Abstract
Objectives
Oral mucositis (OM) is a common debilitating complication of chemoradiotherapy treatment of head and neck cancers. This randomized placebo-controlled double-blind clinical trial study was performed to evaluate the effectiveness of Zataria multiflora (ZM) extract mouthwash in the prevention and reduction of OM related to local radiotherapy in the treatment of head and neck cancer patients.
Methods
Sixty-three patients with head and neck cancers, who underwent a conventional fractionated radiotherapy regimen, were entered into the study. Patients gargled the ZM mouthwash or a placebo before the beginning of the treatment three times daily and before each radiotherapy session. The assessment of OM was conducted according to WHO and Oral Mucositis Assessment Scale.
Results
The OM intensity trends in the ZM group during these weeks of treatment were detected 3.152 times less frequently than in the placebo group. A twofold decrease in the incidence of grades 3–4 OM was observed in the ZM group compared to the placebo. The use of the ZM mouthwash affected the incidence of grades 3–4 OM to a relative risk ratio of 0.432. The pain score was significantly decreased in the ZM group compared to the placebo group.
Conclusion
The present study revealed that ZM mouthwash effectively decreases the severity of OM and mouth pain in patients with head and neck cancer treated with radiotherapy.
Clinical relevance
The use of ZM mouthwash effectively decreases the severity of oral complications induced by ionizing radiation in patients during radiotherapy and resulted in high oral quality care.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of cancer is rapidly increasing in the world, and is recognized as the second leading cause of death in developed countries. Head and neck cancers (HNC) account for about 4% of all malignancies [1]. Fractionated radiotherapy concurrent with chemotherapy is the standard treatment for most HNC patients, as it destroys the malignant cells by intervening in their proliferation and survival [2]. However, ionizing radiation has adverse effects on normal tissues. Those cells with higher proliferation rates (hematopoietic, epithelial, and endothelial cells) are more sensitive to the damaging effects of radiation [3]. Even though the combination of radiotherapy and chemotherapy has the best overall outcome, it also significantly increases the toxicity of the treatment, especially oral mucositis (OM) [1]. The severe and debilitating pain resulting from OM considerably increases cancer-related morbidity. There is an increased need for opioid analgesics due to the severe and intractable pain caused by these lesions, as well as the need for parenteral or enteral nutrition for patients who cannot obtain nutrition orally [4]. Severe mucositis may affect the patient’s treatment plan by necessitating a break in the treatment schedule and increasing infection susceptibility and finally reducing the length of patient survival [5,6,7], and mucositis can cause symptoms and complaints such as nausea, vomiting, and pain, as well as reduce the quality of life for patients by leading to sleep disorders, anorexia, and weight loss [5,6,7]. Severe mucositis can increase the length of hospitalization, the need for specialized interventions, and the associated costs [5,6,7,8]. Although there is no general consensus on the optimal treatment for and prevention of OM, the currently recommended measures include an emphasis on oral hygiene, the use of various mouthwashes, local anesthetics (such as lidocaine, magnesium-containing antacids, diphenhydramine, and sucralfate), sucking ice, growth factors, as well as non-steroidal and steroidal anti-inflammatory agents [6].
As mentioned previously, although several approaches to reducing radiation toxicity exist, it is probable that no single strategy is sufficient to eliminate complications.
However, current measures have not had a considerable therapeutic effect and sometimes cause adverse effects for patients. Considering the importance of this issue for patients’ well-being, it is necessary to identify the most effective strategy with the least complications for the prevention and control of OM, which is an important step toward changing the severity of this complication. To this end, the use of natural products is being proposed as a potential strategy for ameliorating OM severity.
Zataria multiflora Boiss (Lamiaceae; Avishan-e-Shirazi (Shirazi thyme)) (ZM) [9] has a long history of medicinal use via decoction and vapor in aboriginal areas, and it has been traditionally used as a carminative, stimulant, diaphoretic, diuretic, antiseptic, anesthetic, antispasmodic, antihermitic, antidiarrheal, and analgesic [9]. Although ZM is containing thymol, carvacrol, zatrinal, oleanolic acid, betulic acid, rosmarinic acid, and monoterpenoids such as sesquiter-penoids, p-cymene, and y-terpinene, the biological effects of ZM are mainly associated to its phenolic compounds, especially thymol and carvacrol [10]. Several studies have shown that ZM essential oil and hydroalcoholic extract contain a large amount of thymol and carvacrol [11,12,13]. In previous studies, the preventive effect of ZM on the genotoxic effects induced by ionizing radiation was examined on normal human lymphocytes in vitro. The results revealed that lymphocytes treated with ZM had a significant reduction in the incidence of DNA damage [14].
