Abstract
Background
Patients with hepatocellular carcinoma (HCC) who undergo liver resection and transplantation are predicted to have a poor outcome if the disease is associated with vascular invasion. This study aimed to identify preoperative predictors of microvascular invasion in patients with HCCs larger than 5 cm.
Methods
From May 1992 to October 2005, 231 patients underwent curative hepatic resection for HCC. Of these, 96 patients had HCCs larger than 5 cm. Analysis was limited to patients without macroscopic vascular invasion (n = 65).
Results
Multivariate analysis showed that patients with tumors larger than 7 cm and type 2 (single nodular type with extranodular growth) and type 3 (contiguous multinodular type formed by a cluster of small and contiguous nodules) tumors had an increased risk of microscopic vascular invasion. The overall incidence of microscopic vascular invasion was 46.2% (n = 30), but only 12.5% (2/16) in patients with type 1 tumors (single nodular type that is approximately round with a clear demarcation) measuring less than 7 cm.
Conclusion
Larger tumors (>7 cm) and type 2 and type 3 tumors are strong predictors of microvascular invasion in patients with HCCs larger than 5 cm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver resection and liver transplantation have been accepted as the only chance of cure for patients with primary hepatocellular carcinoma (HCC). However, long-term prognosis remains unsatisfactory due to a high incidence of intrahepatic recurrence after hepatectomy. Furthermore, early experience of orthotopic liver transplantation (OLT) in unselected patients was associated with a poor outcome. Subsequent studies have shown that vascular invasion is a major determinant of outcome after resection or transplantation for HCC [1–5].
Mazaferro’s criteria [6] for HCC have been widely used as guidelines for the selection of OLT candidates in many transplantation centers. However, none of the patients in this small-scale study had evidence of microscopic vascular invasion. Llovet et al. [7] reported that microscopic vascular invasion detected during pathologic examination of explant specimens was associated with a lack of disease-free survivors after three years, whereas 94% of patients without vascular invasion were disease-free after three years. Tumor size appears to have no significant impact on patient survival when microscopic vascular invasion is absent, since survival rates after resection of T1 tumors larger than 10 cm in diameter are similar to those following resection of T1 tumors less than 5 cm [7, 8].
In this study, we analyzed the preoperative predictor of microscopic vascular invasion to determine stricter criteria for liver transplantation as a treatment for patients with HCCs larger than 5 cm in diameter.
Patients and methods
Between January 1992 and December 2005, 231 consecutive patients underwent hepatic resection for HCC at the Yokohama City University Graduate School of Medicine, Japan. All patients underwent complete clinical, laboratory, and radiologic testing in order to stage their disease. Of these patients, 96 had large HCCs greater than 5 cm in diameter. No patients in the final cohort had evidence of lymph node metastasis or lung metastasis. The overall incidence of macroscopic vascular invasion was 31 patients according to preoperative radiologic examinations, so we limited our analysis to the remaining patients without major vascular invasion (n = 65). The overall incidence of histologic microvascular invasion was 46.2% (n = 30).
The serologic presence of hepatitis B antigens or antibodies and of hepatitis C antibodies was considered positive evidence of hepatitis B or C exposure, respectively. Preoperative imaging for tumor staging included chest X-rays, abdominal ultrasonography (US), computed tomography (CT), and magnetic resonance imaging, if indicated. Tumor size was defined as the largest diameter of the tumor in the resected specimen. In patients with multiple tumors, the largest lesion was used as the index lesion.
Operative procedures have been described previously [8, 9]. Resected specimens were cut into 10-mm slices, and histopathologic examination revealed microscopic invasion as the presence of cancer cell clusters floating in the vascular space lined by endothelial cells. Tumor differentiation and histology of the noncancerous surrounding parenchyma were defined according to the Liver Cancer Study Group of Japan guidelines [10]. Tumor grade was defined by the poorest degree of differentiation.
