Abstract
Background
Chemotherapy-induced nausea vomiting (CINV) is a common and significant problem in oncology patients and rated as one of cancer chemotherapy’s most distressing side effects. The objectives of this study are to describe the incidence of CINV in highly and moderately emetogenic chemotherapy-treated patients and the prescribing pattern of CINV prophylaxis.
Methods
This retrospective, cross-sectional single-center study randomly collected data on demographics, CINV episodes, and prescribing patterns for adult oncology patients receiving intravenous highly or moderately emetogenic chemotherapy (HEC/MEC) between January and December 2019.
Results
A total of 419 randomly selected records of HEC/MEC recipients with 2388 total chemotherapy cycles were included. The mean age was 53.6 ± 12.6 years old. The majority was female (66%), Malay (54.4%), diagnosed with cancer stage IV (47.7%), and with no comorbidities (47%). All patients were prescribed with IV granisetron and dexamethasone before chemotherapy for acute prevention, whereas dexamethasone and metoclopramide were prescribed for delayed prevention. Aprepitant was not routinely prescribed for the prevention of CINV. CINV incidence was 57% in the studied population and 20% in the total cycle. This study found a significant association between CINV incidence with performance status and cisplatin-based chemotherapy (OR = 3.071, CI = 1.515–6.223, p = 0.002; OR = 4.587, CI = 1.739–12.099, p = 0.02, respectively).
Conclusion
CINV incidence was rather high per patient but relatively low per cycle. Most patients were prescribed with dual regimen antiemetic prophylaxis.
Impact
This study provides evidence that there was suboptimal use of recommended agents for CINV, and there is a clear need for further improvements in CINV management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea vomiting (CINV) is a common and significant problem in oncology patients. CINV is rated as one of cancer chemotherapy’s most distressing side effects [1, 2]. It has a major impact on cancer patients’ well-being and overall response to chemotherapy [3]. The lack of careful intervention in preventing CINV can result in poor performance status, anticipatory CINV, non-compliance to subsequent chemotherapy cycles, as well as incur a substantial cost of unnecessary hospital admissions [4,5,6].
Generally, the latest guidelines published by the American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO) classified CINV into three categories, which are acute onset, delayed onset, and anticipatory onset CINV [7]. Acute onset-CINV occurs within 24 h of initial chemotherapy, while delayed onset occurs more than 24 h following initial chemotherapy and may last up to 5 days. Anticipatory onset CINV is triggered by a conditioned response due to severe nausea and vomiting [7].
The prevention of CINV is an important element of supportive care in cancer. Prophylactic antiemetic agents are prescribed according to the perceived emetogenic potential of chemotherapy agents. Emetogenicity is defined as the propensity of an agent to cause nausea, vomiting, or retching [8]. In the absence of CINV prophylaxis, it is estimated that over 90 percent of patients administered with highly emetogenic chemotherapy (HEC) and 30 to 90 percent of patients administered with moderately emetogenic chemotherapy (MEC) will experience CINV [9, 10]. With antiemetic prophylaxis, CINV incidence varies widely from 20 to 70 percent across different types of populations, geographical areas, prescribing practices, antiemetic institutional reimbursement, and/or local drug regulatory [11,12,13].
In general, antiemetic therapy should be initiated before treatment with chemotherapy agents to provide maximum protection against CINV. It should be continued for a certain length of time to prevent delayed-onset CINV. ASCO, NCCN, and MASCC/ESMO guidelines share fundamental similarities for acute CINV prophylaxis, which uses a combination of a 5-HT3 receptor antagonist, dexamethasone, and the NK1 receptor antagonist within the first 24 h of chemotherapy in HEC and MEC [7].
Local previous research has focused on a certain type of cancer associated with CINV and involved a limited number of population [14,15,16]. The overall and considerable data for all types of cancer on CINV has not been fully investigated [14, 17]. A comprehensive data of CINV occurrence in each complete cycle were seldom reported in previous studies [11, 18]. Our study aims to provide such information that is crucial to plan for interventions and allocate limited resources, especially for a middle-income country like Malaysia. We also aim to evaluate prescribing practices that reflect CINV outcome in actual clinical practice and the factors associated with CINV as it is important to reduce its incidence and improve the management of this side effect.