While recent studies have shown the chemoprotective/radioprotective properties of ZM, the current randomized double-blind clinical trial study assessed the efficacy of ZM mouthwash in reducing the incidence of OM in HNC patients undergoing radiotherapy.
Material and methods
Patients
Participants were among all patients with head and neck cancer who were referred to Shahid Rajaee Hospital in Babolsar and Imam Khomeni Hospital in Sari (both in Iran). Sixty-three patients who had the inclusion criteria were entered into the study after the goals of the study were explained to them; they had signed the consent form, and it was clarified to them that they can refuse to participate at any time. The inclusion criteria involved receiving a planning target volume of the whole oropharynx, naopharynx, and oral cavity at least 3 cm anterior to the retromolartrigone (more than one third of the oral cavity) in the primary beam, a total radiotherapy dose of at least 6000 cGy (200 cGy daily for 5 days per week), and having a Karnofsky Performance Status (KPS) greater than 70. They were treated with radiotherapy alone or radiotherapy with chemotherapy. They are recruited before the simulation.
The exclusion criteria included the following cases: a history of head and neck radiotherapy, poor oral hygiene, suffering from active infectious lesions of the mouth, under the age of 18 years or over the age of 70 years, as well as having liver, kidney, or diabetes problems, autoimmune diseases, any systemic diseases interfering with healing, and a history of allergies to ZM and plants of the Lamiaceae family. Anti-inflammatory and analgesics as standard of care were given to both arms if they were needed.
Our study was performed in accordance with the precepts established by the Declaration of Helsinki. This protocol was approved by the Ethical and Research Committee of Mazandaran University of Medical Sciences (code 92-48). This study was registered in the Iranian Registry of Clinical Trials with the IRCT number IRCT201305285830N3.
Study design
The study was a randomized double-blind clinical trial. Patients were randomly (using the balance block method) divided into two groups: intervention and control [2]. Mouthwashes were numbered 1 to 70. The intervention group used mouthwash containing ZM extract, and the control group received mouthwash as a placebo without ZM. The medicinal mouthwash bottles were designed as double blind. One radiotherapist gave numbered mouthwashes to patients and evaluated them. Both patients and researchers did not know the contents of mouthwashes.
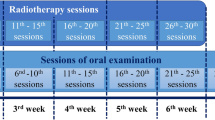
Patients received radiotherapy or chemoradiotherapy for treatment. They were treated with a 6 MV photon beam Siemens Primus linear accelerator (Siemens Primus German) at 100-cm source-to-surface distance (SSD). For treatment, two parallel-opposed lateral fields and a low anterior neck field were used daily with an average dose of 200 cGy and a total dose of 6000 to 7000 cGy in 30 to 35 sessions. The spinal cord dose should be less than 4600 cGy, and shielding blocks were used as needed.
The chemoradiotherapy group in both arms, placebo and ZM groups, received cisplatin (100 mg/m2) with or without 5-fluorouracil (1000 mg/m2) during the first 3 days of the first week of treatment, and then, they received it every 3 weeks during the course of treatment. Oral examination was performed on patients before the start of the treatment and weekly during the treatment course. Monitoring is only up to the last of radiotherapy (RT). Questionnaires were filled out by the study researchers through interviews with the patients and reviewing of the completed records (after providing an explanation of the study to the patient). Moreover, guide and record forms for making note of the times that the mouthwash was used, as well as a soft toothbrush, toothpaste, and mouthwash solution were delivered to each of the subjects. In addition to oral hygiene, patients were also encouraged to gargle the mouthwash 5 mL (concluded 136 mg ZM extract and 0.816 mg thymol) for 1 min—three times a day (after breakfast, after lunch, and before bedtime after brushing) and also once half an hour before each radiotherapy session. After gargling and spitting, the subjects were instructed not to wash their mouth with water for up to an hour, to avoid eating or drinking and to mark the time on the record form. This was maintained until 7 weeks after the conclusion of the treatment. They were also forbidden to smoke or to eat spicy, hot, or very cold foods. The taste, smell, and shape of the ZM and placebo mouthwash were the same in this double-blind study. In both groups, OM status and pain scores were examined and followed weekly from the first until the last session of radiotherapy.
