Abstract
Purpose
Considering the complex pathobiology of oral mucositis, especially in oral cancer patients, the prevention and treatment of oral mucositis in patients undergoing radiotherapy remains an essential and clinically crucial unmet need. The present study aims to investigate and compare the effects of synbiotic mouthwash with normal saline mouthwash on the prevention and control of radiotherapy-induced oral mucositis in oral cancer patients.
Methods
Double-blind, randomized clinical trial (RCT) performed on 64 oral cancer patients who underwent radiotherapy (IRCT20201106049288N1, registration date: 2020–12-23). Patients were divided randomly into the case (32 subjects) and control (32 subjects) groups. All patients underwent intensity-modulated radiotherapy and received 6000 cGY of radiotherapy in 34 fractions. All patients received the usual treatment for mucositis, but in the case group, synbiotic mouthwash was prescribed and in the control group, normal saline mouthwash was prescribed from a day before the start to the end of radiotherapy treatment. Patients were monitored every session for 6 weeks to check the progression, oral involvement severity, and mucositis grade.
Results
The case group showed a significant reduction in the oral mucositis severity. The mucositis grade in the case group from the 7th session of oral examination was significantly lower than the control (p < 0.05), and this significant difference persisted until the last session of oral examination. Incidence rates of severe oral mucositis (grade 3) during the treatment period were 11.59% in the case and 36.45% in control (p < 0.001).
Conclusion
Synbiotic mouthwash significantly reduces and prevents oral mucositis intensity in oral cancer patients undergoing radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Oral cancers include a variety of malignant neoplasms that include tumors in the oral cavity [1]. Radiation therapy is one of the treatment protocols for oral cancer, and it kills cells by directly damaging the DNA [2]. Radiotherapy side effects in cancer patients are associated with oxygen-free radicals and oxidative damage to healthy cells [3, 4]. Oral mucositis (OM) is a severe side effect of radiotherapy that refers to mucosal lesions of the oral cavity and the consequent functional problems [5, 6]. The mean incidence of OM in patients undergoing fractionation radiotherapy is approximately 80% for oral cancer [7, 8]. Recent studies have shown that changes in microbiota and mucin cause mucositis following radiotherapy. This change in microbiota risks compromising the immune system’s protection against the invasion of pathogens [9,10,11,12]. Considering the complex pathobiology of OM, especially in oral cancer patients, the prevention and treatment of OM in patients undergoing radiotherapy remain an essential and clinically crucial unmet need.
Probiotics are living microorganisms that improve health by inhibiting or reducing pathogenic microorganisms [13, 14]. Previous studies have confirmed the role of probiotics in altering the oral flora (such as Streptococcus mutans) and, consequently, the associated diseases [15,16,17]. The mechanisms of probiotic action in the oral cavity are the production of antibacterial agents, attachment to dental surfaces, changing the conditions of the oral environment, and reducing the inflammatory response [18,19,20]. The rationale for using probiotics against mucositis includes anti-inflammatory effects, maintaining mucosal permeability, eliminating pathogenic bacteria, preventing cellular apoptosis, and reinforcing the mucosal barrier [21,22,23,24,25,26]. Previous studies have also confirmed the effectiveness of probiotics and synbiotics in preventing and treating gastrointestinal mucositis [24, 27, 28].
Despite several treatment modalities for radiotherapy-induced OM, there is no perfect treatment strategy, so new therapeutic interventions are needed [29]. Based on the best of our knowledge, there has been no study related to the effect of synbiotic mouthwash on OM. The present study aims to compare the effects of synbiotic mouthwash with normal saline mouthwash on the prevention and control of radiotherapy-induced OM in oral cancer patients, and the incidence of mucositis and mucositis grade were assessed.