Gross classification
Nodular types of HCCs were characterized by a clear border between the tumor and the surrounding parenchyma. Kanai’s classification [11] was then used to subdivide them into three categories based on explant: type 1, single nodular type that is approximately round with a clear demarcation; type 2, single nodular type with extranodular growth (a tumor like type 1 but showing extranodular growth); and type 3, contiguous multinodular type formed by a cluster of small and contiguous nodules. This classification has been accepted by the Liver Cancer Study Group of Japan [10] and is widely used. We classified tumors preoperatively according to this classification using preoperative dynamic CT and US images.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation and compared using the Mann–Whitney U test. Categorical variables were compared using the χ2 test or Fisher’s exact test where appropriate. Overall survival and disease-free survival were calculated by the Kaplan-Mayer method, and the differences between groups were compared using the log-rank test. Univariate predictive factors (p < 0.1) were entered into a stepwise logistic regression model to identify the independent predictors of microvascular invasion. All statistical analyses were performed using the SPSS computer software package (version 10.0 for Windows, SPSS, Inc., Chicago, IL), and p < 0.05 was taken as statistically significant.
Results
The 65 patients (52 men and 13 women, male: female ratio = 4:1) included in the study had a median age of 64.2 years (range = 39–79 years). Forty-six patients had solitary tumors with the median tumor size 8.69 cm (range = 5.0–25.0 cm). Most tumors were moderate or well-differentiated HCCs; only 7.7% of patients had poorly differentiated HCCs. Hepatitis B and C serology was 24.6% and 52.3%, respectively. No patients were classified as Child-Pugh C, while 86.2% were classified as Child-Pugh A. Thirty-five cases had type 1 tumors and 13 cases had type 2 tumors, and 17 cases had type 3 tumors.
Both univariate and multivariate predictors of microvascular invasion were shown to be a tumor size greater than 7 cm (odds ratio [OR] = 3.42, 95% confidence interval [CI] = 1.1–11.2, p < 0.05), a type 2 tumor, and a type 3 tumor (OR = 6.9, 95% CI = 2.2–21.8, p < 0.001) (Table 1).
Increasing tumor size was associated with higher rates of microvascular invasion: tumors 5–6.9 cm had a 26.9% rate, tumors 7–8.9 cm had a 42.9% rate, while tumors greater than 9 cm in diameter had a 66.7% rate (Fig. 1). Evidence of microvascular invasion was in 57.9% of patients with tumors measuring more than 7 cm compared to 26.9% of patients with tumors measuring less than 7 cm (p = 0.043).
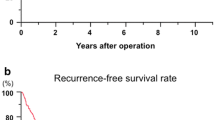
Microvascular invasion was present in 12.5% (2/16) of patients with type 1 tumors measuring less than 7 cm; this percentage rose to 78.9% (15/19) in patients with larger tumors (more than 7 cm), type 2 tumors, and type 3 tumors (Fig. 2). In addition, the overall cumulative survival results for patients with type 1 tumors less than 7 cm undergoing hepatectomy were markedly better than in patients with larger type 2 and type 3 tumors. Survival rates for patients with type 1 tumors measuring less than 7 cm were 94.1% for 1 year, 48.9% for 3 years, and 39.1% for 5 years, with a median survival of 36 months compared with 44.8% for 1 year, 29.9% for 3 years, and 14.9% for 5 years. There was a median survival of 11 months for patients with larger type 2 and type 3 tumors (p = 0.047) (Fig. 3).
Discussion
Vascular invasion is a strong predictor of outcome in HCC patients treated with OLT and liver resection [12–14]. Mazzaferro et al. [6] performed a prospective trial of transplantation for early-stage HCC and established the Milan enrollment criteria based on preoperative imaging studies of a single tumor less than 5 cm in diameter or a maximum of three tumors all less than 3 cm in diameter. These criteria became the standard for patient selection, and the United Network of Organ Sharing and other procurement agencies granted waiting-list priority for patients meeting their criteria. However, none of the patients in that small-scale study had evidence of microscopic vascular invasion.