Patient assessment
The severity of OM was assessed based on the World Health Organization (WHO) mucositis scale [15] and the Oral Mucositis Assessment Scale (OMAS).
WHO scale defines the state of the mucositis injuries according to their severity (scale 0–4): grade 0, absence of symptoms; grade 1, soreness and erythema, no further symptoms; grade 2, ulcers present, but solid diet possible; grade 3, only liquids can be swallowed; and grade 4, oral alimentation impossible.
As previously verified, the OMAS is a reliable and valid method to assess tissue ulcers [16]. Using this methodology, the oral cavity is divided into nine regions: upper and lower lips, right and left buccal mucosa, right and lateral tongue, left ventral and lateral tongue, floor of the mouth, and the soft and hard palate. Each region is assessed and rated in terms of erythema and ulceration. If the size of the ulcer is less than 1 cm, between 1 and 3 cm, or larger than 3 cm, the corresponding scores are 0, 2, and 3, respectively. In terms of erythema, if the mucosa is normal, moderate, or severe, the scores are 0, 1, and 2, respectively. The maximum score is 45, and the minimum is 0.
A researcher and a consultant who were experts in oncology and radiotherapy measured OM weekly, and six scores were calculated for each patient throughout the 7 weeks. A modified visual analog scale (VAS) for pain assessment was used [17].
Required opioid analgesic, anti-inflammatory drug and antibiotics, and body weight were assessed weekly from the first day of treatment.
Preparation of plant extract
Dried aerial parts of ZM were collected in the flowering season at the end of June 2012 and brought from the city of Firozabad in the Fars province of Iran (28.8194258° N, 52.5518705° W). This plant was approved by a senior pharmacognosist (Herbarium number F-1-8-4-21). Homogenous powder (1 mm in diameter mesh no. 18) was macerated in 70% ethanol for 48 h (1:10 w/v), after which the hydro alcoholic extract of dried ZM was processed by removing the solvent using a rotary (Heidolph, Germany). The ZM extract was standardized based on thymol as the major active ingredient according to our previous reports [18, 19].
Mouthwash preparation
Various formulations for mouthwash were made through keeping the pharmaceutical compounds and changing the components to obtain the best formulation. The best formulation for providing mouthwash with 2.72% ZM dried extract (W/V) was adjusted as water 50%, polyethylene glycol 25%, and glycerin 25%. Other ingredients added to the mouthwash were Tween 80 (4.7%), methyl paraben (0.1%), propyl paraben (0.01%), and sodium saccharin (0.1%). The ZM mouthwash was processed through several stages. First, 60 g of dried ZM extract was added to 500 ml of polyethylene glycol, and then 500 ml of glycerin followed by 2 g methyl paraben and 0.2 g propyl paraben. After that, the mixture was kept in a shaker for 24 h to form a homogeneous suspension, after which 100 g Tween 80 and 2 g sodium saccharin were added to the materials being mixed. Finally, 1000 ml water was slowly added to the mixture. All of the containers were sterilized before use, and the mouthwash was prepared at room temperature. The placebo contained all of the aforementioned ingredients except for the ZM extract. The researcher tested the smell and the taste of the ZM mouthwash and the placebo for sustainability.
Statistical analysis
The Student’s t test and repeated measures were applied to evaluate OM and pain score. The chi-squared test, Mann-Whitney U test, and Fisher exact test were used to evaluate the baseline characteristics of the patients, required analgesic, weight loss, and required antibiotics. Kolomogrov Smirnov (KS) was used for normality test in each group.
Analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). A P value less than 0.05 was considered significant.
Result
Overall assessment
The ZM extract was standardized based on thymol content. The thymol content was 6 mg/g in ZM extract [18, 19].
This study was as treated analysis. Sixty-three patients were included in this study and divided randomly into two groups. Five patients from the placebo group were excluded—two had severe mucositis and were treated with other drugs, one left the study, one passed away in the middle of treatment, and one used the drug irregularly. Six patients from the ZM group were excluded—one had severe mucositis and wanted to be treated with other drugs, one left the study, and four used the drug irregularly. Finally, statistical analysis was performed on 52 patients—25 in the ZM group and 27 in the placebo group (Fig. 1). There were no differences between the two groups in sex, age, BMI (body mass index), or education in the beginning of the study (Table 1).
Weight loss and the need for analgesics and antibiotics were significantly lower in the ZM group compared to the placebo group (Table 2).