Material and methods
Study design and patient enrollment
The double-blind, randomized clinical trial (RCT) was conducted at the Institute Cancer of Imam Khomeini Hospital (Tehran, Iran). Sixty-four individual subjects between the ages of 20–70 years with newly pathologically diagnosed oral squamous cell carcinoma (OSCC), referred to as intensity-modulated radiotherapy (IMRT) for receiving 6000 cGY of radiotherapy in 34 fractions (150–200 cGY/day, 5 day/week) in the oral cavity area, with the gross tumor volume (total of 6–7 weeks), were enrolled in this study. The clinical stages of the enrolled patients were determined according to the 8th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual [30]. Patients were divided by the simple randomization method into case and control groups. The case and control patients were matched for age and gender. The study was conducted using a double-blind method, and the mouthwash distributor did not know the type of mouthwash used (whether it was synbiotic or normal saline). The patients did not know about the type of mouthwash that they used. Before enrollment, all patients received medical evaluations, including medical history and a physical examination. The exclusion criteria for patients were as follows:
-
1- Autoimmune diseases
-
2- Any history of OM before radiotherapy
-
3- Using an antimicrobial agent
-
5- Patients with a known history or coexisting any other tumors or malignancies
-
6- Any obvious inflammatory diseases
-
8- Allergies to probiotics
-
9- Patients with known active dental and periodontal infections
Ethics approval and consent to participate
Before starting radiotherapy, the purpose of the study and its steps were explained in both case and control groups. After obtaining the patient’s informed consent in accordance with the ethics committee requirements at the participating institutes and the Declaration of Helsinki, the study was started. This study was approved by the Tehran University of Medical Sciences Ethical Committee (Ethical code: IR.TUMS.DENTISTRY.REC.1399.176), and this study was registered in the Clinical Trial Registration Center of Iran (IRCT code: IRCT20201106049288N1). All methods were performed under the relevant guidelines and regulations, and this study was conducted in accordance with the Declaration of Helsinki [31]. Randomization and masking of the mouthwash type.
The random assignment of patients was performed with a computer-generated random number code to receive synbiotic or normal saline mouthwash. The drug was packaged and distributed according to random numbers and blinding codes. The blinding codes were not disclosed during all stages of experiments.
Mouthwash preparation
To prepare synbiotic mouthwash, 3 g of FamiLact powder (Familact, Zist Takhmir Company, Tehran, Iran) with the approval number of 0347756442342525 from the Food and Drug Department of Iran’s Ministry of Health and Medical Education was used, and dissolved in 100 ml of sterile normal saline (0.9%). The FamiLact is a synbiotic (probiotic + prebiotic) formulation which contains seven different gram-positive organisms strains (2 × 1010 colony forming units [CFU] Bifidobacterium breve, 7 × 109 CFU Bifidobacterium longum, 2 × 109 CFU Lactobacillus acidophilus, 7 × 109 CFU Lactobacillus casei, 2 × 108 CFU Lactobacillus bulgaricus, 1.5 × 109 CFU Lactobacillus rhamnosus, 1.5 × 1010 CFU Streptococcus salivarius subsp. thermophiles) and 40 mg fructooligosaccharide as a prebiotic, lactose, magnesium stearate, and talc as carrier substances. The mouthwashes were kept in a 100-ml bottle and checked for physical stability for 2 months without changing color, odor, and flavor. There was a minor difference between the two types of mouthwash tastes. However, the clinicians did not explain the taste difference between the two types of mouthwash to patients. Also, we avoided encountering two groups (case and control) with each other.
Trial protocol
All patients underwent radiotherapy using the National Comprehensive Cancer Network guidelines. Patients received 6000 cGY of radiotherapy in 34 fractions (150–200 cGY/day, 5 day/week), with the gross tumor volume (total of 6–7 weeks). Patients were trained regarding the dietary regime and oral hygiene instructions. We advise patients to use a soft or ultra-soft toothbrush and the same toothpaste without allergic flavor at least twice a day and once using gentle flossing [32]. Dietary considerations include avoiding allergenic foods such as cinnamon, tomatoes, strawberries, liqueurs, walnuts, onions, and raw garlic, celery [33]. Patients were asked to avoid smoking or drinking alcoholic beverages [34]. Nystatin mouthwash was prescribed for all patients in both groups (nystatin suspension 100/000 U/mL, 5–10 mL rinse), then spit (3–4 times daily in 1 to 3 min). Since nystatin suspension contains sugar, patients are instructed to use mouthwash before oral hygiene measures such as brushing and dental flossing [32, 35]. They recommended keeping the oral environment moist by sipping water frequently and using sugar-free chewing gum [36, 37]. All patients were instructed to use the modified Bass technique for brushing and to avoid gingival and soft tissue injury. During their treatment, individuals in both case and control groups were instructed not to use probiotic products such as bread, cakes, yogurt, or dairy. In the case group, in addition to the usual treatment of mucositis, synbiotic mouthwash was prescribed a day before the start of radiotherapy treatment to the end of the radiotherapy treatment. They gargled 10 ml of mouthwash three times a day for 3 min. In the control group, besides the usual treatment of mucositis, a normal saline mouthwash was prescribed for 10 ml to be used three times a day for 3 min. Mouthwashes were given to each treatment group in similar bottles (shape, size, and color). During radiotherapy, patients were monitored every day or every 2 days for 6 weeks to check the progression of mucositis, severity of oral involvement, the occurrence of mucositis and its grading, and occurrence were assessed daily by three oral medicine specialists based on the World Health Organization (WHO) grading system [38], National Cancer Institute’s general criteria for adverse events (version 5.0), and updated MASCC/ISOO Guidelines were used to score and manage mucositis in patients [39]. For this purpose, patients were examined every session of radiotherapy treatment for 6 weeks to check the progression of mucositis; this oral examination (OE) started precisely after the fifth session of radiotherapy and continued after each radiotherapy treatment session. Consequently, the last follow-up OE session was done 1 week after the last radiotherapy session (Fig. 1). The patient’s oral hygiene was examined in each follow-up session by plaque index. A checklist asked patients about the frequency of toothbrushing and dental flossing daily after radiotherapy. During the study, no unwanted side effects or symptoms, such as allergic reactions, were seen in patients under treatment.