At present, the adoption of expanded criteria has been suggested by a number of centers because some patients who do not meet the Milan criteria are excluded from potentially curative surgery. The University of California, San Francisco (UCSF) criterion is a single lesion 6.5 cm or smaller in diameter or three lesions with the largest 4.5 cm or smaller and with a total tumor diameter of 8 cm or smaller[15]. Patients with between two and four tumors 5 cm or smaller in diameter or a single lesion 6 cm or smaller[16] had recurrence-free survival rates equivalent to patients meeting the Milan criteria.
The use of tumor size alone to prioritize patients for transplantation is problematic, since size has no significant impact on survival in the absence of microscopic vascular invasion. Survival after resection of T1 tumors larger than 10 cm in diameter is similar to survival following resection of T1 tumors less than 5 cm [7, 8], and patients with tumors larger than 5 cm have recurrence-free survival rates greater than 80% [17]. Indeed, tumor size alone may not be a contraindication for transplantation.
Recent studies suggest that vascular invasion is a strong predictor of outcome in HCC patients treated with OLT, so it is important to identify the vascular invasion preoperatively. While major vascular invasion can be identified preoperatively in the majority of cases, microscopic vascular invasion is impossible to rule out before transplantation. Recognition of surrogate markers of microscopic vascular invasion that can be obtained in the preoperative/pretransplant setting could improve the selection of patients for surgical treatment of larger HCCs. Although increasing tumor size is believed to be associated with microscopic vascular invasion, only a few studies have examined the correlation between tumor diameter and microscopic vascular invasion in detail [18–22].
Esnaola et al. [18] reported that tumor sizes above 4 cm and tumor grade were strong predictors of microvascular invasion in HCC patient OLT candidates who met the Milan criteria. Pawlik et al. [19] reported that high histologic grade, AFP level of at least 1000 ng/ml, and multiple nodule tumors each predicted occult vascular invasion in tumors larger than 5 cm. In the present study, a tumor diameter greater than 7 cm was one of the independent risk factors for microscopic vascular invasion. Indeed, the incidence of microscopic vascular invasion was almost twice as high in tumors larger than 7 cm (61%) than in smaller tumors (32%) and continued to rise with increasing size, even above 10 cm.
Kannai’s classification [8], which is based on gross patterns of tumor growth and spread, is widely used and has been accepted by the Liver Cancer Study Group of Japan [10]. Hui [21] reported that type 2 and type 3 tumors were significant univariate and multivariate risk factors for tumor recurrence and disease-specific death, and that microscopic vascular invasion was more frequent in type 3 than in type 1 and type 2 tumors. Inayoshi [22] also reported that microscopic vascular invasion and subclinical intrahepatic metastases were more frequent in type 2 and type 3 HCC than type 1 HCC.
In the present study, type 2 and type 3 tumors were independently associated with increased microscopic vascular invasion; specifically, microscopic vascular invasion was significantly more common in patients with type 2 and type 3 tumors (70%). These data suggest that tumor gross classification should be considered as a strict selection criterion rather than size alone. As microvascular invasion was present in 12.5% of patients with type 1 tumors measuring less than 7 cm in diameter, the OLT criteria for large HCC tumors appears to include patients with type 1 tumors and those with tumors of under 7 cm diameter.
In a comparative genomic hybridization (CGH) study, Pang et al. [23] reported that the amplification of chromosomes 1q, 6p, and 17q and the deletion of 11p were significantly associated with venous invasion. Several studies of the molecular mechanism of HCC have suggested a relationship between HCC vascularity and VEGF expression [24]. VEGF levels in HCC specimens from patients with tumor emboli and poorly encapsulated tumors (both adverse prognostic features) were higher than in specimens without, suggesting that VEGF expression may correlate with HCC tumor invasion and metastasis [25]. As percutaneous fine-needle biopsy can be used to obtain liver tissue, future studies should attempt to determine the relative value of genetic and epigenetic alterations and novel molecular markers in predicting the presence of microvascular invasion in HCC patients treated with resection or OLT.