Evaluation of OM
Assessment of OM was done according to WHO and OMAS criteria at the end of each week from the beginning of treatment in both groups. According to the OMAS criteria, the results of inter-group comparison showed that OM in placebo group (P = 0.007) and ZM group (P = 0.000) was significantly different in weeks of treatment and the results of comparison between two groups showed that OMAS scores were significantly lower in the ZM group compared to the placebo group from the first to the sixth week of the study. The mean of the OMAS scores in week 6 was 10.00 ± 8.45 in the placebo group versus 4.96 ± 7.85 in the ZM group, and a twofold (50%) decrease in OM intensity was observed in the ZM group (Table 3).
According to the repeated measures ANOVA, the differences between the OMAS of the ZM versus placebo groups during the weeks of evaluation were statistically significant, and OM intensity was significantly decreased in the ZM group compared to the placebo group. The incidence of OM in the ZM group was detected 3.152 times less than in the placebo group (Fig. 2). For example, these trends in OM intensity were observed in randomly selected patients’ oral images in weeks 3 and 6 in the ZM and placebo groups.
According to the WHO criteria, the results of inter-group comparison showed that OM in placebo group (P = 0.000) and ZM group (P = 0.000) were significantly different in weeks of treatment and the results of comparison between two groups showed that the incidence of OM in the ZM group was significantly lower than in the placebo group at weeks 3, 4, and 6 of the study (Table 3). The mean of the WHO scores in week 6 was 2.03 ± 1.31 in the placebo group compared to 1.28 ± 1.27 in the ZM group, and a twofold decrease was observed in the WHO scores of the ZM group. The OMAS criteria results were equivalent. Moreover, a twofold decrease in the incidence of grades 3–4 OM was detected in the ZM group compared to the placebo (24 versus 55.5%). Finally, ZM influenced the incidence of grades 3–4 OM to a relative risk ratio of 0.432 (95% confidence interval [CI] 0.199–0.938). The repeated measures ANOVA showed that OM intensity during the weeks of evaluation was significantly decreased in the ZM group compared to the placebo group, and the incidence of OM in the ZM group was detected 0.540 times less than in the placebo group (Fig. 3).
Assessment of oral pain intensity
The results of inter-group comparison showed that pain score in placebo group (P = 0.000) and ZM group (P = 0.003) were significantly different in weeks of treatment. Patients in the ZM group had a lower pain score than the placebo group at weeks 2 to 6 of treatment. The repeated measures ANOVA showed that pain intensity during the weeks of evaluation was significantly decreased in the ZM group compared to the placebo group and the pain scores in the ZM group were detected 0.961 times less than in the placebo group. The mean of the pain scores in week 6 was 2.77 ± 2.59 in the placebo group compared to 0.92 ± 2.17 in the ZM group, and a threefold decrease in pain scores in the ZM group was observed (Fig. 4).
Discussion
Our study showed that ZM can reduce the incidence of OM in HNC patients treated with radiotherapy. Patients using ZM had a significantly lower OM intensity than those in the placebo group from weeks 1 to 6 of treatment. The pain score, the weight loss, and the need for analgesics and antibiotics were significantly lower in the ZM group compared to the placebo group. The results of OMAS scale and WHO scale are approximately the same. Because in OMAS scale, observer measure OM is according to the presence of erythema and ulceration, but in WHO, observer measure is not only according to the presence of erythema and ulceration but also to the patient’s capacity to eat; our results in these two methods were slightly different.
Nevertheless, OM remains among the most intolerable complications of radiotherapy treatment for HNC patients. It causes pain, difficulties with swallowing, weight loss, and treatment interruptions [20].