Statistical analysis methods
Statistical analyses were performed using the statistical software SPSS 24.0.0. (SPSS Inc., USA) and GraphPad Prism 9.0.00 (GraphPad Software, USA). P-values (p) less than 0.05 were considered significant. Descriptive statistics were reported as mean ± SD for quantitative variables, and all the data were analyzed using Mann–Whitney and Wilcoxon W tests and non-linear regression.
Results
Patients’ characteristics
One hundred and forty-five patients with oral cancer diagnosed by histopathology were assessed as eligible for enrollment in this study from February 2020 to May 2020. This study was a double-blind, randomized clinical trial in patients with oral cancer undergoing head and neck radiotherapy. Sixty-four patients were excluded from the study because of protocol deviations from the study protocol. Three patients without oral discomfort, 19 patients, who did not meet inclusion criteria, 27 patients because of exclusion criteria, six patients who declined to participate, seven patients who declined before randomization, and two patients for other reasons (two patients died during the treatment and followed up the process). During the follow-up period, nine patients who did not complete radiotherapy treatment were omitted from the study. Finally, 32 patients in each group of synbiotic and normal saline mouthwash were analyzed (Fig. 2).
All patients enrolled in the analysis step completely received their planned radiation treatment dose, and there was no significant difference in the radiotherapy dose received between the case and control groups. In both the control and case groups, 23 subjects (72.88%) were male, nine subjects (28.12%) were female, and there was no difference in the case and control groups regarding sex and age. The age of participants, tumor stage, node stage, and site of cancer development are stated in Table 1.
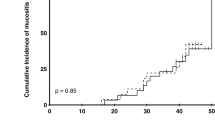
First, OMs were observed 1 week after the first radiotherapy. Tongue, floor of the mouth, and buccal mucosa were the most commonly found sites of OM in the case and control. The minimum and maximum mucositis grading of each person receiving normal saline and each person receiving synbiotic recipient for 6 weeks and 30 sessions of radiotherapy were the same; without OM and grade 3, respectively. The incidence rates of severe OM (grade 3) during the treatment period were 11.59% in the case and 36.45% in the control group, and the difference was statistically significant (p < 0.001). Incidence rates of grades 1 and 2 in the case group were 25.36% and 60.97%, respectively, and in the control group were 14.49% and 47.93%, respectively. OM grades in the intervention and control groups, represented as a percentage in each session from the first to the end of the OE session, are shown in Fig. 3.
The mean mucositis grade of each normal saline recipient and synbiotic mouthwash in the first OE session was 0.84 and 0.75 (p > 0.05), respectively. The mean mucositis grade in the 30th OE session of the case group was 1.75, significantly lower than the mean mucositis grade in the 30th OE session of the control group (2.4, p < 0.00.1). The mean mucositis grade in the intervention and control group in each OE session is shown in Fig. 4. However, as shown in Fig. 4, the trend of the graphs is the same in both groups (case and control groups), and severe OM was mostly observed in the 15th to 22nd OE sessions.
Using the Mann–Whitney U test, a comparison was made of the mean mucositis grade of both case and control groups at similar weeks. Mann–Whitney’s analysis showed that the mean mucositis grade has a significant statistical difference between the two groups from the 7th session of OE onwards. The present study results showed that the mucositis grade in the first and second sessions of OE in both case and control groups was similar. The mucositis grade differed between the two groups from 3 to 7th session of the OE, but this difference was not statistically significant (p > 0.05). The mucositis grade of both groups after the 7th OE session was statistically significant (p < 0.05). Mucositis grading was significantly different in the case and control groups from the seventh OE session to the 30th session of OE (Table 2). Mucositis grade in the synbiotic group was significantly lower than in the normal saline group (p < 0.05). These results show that mouthwash containing synbiotic strains significantly reduces the incidence of grade 3 OM.
To construct models to predict the intensity of OM, after surveying and using the curve fitting tool of GraphPad Prism 9.0.00 (GraphPad Software, USA), we chose the substrate inhibition equation regression model (Eq. 1), where Y represents the mean value of OM grade, and X represents session of OE, and also A, B, and C are the constant value that was computed by curve fitting.
