risk factors for poor PFS (Table 3). In logistic analysis, Type III tumor is a factor deciding poorly differentiated carcinoma, though the factors found before surgery such as the invasion to adjacent organ, tumor size >2 cm, tumor functionality, simultaneous liver metastases were not (data not shown). These results strongly support the link between morphology and the malignant potential of P-NETs. In this context, gene expression in Type II/III P-NETs should be examined in the near future, as this may suggest pathways that contribute to poor prognosis. Moreover, in patients with no surgical treatment, Type III had significantly poorer survival than Type I (supplemental figure).
On the other hand, Ki-67 index, mitotic index and tumor size were not associated with PFS, as shown in Table 3. They might be the confounding factors of synchronous liver metastases and/or morphology. They have been regarded as important factors in both WHO 2010 grade and TNM staging, though it has been established that there are several problems in the construction of TNM staging. For example, Luo et al. indicated that patients with stage I disease had a similar prognosis to patients with stage IIA disease, and patients with stage IIIB disease had a lower HR for death than did patients with stage IIIA disease by ENETS staging [18]. In the present study, synchronous liver metastases and/or morphology were the important factors in considering the malignancy of the disease. Aggregating further evidence, the morphological classification may solve these problems in the near future.
Recent findings suggest that CT plays an important role in the early detection and precise diagnosis of P-NETs [14]. Rodallec et al. reported that tumors with low enhancement on CT were correlated with poor differentiation and worse overall survival [15]. D’Assignies et al. reported that tumor blood flow as assessed by CT was strongly correlated with intra-tumoral microvascular density and WHO classification [16]. Yamada et al. also showed that receiver operating characteristic analysis, using corrected CT attenuation values in the pancreatic phase, showed area under the curves higher than the tumor size, allowing for the prediction of G2 P-NETs [17]. These attempts improve our understanding of P-NETs physiology. In this context, the classification system we present, constructed by the pathological findings, has high versatility without any additional diagnostic imaging or complicated analysis [8]. In this study, this simple and non-invasive morphological classification system accurately predicted the prognosis and malignant potential of patients.
In conclusion, we established a non-invasive morphological classification using MDCT findings in P-NETs. This novel simple morphological classification system can be used to predict Type II and III tumors that may have higher malignant potential than Type I tumors.
References
Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12.
Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64.
Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol. 2012;47:941–60.
Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18(Suppl 1):S1–16.
Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–34.
Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. pp.13–14.
Katsuta E, Kudo A, Akashi T, et al. Macroscopic morphology for estimation of malignant potential in pancreatic neuroendocrine neoplasm. J Cancer Res Clin Oncol. 2016;142:1299–306.
Nakayama H, Takayama T, Okubo T, et al. Proposal of objective morphological classification system for hepatocellular carcinoma using preoperative multiphase computed tomography. J Gastroenterol. 2014;49:1430–7.
Inayoshi J, Ichida T, Sugitani S, et al. Gross appearance of hepatocellular carcinoma reflects E-cadherin expression and risk of early recurrence after surgical treatment. J Gastroenterol Hepatol. 2003;18:673–7.
Nagano Y, Shimada H, Takeda K, et al. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg. 2008;32:2218–22.
Shimada M, Rikimaru T, Hamatsu T, et al. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg. 2001;182:177–82.
Murakata A, Tanaka S, Mogushi K, et al. Gene expression signature of the gross morphology in hepatocellular carcinoma. Ann Surg. 2011;253:94–100.
Horton KM, Hruban RH, Yeo C, Fishman EK. Multi-detector row CT of pancreatic islet cell tumors. Radiographics. 2006;26:453–64.
Rodallec M, Vilgrain V, Couvelard A, et al. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology. 2006;6:77–85.
d’Assignies G, Couvelard A, Bahrami S, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250:407–16.
Yamada S, Fujii T, Suzuki K, et al. Preoperative identification of a prognostic factor for pancreatic neuroendocrine tumors using multiphase contrast-enhanced computed tomography. Pancreas. 2016;45:198–203.
Luo G, Javed A, Strosberg JR, et al. Modified staging classification for pancreatic neuroendocrine tumors on the basis of the american joint committee on cancer and european neuroendocrine tumor society systems. J Clin Oncol. 2017;35:274–80.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (C) Grant Number 15K10046.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2017_1349_MOESM2_ESM.tif
Supplementary figure Overall survival rates of non-surgical patients with pancreatic neuroendocrine tumors according to morphological classification. Note the significant difference between Types I and III (TIFF 6604 kb)
Rights and permissions
About this article
Cite this article
Oba, A., Kudo, A., Akahoshi, K. et al. A simple morphological classification to estimate the malignant potential of pancreatic neuroendocrine tumors. J Gastroenterol 52, 1140–1146 (2017). https://doi.org/10.1007/s00535-017-1349-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1349-7