A number of locally and systemically applied agents, as well as new and unproved agents, have been investigated for the purpose of decreasing or preventing radiotherapy-induced OM. These include antibiotics, anti-inflammatory agents, cytokines, mouth-coating agents, vitamins, anticholinergic agents, antioxidants, antiviral agents, immunomodulatory drugs, amino acids, angiogenesis inhibitors, cytoprotectors, hormones, and other modalities. Nonetheless, there is no widely accepted prophylaxis or effective treatment available for this complication [5]. Several studies performed on natural product for preventing or reducing chemotherapy and radiotherapy-induced oral mucositis. The clinical results of these natural products were presented in a literature review [21]. Calendula officinalis mouthwash significantly decreased the intensity of OM compared to placebo at week 2 (score 5.5 vs. 6.8), week 3 (score 8.25 vs. 10.95), and week 6 (score 11.4 vs. 13.35). The antioxidant property was the main proposed mechanism of this natural product for radioprotection [2]. Mouthwash containing 1% chamomilla recuita extract reduced incidence, intensity, and duration of mucositis in patients undergoing hematopoietic stem cell transplantation. This natural product prevented OM by the anti-inflammatory activity, inhibiting leukotriene synthesis and antioxidative effects [22]. Oral mucositis rates and severity after 2 weeks in patients during chemotherapy were significantly lower in the olive leaf extract treatment compared to the placebo group. The IL-1β and TNF-α levels were significantly decreased in the olive leaf extract group compared to the placebo group [23]. Oral topical application of Glycyrrhiza glabra reduced intensity of radiation and chemotherapy-induced mucositis in patients. Glycyrrhiza glabra has antiulcer, anti-inflammatory, and skin regeneration activities [24]. Topical gel, containing Curcuma longa, effectively reduced the oral symptoms of mucositis in patients undergoing head and neck cancer. Oral lesions in case group were significantly smaller than that control group. Curcuma longa can inhibit generation of superoxide anion and hydroxyl radicals [25]. Indigowood root reduced oral mucositis, anorexia, and swallowing difficulty induced by radiation in patients. This natural product has anti-inflammatory ability to reduce the mucosal damage caused by radiation [26]. Amifostine is the only drug that has been approved by the FDA for decreasing the occurrence of xerostomia in HNC patients treated with radiotherapy [27]. However, this drug causes adverse effects that lead to usage difficulties and is also expensive and only available as an injection [28].
The beneficial effects of ZM for antinociceptive [29], anti-inflammatory [30], antioxidant [14], immunostimulatory [31], antiulcerogenic [32], and antiaphthous activity have been demonstrated [33]. Many of the beneficial activities reported for ZM are mainly related to the contents of various secondary metabolites such as thymol and carvacrol that are phenolic compounds [9]. In this study, we showed that ZM significantly reduces radiotherapy-induced OM in HNC patients. The high thymol and carvacrol contents in ZM and its antioxidant activity support its protective effect in radiotherapy-induced OM. In the initial stages of OM, irradiation or chemotherapy produces free radicals and reactive oxygen species (ROS) that harmfully influence the cells and strands of DNA in the basal epithelium and the submucosa, which causes the lesions. This is a critical first step in the development of OM [34]. The chemical composition of ZM reveals several phenolic compounds such as thymol, hydroxybenzoic acid, and cymene [13]. Thymol provides a protective effect against radiation-induced oxidative stress and lipid peroxidation. This protective effect is mainly due to its antioxidant activity. Considering the antioxidant properties of ZM [9, 11, 14, 35], it may act against ROS and prevent or delay the initiation phase. In the next stage of OM, in addition to free radicals and ROS, damaged cells start a waterfall of reactions and pro-inflammatory cytokines lead to lesions and basal cell apoptosis. These products strengthen the lesions [34]. Recent studies reported that ethanolic extracts from the aerial parts of ZM provided protection against acute and, more specifically, chronic inflammation [30, 36]. The anti-inflammatory properties of ZM play a role in its protective effect in OM induced by radiotherapy. In the third stage of OM induced by irradiation or chemotherapy, painful lesions appear and are colonized by bacteria. Bacterial colonization can lead to the release of new pro-inflammatory cytokines [34].
Previously, we investigated the cell killing effect of ZM extract on cell death induced by ionizing radiation in human glioblastoma cell line (A172) and human non-malignant fibroblasts (HFFF2) in vitro. ZM exhibited a synergistic effect on cell killing by ionizing radiation on cancer cells. It was not observed any statistically significant difference in non-malignant HFFF2 cell growth in ZM alone and with radiation. It seems that ZM has a selective radiosensitive effect on cancer cell and radioprotective effect on normal cell [19].
The results of our study showed that the need for antibiotics and analgesics was significantly lower in the ZM group compared to the placebo group, and patients in the ZM group had a lower pain score than the placebo group at weeks 2 to 6 of treatment. Recent studies have demonstrated that ZM oil provides beneficial effects in the treatment of recurrent aphthous stomatitis and also provides pain-relieving effects [33, 37]. Several studies have described the antifungal activity of ZM oil against Candida species [38]. The antibacterial effects of ZM were tested on different strains of bacteria. Researchers reported that this plant is a good source of oxygenated monoterpenes, particularly thymol and carvacrol, and possesses significant antimicrobial and antifungal properties [9].