The parameters of Eq. 1 and the fitted equation on the mean grade of OM are shown in Fig. 5.
Discussion
Current study results show that synbiotic mouthwash significantly prevent OM induced by radiotherapy in oral cancer patients. The pathobiology of OM is complex and multifaceted, and the role of oral microbiota as an effective preventive and protective factor seems to be very important [40]. Some studies have shown that despite preventive oral care, patients undergoing cancer treatment developed OM, and the time of onset and the severity of OM were associated with normal oral flora before and during treatment [41].
Several studies have also investigated the role of gastrointestinal microbiota and intestinal mucosa and their effects on the occurrence of oral mucosa [42]. The intestinal microbiota is effective in the pathogenesis of OM, especially in the recovery phase, by modulating pro-inflammatory pathways [43]. There is also evidence linking the role of the gut microbiome in the pathogenesis of OM by regulating immune responses. In addition, bowel dysfunction due to cancer treatment and changes in the gut microbiome can exacerbate the severity of OM [44]. Probiotic supplements can effectively reduce intestinal microbial disorders caused by radiochemotherapy, and this can ultimately reduce OM in patients with nasopharyngeal cancer [7].
Probiotics may reduce the incidence and severity of OM caused by cancer treatment [26]. Cordeiro et al. showed that probiotic drinks effectively prevented mucositis in mice and concluded that the prevention of mucositis depends on the bacterial strain [45]. They also showed that probiotic products could be used to reduce the side effects of chemotherapy using generally recognized as safe (GRAS) bacteria by strengthening the immune system [46]. Streptococcus salivarius K12 can reduce radiotherapy-induced OM in mice and is a promising treatment for cancer patients undergoing radiation therapy [47]. They also showed that Bacillus clausii UBBC-07 effectively delayed the onset and reduced the recovery time of OM, especially in the prevention of acute OM [48].
Studies have emphasized the need to use specific probiotics to reduce the OM severity in clinical trial studies of diverse populations undergoing cancer treatments [49]. Two studies focused on lactobacillus and lactococci administered systemically or topically [50, 51]. Both studies effectively prevent OM in patients with H and N cancer. However, no instructions were provided because of the limited data for these probiotics [52].
Jiang et al. [7] and Xia et al. [53] showed that probiotics have a significant role in preventing OM caused by radiotherapy and chemotherapy by strengthening the immune system and increasing the number of T cells and lymphocytes [7, 53]. Besides the role of probiotics in oral health [54, 55] and new hopes in cancer treatments [56], probiotics strengthen the immune system by regulating the balance of microbial flora [57] and preventing the growth of pathogenic bacteria, which plays an essential role in the prevention and control of OM [26]. Wei et al. stated that probiotics could effectively prevent or treat diarrhea caused by chemotherapy or radiotherapy, which is consistent with ours [58]. Shu et al. concluded that probiotics reduce the incidence and severity of OM in cancer treatment. However, more clinical trials with a double-blind, randomized study design have been recommended to confirm the effects of probiotics [26].
Chang et al. showed that probiotic administration inhibits mucositis and reduces the severity of diarrhea and intestinal toxicity in mice with colorectal cancer undergoing chemotherapy [59]. Other studies have shown that synbiotics are involved in preventing mucositis by increasing the expression of toll-like receptors (TLRs) at the level of macrophages [60]. Lipoteichoic acid on the surface of the bacterium Lactobacillus rhamnosus can bind to toll-like receptor 2 (TLR2) on the surface of peristaltic macrophages, producing the chemokine CXCL12 [26, 60]. Yeung et al. used oral lactobacilli probiotics in mice with chemotherapy-induced intestinal mucositis. They suggested that probiotics may be used to improve mucositis as an alternative therapeutic solution to prevent or manage mucositis caused by chemotherapy, and their results were in line with ours [61]. Stamatova et al. used probiotics in dairy products to improve the signs and symptoms of Crohn’s disease. They mentioned that probiotic bacteria in the mouth are essential to determine their safety and properties [62].
Several studies suggested that Bifidobacterium breve could effectively improve intestinal environments in clinical use [63, 64]. Pre-clinical studies after chemotherapy showed that a decrease in some bacteria species, including Bifidobacterium, results in lipopolysaccharide (LPS) that initiates the inflammatory pathways [65]. Using anti-inflammatory agents continues to be a promising strategy for the prevention and treatment of OM in cancer patients [66, 67]. Jiang et al. used a Bifidobacterium longum supplement to reduce the severity of OM by chemoradiotherapy in patients with nasopharyngeal carcinoma [7]. Picó-Monllor concluded that B. longum was the most frequently used probiotic for mucositis treatment. A combination of other probiotics resulted in the successful treatment of mucositis-associated with colorectal cancer [21, 68].