In conclusion, our study suggests that a tumor diameter greater than 7 cm and a type 2 or type 3 tumor classification could be used as preoperative predictors of microvascular invasion in patients with HCCs larger than 5 cm.
References
Kosuge T, Makuuchi M, Takayama T et al (1993) Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology 40:328–332
Izumi R, Shimizu K, Ii T et al (1994) Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 106:720–727
Vauthey JN, Lauwers GY, Esnaola NF et al (2002) A simplified staging for hepatocellular carcinoma. J Clin Oncol 20:1527–1536
Iwatsuki S, Dvorchik I, Marsh JW et al (2000) Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 191:389–394
Marsh JW, Dvorchik I, Bonham CA et al (2000) Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer 88:538–543
Mazzaferro V, Regalia E, Doci R et al (1996) Liver transplantation for the treatment of small hepatocellular carcinomas inpatients with cirrhosis. N Engl J Med 334:693–699
Llovet JM, Bruix J, Fuster J et al (1998) Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatology 27:1572–1577
Nagano Y, Tanaka K, Togo S et al (2005) Efficacy of hepatic resection for hepatocellular carcinomas larger than 10 cm. World J Surg 29:66–71
Nagano Y, Tanaka K, Togo S et al (2005) The role of median sternotomy in resections for large hepatocellular carcinomas. Surgery 137:104–108
Liver Cancer Study Group of Japan (2000) The general rules for the clinical and pathological study of primary liver cancer, 4th edn. Kanehara and Co. Ltd, Tokyo
Kanai T, Hirohashi S, Upton MP et al (1987) Pathology of small hepatocellular carcinoma: a proposal for a new gross classification. Cancer 60:810–819
Shetty K, Timmins K, Brensinger C et al (2004) Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl 10:911–918
Salizzoni M, Romagnoli R, Lupo F et al (2003) Microscopic vascular invasion detected by anti-CD34 immunohistochemistry as a predictor of recurrence of hepatocellular carcinoma after liver transplantation. Transplantation 76:844–848
Tsai TJ, Chau GY, Lui WY et al (2000) Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 127:603–608
Yao FY, Ferrell L, Bass NM et al (2002) Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TMN criteria. Liver Transpl 8:765–774
Oncona N, Davis GL, Goldstein RM et al (2007) Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl 13:391–399
Roayaie S, Frischer JS, Emre SH et al (2002) Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg 235:533–539
Esnaola NF, Lauwers GY, Mirza NQ et al (2002) Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg 6:224–232
Pawlik TM, Delman KA, Vauthey JN et al (2005) Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 11:1086–1092
Yuki K, Hirohashi S, Sakamoto M et al (1990) Growth and spread of hepatocellular carcinoma: a review of 240 consecutive autopsy cases. Cancer 66:2174–2179
Hui AH, Takayama T, Sano K et al (2000) Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol 33:975–979
Inayoshi J, Ichida T, Sugitani S et al (2003) Gross appearance of hepatocellular carcinoma reflects E-cadherin expression and risk of early recurrence after surgical treatment. J Gastroenterol Hepatol 18:673–677
Pang A, Ng IO, Fan ST et al (2003) Clinicopathologic significance of genetic alterations in hepatocellular carcinoma. Cancer Genet Cytogenet 146:8–15
Shimoda K, Mori M, Shibuta K et al (1999) Vascular endothelial growth factor/vascular permeability factor mRNA expression in patients with chronic hepatitis C and hepatocellular carcinoma. Int J Oncol 14:353–359
El-Assal ON, Yamanoi A, Soda Y et al (1998) Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology 27:1554–1562
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagano, Y., Shimada, H., Takeda, K. et al. Predictive Factors of Microvascular Invasion in Patients with Hepatocellular Carcinoma Larger Than 5 cm . World J Surg 32, 2218–2222 (2008). https://doi.org/10.1007/s00268-008-9585-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-008-9585-x