The antibacterial and antifungal properties of ZM support its protective effect in radiotherapy-induced OM. Our results revealed that ZM significantly delayed the onset and reduced the severity of radiotherapy-induced complication. Moreover, ZM-medicated mouthwash is both inexpensive and easy to administer, and all patients were able to tolerate it without any significant side effects.
We have some limitation in our study. Our study was unable to measure and compare the possible follow-up with patients after the conclusion of the radiotherapy treatment, and because of this, we could not measure the effect of ZM in patient survival.
Conclusion
In this clinical trial study, we showed that the incidence of OM in patients using ZM-medicated mouthwash was significantly lower than in the placebo group at 6 weeks into the treatment. Throughout the weeks of treatment, the OM intensity trends in the ZM group were lower than those in the placebo group, and a 50% decrease in OM was observed in patients treated with the ZM mouthwash compared to control group.
Ultimately, ZM decreased the pain score, weight loss, and need for analgesics and antibiotics during the radiotherapy treatment. The usage instructions of this medication are simple: gargle three times per day. More investigations are recommended to clarify the optimal dose and administration frequency to have better outcomes in radiotherapy-induced OM.
Limitation: Because there are just two radiotherapy centers in our state, patients are from different cities and they went to their cities after their treatment finished, and the limitation of our study was that patients following after the end of their treatment was not possible, and we could not assess the long outcomes.
Heading
Zataria extract mouthwash reduced oral mucositis induced by radiotherapy in patients.
Zataria extract mouthwash reduced oral pain induced by radiotherapy in patients.
The use of Zataria extract mouthwash resulted in less needed antibiotics and analgesia.
Abbreviations
- OM:
-
Oral mucositis
- ZM :
-
Zataria multiflora
- HNC:
-
Head and neck cancers
- FDA:
-
Food and Drug Administration
- KPS:
-
Karnofsky Performance Status
- SSD:
-
Source-to-surface distance
- WHO:
-
World Health Organization
- OMAS:
-
Oral Mucositis Assessment Scale
- VAS:
-
Visual analog scale
- HPLC:
-
High-performance liquid chromatography
- ANOVA:
-
Analysis of variance
- SPSS:
-
Statistical Package for the Social Sciences
References
Rodríguez-Caballero A, Torres-Lagares D, Robles-García M, Pachón-Ibáñez J, González-Padilla D, Gutiérrez-Pérez JL (2012) Cancer treatment-induced oral mucositis: a critical review. Int J Oral Maxillofac Surg 41(2):225–238. https://doi.org/10.1016/j.ijom.2011.10.011
Babaee N, Moslemi D, Khalilpour M, Vejdani F, Moghadamnia Y, Bijani A, Baradaran M, Kazemi MT, Khalilpour A, Pouramir M, Moghadamnia AA (2013) Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: a randomized controlled clinical study. Daru 21(1):18. https://doi.org/10.1186/2008-2231-21-18
Eilers J, Million R (2011) Clinical update: prevention and management of oral mucositis in patients with cancer. Semin Oncol Nurs 27(4):e1–16. https://doi.org/10.1016/j.soncn.2011.08.001
Bardy J, Molassiotis A, Ryder WD, Mais K, Sykes A, Yap B, Lee L, Kaczmarski E, Slevin N (2012) A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br J Oral Maxillofac Surg 50(3):221–226. https://doi.org/10.1016/j.bjoms.2011.03.005
Ertekin MV, Koc M, Karslioglu I, Sezen O (2004) Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys 58(1):167–174. https://doi.org/10.1016/S0360-3016(03)01562-1
Plevova P (1999) Prevention and treatment of chemotherapy- and radiotherapy-induced oral mucositis: a review. Oral Oncol 35(5):453–470. https://doi.org/10.1016/S1368-8375(99)00033-0
Quinn B, Potting CM, Stone R, Blijlevens NM, Fliedner M, Margulies A, Sharp L (2008) Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplant patients. Eur J Cancer 44(1):61–72. https://doi.org/10.1016/j.ejca.2007.09.014