Oh et al. investigated the effects of Lactobacillus acidophilus on the intestinal mucositis of rats and concluded that L. acidophilus could be used for mucositis prevention and treatment [69]. Cordeiro et al. reported the effects of L. acidophilus on preventing mucositis in mice [45]. Similar results were reported by Quaresma et al. regarding the mixture containing Lactobacillus spp. and Bifidobacterium spp. It could lead to intestinal mucositis in mice [70]. The combination of B. breve, B. longum, and L. acidophilus suggested reducing mucositis incidence rates or ameliorating its symptoms in chemo- or radiotherapy treated patients [21]. Yeung et al. showed the immune-modulating effects of Lactobacillus casei in a chemotherapy-induced intestinal mucositis mouse model [71]. Several studies have used synbiotics to control, treat, and prevent mucositis. Also, they reported that synbiotic consumption in breast cancer survivors had beneficial effects on adiponectin, TNF-α, and hs-CRP [72, 73]. Trindade et al. showed that synbiotic administration (including fructooligosaccharide) could decrease mucosal damage in an experimental murine model affected by mucositis [74].
A similar trend of OM grade intensity was reported in previous studies that found it increased during the second to the fourth week from the beginning of the treatment, and after a peak, it decreased [75, 76]. Oral probiotics can potentially reduce the incidence of OM due to chemotherapy. However, clinical trials should further confirm the effectiveness of oral probiotics against side effects. The species and number of probiotics should be optimized and standardized before clinical applications [49]. Our study had limitations, including that dietary changes during treatment were not completely controllable, although patients were given detailed recommendations. Future independent studies with larger sample sizes will be necessary to confirm the findings and possibly identify confounding factors and potential side effects. Our results showed that synbiotic mouthwash decreased the peak of mean OM grade intensity. Equation 1 could be used for modeling, prediction, and comparison of various treatment modalities of OM.
Conclusion
To our knowledge, the present study is the only one that investigates the effect of the topical application of synbiotic mouthwash on the management of OM induced by radiotherapy in oral cancer patients. Our results showed that synbiotic mouthwash significantly reduces OM intensity and prevents mucositis in oral cancer patients undergoing radiotherapy. However, it is suggested that, in future studies, the mechanism of mucositis prevention by probiotic and synbiotic mouthwash with higher sample sizes be investigated.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- IMRT:
-
Intensity-modulated radiotherapy
- GRAS:
-
Generally recognized as safe
- LPS:
-
Lipopolysaccharide
- OE:
-
Oral examination
- OM:
-
Oral mucositis
- OSCC:
-
Oral squamous cell carcinoma
- p :
-
P-Value
- RCT:
-
Randomized clinical trial
- TLR2:
-
Toll-like receptor 2
- TLRs:
-
Toll-like receptors
- WHO:
-
World Health Organization
References
Ellington TD, Henley SJ, Senkomago V, O’Neil ME, Wilson RJ, Singh S et al (2020) Trends in incidence of cancers of the oral cavity and pharynx—United States 2007–2016. Morb Mortal Wkly Rep 69(15):433
Wang K, Tepper JE (2021) Radiation therapy-associated toxicity: etiology, management, and prevention. CA: A Cancer J Clin 71(5):437–54
Cruz-Gregorio A, Martínez-Ramírez I, Pedraza-Chaverri J, Lizano M (2019) Reprogramming of energy metabolism in response to radiotherapy in head and neck squamous cell carcinoma. Cancers 11(2):182
Lin A, Maity A (2015) Molecular pathways: a novel approach to targeting hypoxia and improving radiotherapy efficacy via reduction in oxygen demand. Clin Cancer Res 21(9):1995–2000
Sonis ST (2013) Oral mucositis: Springer Science & Business Media
Mallick S, Benson R, Rath G (2016) Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol 273(9):2285–2293
Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y et al (2019) A randomized, double-blind, placebo-controlled trial of s to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 125(7):1081–1090
Maria OM, Eliopoulos N, Muanza T (2017) Radiation-induced Oral Mucositis Front Oncol 7:89
Sonis ST (2017) The chicken or the egg? Changes in oral microbiota as cause or consequence of mucositis during radiation therapy. EBioMedicine 18:7–8
Vanhoecke B, De Ryck T, Stringer A, Van de Wiele T, Keefe D (2015) Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis 21(1):17–30