Charalambous M, Raftopoulos V, Lambrinou E, Charalambous A (2013) The effectiveness of honey for the management of radiotherapy-induced oral mucositis in head and neck cancer patients: a systematic review of clinical trials. Eur J Integ Med 5(3):217–225. https://doi.org/10.1016/j.eujim.2013.01.003
Sajed H, Sahebkar A, Iranshahi M (2013) Zataria multiflora Boiss. (Shirazi thyme)—an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol 145(3):686–698. https://doi.org/10.1016/j.jep.2012.12.018
Alizadeh NS, Khoei EM, Fazelimanesh M, Astaneh A (2009) Antibacterial effects of Zataria multiflora boiss (shirazi avishan extract) on urinary tract escherichia coli infections. Res J Biol Sci 4:891–894
Shaiq Ali M, Saleem M, Ali Z, Ahmad VU (2000) Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry 55(8):933–936. https://doi.org/10.1016/S0031-9422(00)00249-1
Shafiee A, Javidnia K (1997) Composition of essential oil of Zataria multiflora. Planta Med 63(4):371–372. https://doi.org/10.1055/s-2006-957707
Ebrahimzadeh H, Yamini Y, Sefidkon F, Chaloosi M, Pourmortazavi SM (2003) Chemical composition of the essential oil and supercritical CO2 extracts of Zataria multiflora Boiss. Food Chem 83(3):357–361 https://doi.org/10.1016/S0308-8146(03)00096-7
Hosseinimehr SJ, Mahmoudzadeh A, Ahmadi A, Ashrafi SA, Shafaghati N, Hedayati N (2011) The radioprotective effect of Zataria multiflora against genotoxicity induced by gamma irradiation in human blood lymphocytes. Cancer Biother Radiopharm 26(3):325–329. https://doi.org/10.1089/cbr.2010.0896
Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, Jvd Z, van Putten WLJ, van Rhoon GC, van Dijk JDP, González DG, Princess Margaret Hospital/Ontario Cancer I, Liu F-F, Goodman P, Sherar M (1996) Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. Int J Radiat Oncol Biol Phys 35(4):731–744. https://doi.org/10.1016/0360-3016(96)00154-X
Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH Jr, Mulagha MT, Peterson DE, Rose AH, Schubert MM, Spijkervet FK, Wittes JP (1999) Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer 85(10):2103–2113. https://doi.org/10.1002/(SICI)1097-0142(19990515)85:10<2103::AID-CNCR2>3.0.CO;2-0
Svanberg A, Ohrn K, Birgegard G (2015) Caphosol((R)) mouthwash gives no additional protection against oral mucositis compared to cryotherapy alone in stem cell transplantation. A pilot study. Eur J Oncol Nurs 19(1):50–53. https://doi.org/10.1016/j.ejon.2014.07.011
Aghamohammadi A, Azadbakht M, Hosseinimehr SJ (2016) Quantification of thymol content in different extracts of Zataria multiflora by HPLC method. Pharm. Biomed Res 2(1):8–13
Aghamohammadi A, Hosseinimehr SJ, Ghasemi A, Azadbakht M, Pourfallah TA (2015) Radiosensitization effects of a Zataria multiflora extract on human glioblastoma cells. Asian Pac J Cancer Prev 16(16):7285–7290. https://doi.org/10.7314/APJCP.2015.16.16.7285
CK S, Mehta V, Ravikumar L, Shah R, Pinto H, Halpern J, Koong A, Goffinet D, Le QT (2004) Phase II double-blind randomized study comparing oral aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms. Int J Radiat Oncol Biol Phys 60(1):171–177. https://doi.org/10.1016/j.ijrobp.2004.02.012
Aghamohamamdi A, Hosseinimehr SJ (2016) Natural products for management of oral mucositis induced by radiotherapy and chemotherapy. Integr Cancer Ther 15(1):60–68. https://doi.org/10.1177/1534735415596570
Braga FT, Santos AC, Bueno PC, Silveira RC, Santos CB, Bastos JK, Carvalho EC (2015) Use of Chamomilla recutita in the prevention and treatment of oral mucositis in patients undergoing hematopoietic stem cell transplantation: a randomized, controlled, phase II clinical trial. Cancer Nurs 38(4):322–329. https://doi.org/10.1097/NCC.0000000000000194
Ahmed KM (2013) The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer. A randomized clinical trial. Saudi Dent J 25(4):141–147. https://doi.org/10.1016/j.sdentj.2013.09.001
Das D, Agarwal S, Chandola H (2011) Protective effect of Yashtimadhu (Glycyrrhiza glabra) against side effects of radiation/chemotherapy in head and neck malignancies. Ayu 32(2):196–199. https://doi.org/10.4103/0974-8520.92579
Mansourian A, Amanlou M, Shirazian S, Jahromi ZM, Amirian A (2015) The effect of “Curcuma Longa” topical gel on radiation-induced oral mucositis in patients with head and neck cancer. Inter. J Radiat Res 13(3):269–274