Thorpe D (2019) The role of mucins in mucositis. Curr Opin Support Palliat Care 13(2):114–118
Zhu X-X, Yang X-J, Chao Y-L, Zheng H-M, Sheng H-F, Liu H-Y et al (2017) The potential effect of oral microbiota in the prediction of mucositis during radiotherapy for nasopharyngeal carcinoma. EBioMedicine 18:23–31
Dawood MA, Koshio S, Abdel-Daim MM, Van Doan H (2019) Probiotic application for sustainable aquaculture. Rev Aquac 11(3):907–924
Khaneghah AM, Abhari K, Eş I, Soares MB, Oliveira RB, Hosseini H et al (2020) Interactions between probiotics and pathogenic microorganisms in hosts and foods: a review. Trends Food Sci Technol 95:205–218
Laleman I, Detailleur V, Slot DE, Slomka V, Quirynen M, Teughels W (2014) Probiotics reduce mutans streptococci counts in humans: a systematic review and meta-analysis. Clin Oral Invest 18(6):1539–1552
Koopaie M, Fatahzadeh M, Jahangir S, Bakhtiari R (2019) Comparison of the effect of regular and probiotic cake (Bacillus coagulans) on salivary pH and Streptococcus mutans count. Dental Med Problems 56(1):33–38
Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM (2018) Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J Cell Mole Med 22(3):1972–83
Nguyen T, Brody H, Lin GH, Rangé H, Kuraji R, Ye C et al (2020) Probiotics, including nisin-based probiotics, improve clinical and microbial outcomes relevant to oral and systemic diseases. Periodontology 2000 82(1):173–85
Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol 103(16):6463–6472
Ribeiro F, Rossoni R, de Barros P, Santos J, Fugisaki L, Leão M et al (2020) Action mechanisms of probiotics on Candida spp. and candidiasis prevention: an update. J Appl Microbiol 129(2):175–85
Picó-Monllor JA, Mingot-Ascencao JM (2019) Search and selection of probiotics that improve mucositis symptoms in oncologic patients. A Syst Rev Nutri 11(10):2322
Thomsen M, Clarke S, Vitetta L (2018) The role of adjuvant probiotics to attenuate intestinal inflammatory responses due to cancer treatments. Beneficial Microbes 9(6):899–916
Cereda E, Caraccia M, Caccialanza R (2018) Probiotics and mucositis. Curr Opin Clin Nutr Metab Care 21(5):399–404
Batista VL, Da Silva TF, De Jesus LCL, Dias Coelho-Rocha N, Barroso FAL, Tavares LM et al (2020) Probiotics, prebiotics, synbiotics, and paraprobiotics as a therapeutic alternative for intestinal mucositis running head: alternative treatment for intestinal mucositis. Front Microbiol 11:2246
Miknevicius P, Zulpaite R, Leber B, Strupas K, Stiegler P, Schemmer P (2021) The impact of probiotics on intestinal mucositis during chemotherapy for colorectal cancer: a comprehensive review of animal studies. Int J Mol Sci 22(17):9347
Shu Z, Li P, Yu B, Huang S, Chen Y (2020) The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis: a systematic review and meta-analysis. Oral Oncol 102:104559
Gibson RJ, Keefe DM, Lalla RV, Bateman E, Blijlevens N, Fijlstra M et al (2013) Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 21(1):313–326
Bowen JM, Gibson RJ, Coller JK, Blijlevens N, Bossi P, Al-Dasooqi N et al (2019) Systematic review of agents for the management of cancer treatment-related gastrointestinal mucositis and clinical practice guidelines. Support Care Cancer 27(10):4011–4022
Sonis ST (2021) Treatment for oral mucositis—current options and an update of small molecules under development. Curr Treat Options Oncol 22(3):1–14
Edition S, Edge S, Byrd D (2017) AJCC cancer staging manual. AJCC cancer staging manual
Association WM (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Hong CH, Gueiros LA, Fulton JS, Cheng KKF, Kandwal A, Galiti D et al (2019) Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 27(10):3949–3967
Langius JA, Zandbergen MC, Eerenstein SE, van Tulder MW, Leemans CR, Kramer MH et al (2013) Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo) radiotherapy: a systematic review. Clin Nutr 32(5):671–678
Marziliano A, Teckie S, Diefenbach MA (2020) Alcohol-related head and neck cancer: Summary of the literature. Head Neck 42(4):732–738
Ana GS, Normando AGC, de Toledo IP, Dos Reis PED, Guerra ENS (2020) Topical treatment of oral mucositis in câncer patients: a systematic review of randomized clinical trials. Asian Pacific J Cancer Prevention: APJCP 21(7):1851
Fleming M, Craigs CL, Bennett MI (2020) Palliative care assessment of dry mouth: what matters most to patients with advanced disease? Support Care Cancer 28(3):1121–1129
Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y et al (2015) Basic oral care for hematology–oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer 23(1):223–236
Organization WH (1979) WHO handbook for reporting results of cancer treatment: World Health Organization
Elad S, Cheng KKF, Lalla RV, Yarom N, Hong C, Logan RM et al (2020) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126(19):4423–4431
Triarico S, Agresti P, Rinninella E, Mele MC, Romano A, Attinà G et al (2022) Oral microbiota during childhood and its role in chemotherapy-induced oral mucositis in children with cancer. Pathogens 11(4):448
Reyes-Gibby CC, Wang J, Zhang L, Peterson CB, Do KA, Jenq RR et al (2020) Oral microbiome and onset of oral mucositis in patients with squamous cell carcinoma of the head and neck. Cancer 126(23):5124–5136
Mougeot J-LC, Stevens CB, Morton DS, Brennan MT, Mougeot FB (2019) Oral microbiome and cancer therapy-induced oral mucositis. JNCI Monographs 2019(53):lgz002
Al-Qadami G, Verma G, Van Sebille Y, Le H, Hewson I, Bateman E, et al (2022) Antibiotic-induced gut microbiota depletion accelerates the recovery of radiation-induced oral mucositis in rats. Int J Radiat Oncol* Bio* Phys. In Press (Available online 8 April 2022)
Al-Qadami G, Van Sebille Y, Bowen J, Wardill H (2022) Oral-gut microbiome axis in the pathogenesis of cancer treatment-induced oral mucositis. Front Oral Health 3(881949)
Cordeiro BF, Oliveira ER, da Silva SH, Savassi BM, Acurcio LB, Lemos L et al (2018) Whey protein isolate-supplemented beverage, fermented by Lactobacillus casei BL23 and Propionibacterium freudenreichii 138, in the prevention of mucositis in mice. Front Microbiol 9:2035
Do Carmo FLR, Rabah H, Cordeiro BF, Da Silva SH, Pessoa RM, Fernandes SOA et al (2019) Probiotic Propionibacterium freudenreichii requires SlpB protein to mitigate mucositis induced by chemotherapy. Oncotarget 10(68):7198
Wang Y, Li J, Zhang H, Zheng X, Wang J, Jia X et al (2021) Probiotic Streptococcus salivarius K12 alleviates radiation-induced oral mucositis in mice. Front Immunol 12:2107
Mirza MA, Aruna D, Irukulla M (2022) Efficacy of Bacillus clausii UBBC-07 spores in the amelioration of oral mucositis in head and neck cancer patients undergoing radiation therapy. Cancer Treatment and Research Communications. 100523
Feng J, Gao M, Zhao C, Yang J, Gao H, Lu X, et al (2022) Oral administration of probiotics reduces chemotherapy-induced diarrhea and oral mucositis: a systematic review and meta-analysis. Frontiers in nutrition. 9(823288)
Sharma A, Rath G, Chaudhary S, Thakar A, Mohanti BK, Bahadur S (2012) Lactobacillus brevis CD2 lozenges reduce radiation-and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer 48(6):875–881
Limaye SA, Haddad RI, Cilli F, Sonis ST, Colevas AD, Brennan MT et al (2013) Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer 119(24):4268–4276
Yarom N, Hovan A, Bossi P, Ariyawardana A, Jensen SB, Gobbo M et al (2020) Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines—part 2: honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Support Care Cancer 28(5):2457–2472
Xia C, Jiang C, Li W, Wei J, Hong H, Li J et al (2021) A phase II randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma. Front Immunol 12:545
Allaker RP, Stephen AS (2017) Use of probiotics and oral health. Curr Oral Health Rep 4(4):309–318
Nadelman P, Magno MB, Masterson D, da Cruz AG, Maia LC (2018) Are dairy products containing probiotics beneficial for oral health? A systematic review and meta-analysis. Clin Oral Invest 22(8):2763–2785
Sehrawat N, Yadav M, Singh M, Kumar V, Sharma VR, Sharma AK (2021) Probiotics in microbiome ecological balance providing a therapeutic window against cancer. Semin Cancer Biol 70:24–36
Galdeano CM, Cazorla SI, Dumit JML, Vélez E, Perdigón G (2019) Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 74(2):115–124
Wei D, Heus P, van de Wetering FT, van Tienhoven G, Verleye L, Scholten RJ (2018) Probiotics for the prevention or treatment of chemotherapy-or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst Rev 8:1–73
Chang C-W, Liu C-Y, Lee H-C, Huang Y-H, Li L-H, Chiau J-SC et al (2018) Lactobacillus casei variety rhamnosus probiotic preventively attenuates 5-fluorouracil/oxaliplatin-induced intestinal injury in a syngeneic colorectal cancer model. Front Microbiol 9:983
Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V et al (2019) Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut 68(6):1003–1013
Yeung C-Y, Chan W-T, Jiang C-B, Cheng M-L, Liu C-Y, Chang S-W et al (2015) Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS ONE 10(9):e0138746