You WC, Hsieh CC, Huang JT (2009) Effect of extracts from indigowood root (Isatis indigotica Fort.) on immune responses in radiation-induced mucositis. J Altern Complemt Med 15(7):771–778. https://doi.org/10.1089/acm.2008.0322
Lindegaard JC, Grau C (2000) Has the outlook improved for amifostine as a clinical radioprotector? Radiother Oncol 57(2):113–118. https://doi.org/10.1016/S0167-8140(00)00235-8
Mell LK, Movsas B (2008) Pharmacologic normal tissue protection in clinical radiation oncology: focus on amifostine. Expert Opin Drug Metab Toxicol 4(10):1341–1350. https://doi.org/10.1517/17425255.4.10.1341
Roozbahani N, Jabbari Z, Yazdi S (2006) The comparison of Shirazi Thymus Vulgaris and Mefenamic acid effects on primary dysmenorrhea. Arak Med Univ J 8(3):23–27
Jaffary F, Ghannadi A, AS POUSH (2000) Antiinflammatory activity of Zataria multiflora Boiss. J Res Med Sci 5(4):3538
Khosravi A, Franco M, Shokri H, Yahyaraeyat R (2007) Evaluation of the effects of Zataria multiflora, Geranium pelargonium, Myrthand Lemonessences on immune system function in experimental animals. J Vet Res 62(4):119–123
Minaiyan M, Ghannadi A, Salehi E (2005) Antiulcerogenic effect of Zataria multiflora Boiss. on cysteamine induced duodenal ulcer in rats. Iran. J Pharm Sci 1(4):223–229
Mansoori P, Ghavami R, Shafiei A (2002) Clinical evaluation of Zataria multiflora essential oil mouthwash in the management of recurrent aphthous stomatitis. DARU J Pharmaceutical Sci 10(2):74–77
Bensadoun RJ, Le Page F, Darcourt V, Bensadoun F, Ciais G, Rostom YA, Poissonnet G, Dassonville O, Demard F (2006) Radiation-induced mucositis of the aerodigestive tract: prevention and treatment. MASCC/ISOO mucositis group’s recommendations. Bull Cancer 93(2):201–211
Ali Muhammad S, Saleem M, Ahmad Viqar U (1999) Zatatriol: a new aromatic constituent from Zataria multiflora. Zeitschrift für Naturforschung B 54(6). https://doi.org/10.1515/znb-1999-0616
Hosseinzadeh H, Ramezani M, Salmani G (2000) Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol 73(3):379–385. https://doi.org/10.1016/S0378-8741(00)00238-5
Jafari S, Amanlou M, Borhan-Mojabi K, Farsam H (2003) Comparartive study of Zataria multiflora and Anthemis nobelis extracts with Myrthus communis preparation in the treatment of recurrent aphthous stomatitis. DARU J Pharmaceutical Sciences 11(1):23–27
Khosravi AR, Shokri H, Tootian Z, Alizadeh M, Yahyaraeyat R (2009) Comparative efficacies of Zataria multiflora essential oil and itraconazole against disseminated Candida albicans infection in BALB/c mice. Braz J Microbiol 40(3):439–445. https://doi.org/10.1590/s1517-83822009000300003
Funding
This study was supported by a grant from Mazandaran University of Medical Science, Sari, Iran.
Author information
Authors and Affiliations
Contributions
1. The conception and design of the study, or acquisition of data, or analysis and interpretation of data: Seyed Jalal Hosseinimehr, Azar Aghamohammadi, Daryush Moslemi, Jafar Akbari, Mohammad Azadbakht, Arash Ghesmi, and Askari Asgharpour.
2. Drafting the article or revising it critically for important intellectual content: Seyed Jalal Hosseinimehr, Azar Aghamohammadi, and Daryush Moslemi.
3. Final approval of the version to be submitted: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of research committee of Mazandaran University of Medical Sciences and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This protocol was approved by the Ethical and Research Committee of Mazandaran University of Medical Sciences (code 92-48). This study was registered in the Iranian Registry of Clinical Trials with the IRCT number IRCT201305285830N3.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Aghamohammadi, A., Moslemi, D., Akbari, J. et al. The effectiveness of Zataria extract mouthwash for the management of radiation-induced oral mucositis in patients: a randomized placebo-controlled double-blind study. Clin Oral Invest 22, 2263–2272 (2018). https://doi.org/10.1007/s00784-017-2324-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2324-7