Stamatova I, Meurman JH (2009) Probiotics: health benefits in the mouth. Am J Dent 22(6):329
Wada M, Nagata S, Saito M, Shimizu T, Yamashiro Y, Matsuki T et al (2010) Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support Care Cancer 18(6):751–759
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, des Varannes SB et al (2014) Systematic review: the role of the gut microbiota in chemotherapy-or radiation-induced gastrointestinal mucositis–current evidence and potential clinical applications. Alimentary Pharmacol Ther 40(5):409–21
Secombe KR, Coller JK, Gibson RJ, Wardill HR, Bowen JM (2019) The bidirectional interaction of the gut microbiome and the innate immune system: Implications for chemotherapy-induced gastrointestinal toxicity. Int J Cancer 144(10):2365–2376
Nicolatou-Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P et al (2013) Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer 21(11):3179–3189
Ariyawardana A, Cheng KKF, Kandwal A, Tilly V, Al-Azri AR, Galiti D et al (2019) Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 27(10):3985–3995
Gao Z, Guo B, Gao R, Zhu Q, Wu W, Qin H (2015) Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep 12(4):6119–6127
Oh N, Lee J, Lee J, Lee KW, Kim Y (2017) Mulberry leaf extract fermented with Lactobacillus acidophilus A4 ameliorates 5-fluorouracil-induced intestinal mucositis in rats. Lett Appl Microbiol 64(6):459–468
Quaresma M, Damasceno S, Monteiro C, Lima F, Mendes T, Lima M et al (2020) Probiotic mixture containing Lactobacillus spp. and Bifidobacterium spp. attenuates 5-fluorouracil-induced intestinal mucositis in mice. Nutrition And Cancer 72(8):1355–65
Yeung C-Y, Chiau J-SC, Cheng M-L, Chan W-T, Chang S-W, Jiang C-B, et al (2020) Immune modulation effects and safety of Lactobacillus casei variety rhamnosus in a chemotherapy-induced intestinal mucositis mouse model
Trindade L, Martins V, Rodrigues N, Souza E, Martins F, Costa G et al (2018) Oral administration of Simbioflora®(synbiotic) attenuates intestinal damage in a mouse model of 5-fluorouracil-induced mucositis. Beneficial Microbes 9(3):477–486
Lahiji MR, Zarrati M, Najafi S, Yazdani B, Cheshmazar E, Razmpoosh E et al (2021) Effects of synbiotic supplementation on serum adiponectin and inflammation status of overweight and obese breast cancer survivors: a randomized, triple-blind, placebo-controlled trial. Support Care Cancer 29(7):4147–4157
Trindade LM, Torres L, Matos ID, Miranda VC, de Jesus LCL, Cavalcante G, et al (2021) Paraprobiotic Lacticaseibacillus rhamnosus protects intestinal damage in an experimental murine model of mucositis. probiotics and antimicrobial proteins. 1–13
Ingrosso G, Saldi S, Marani S, Wong AY, Bertelli M, Aristei C et al (2021) Breakdown of symbiosis in radiation-induced oral mucositis. Journal of Fungi 7(4):290
Sonis ST (2021) A hypothesis for the pathogenesis of radiation-induced oral mucositis: when biological challenges exceed physiologic protective mechanisms. Implications for pharmacological prevention and treatment. Supportive Care in Cancer. 1–9
Author information
Authors and Affiliations
Contributions
MK and SM conceived the study idea and led data collection. MK, ZM, and SM created the study protocol and wrote the original draft. MK, ZM and SK contributed to data analysis/interpretation and preparation of the manuscript. MK, SM, and ZM led the writing—review and editing. MK, ZM, and SK interpreted the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Before starting radiotherapy, the purpose of the study and its steps were explained in both case and control groups. After obtaining the patient’s informed consent in accordance with the ethics committee requirements at the psarticipating institutes and the Declaration of Helsinki, the study was started. This study was approved by the Tehran University of Medical Sciences Ethical Committee (Ethical code: IR.TUMS.DENTISTRY.REC.1399.176), and this study was registered in the Clinical Trial Registration Center of Iran (IRCT code: IRCT20201106049288N1). All methods were performed under the relevant guidelines and regulations, and this study was conducted in accordance with the Declaration of Helsinki [31].
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manifar, S., Koopaie, M., Jahromi, Z.M. et al. Effect of synbiotic mouthwash on oral mucositis induced by radiotherapy in oral cancer patients: a double-blind randomized clinical trial. Support Care Cancer 31, 31 (2023). https://doi.org/10.1007/s00520-022-07521-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-022-07521-5