Methods
The study was conducted as a retrospective cross-sectional study in the National Cancer Institute (IKN), Ministry of Health Malaysia. IKN is the referral center for solid cancer and receives cancer patients across the country. Inclusion criteria were adult (≥ 18 years old) oncology patients and received intravenous HEC/MEC in IKN from January 2019 to December 2019. Patients receiving HEC/MEC with concurrent radiation therapy were excluded because of possible contribution to the potential emetogenicity of radiation.
The calculated sample size was 406, based on previous estimates of the percentage of patients experiencing an episode of CINV, which was 40%. The significant level was set as α = 0.05 (two-tailed), 95% confidence interval (CI), and an additional 10% considering for data lost [19]. Records were filtered, extracted, and randomized, and a sufficient number of data as per the calculated sample size were selected and included in the study.
Information such as patient’s demographic data (age, ethnicity, gender, body mass index), patients’ clinical data (diagnosis, type, and stage of cancer, presence of comorbidities), chemotherapy regimens, antiemetic regimen for treatment and prevention, and episodes of nausea and vomiting were obtained and collected from the patients’ electronic medical records (EMR) system. The system used is a complete oncology patient management information system that collects, stores, and centralizes medical oncology, radiation oncology, and patient data into a single user interface, accessible by multidisciplinary teams. Episodes of nausea and vomiting were documented, and their severity was ascertained using Common Terminology Criteria for Adverse Events (CTCAE) Grade [20]. Performance status (PS) as estimated by the Eastern Cooperative Group (ECOG) performance status scale was used in this study [21, 22]. The management for breakthrough CINV was also recorded. Inconsistency in treatment given for a patient was defined as treatment that was not being consistently prescribed in each chemotherapy cycles following breakthrough CINV. Malaysia MOH Systemic Protocol for Cancer (2016) was used to determine the appropriateness of antiemetic choices and emetogenicity level of chemotherapy regimens. The practice pattern was also compared with international guidelines such as ASCO, NCCN, and MASCC/ESMO antiemetic guidelines.
The data obtained from this study were analyzed both descriptively and analytically. Data were analyzed by CINV categories, by patient, and by cycles. For basic characteristics, we used the χ2 or t-test to compare patients with CINV incidence. Binary logistic regression was conducted with any CINV events as the dependent variable. Univariate analysis was run, and outcomes of independent variables with p-values < 0.25 were further included in the multivariate analysis. Backward entry was used to determine the final covariates in the regression model. P-values ≤ 0.05 will be considered statistically significant in the final model. A greater value than the significance level of the Hosmer–Lemeshow was used, which indicates that there is not enough evidence to conclude that the model does not fit the data of the final model. All statistical tests were performed with the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL) version 26 and was based on the two-sided significance level of 5%.
Ethical approval for this study was obtained from the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia (NMRR-20–2988-57474), and the study center permission was obtained from the Director of IKN.
Results
Patients’ demographic and clinical characteristics
Four hundred nineteen selected records of adult IKN patients receiving HEC or MEC with total of 2388 chemotherapy cycles were included in this study. The mean ± SD age was 53.6 ± 12.6 years old. Sixty-six percent were female, 54.4% were Malay, 47.4% were diagnosed with stage IV, and 47% came without comorbidities Three-quarters of the subjects received HEC, and breast cancer patients were the highest MEC/HEC recipients. The top three chemotherapy prescribed for the subjects were anthracycline cyclophosphamide-based (AC-based), carboplatin-based, and cisplatin-based.
CINV incidence
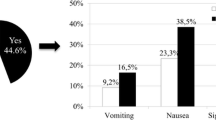
Out of 419 patients, 57 percent experienced CINV throughout the regime administered, and most of them presented with delayed type of CINV. Almost one-third experienced any sort of CINV in cycle 1, and almost half experienced it within the first three cycles. The severity of CINV was mainly mild (grade 1), with no intervention provided. Out of 2388 chemotherapy cycles, about 20% resulted in patients experiencing CINV, and majority (62.7%) were delayed nausea and delayed nausea and vomiting. Treatment mostly was not given for the delayed nature of CINV and with CTCAE grade 1, as showed in Figs. 1 and 2.
CINV prophylaxis
For prevention of acute CINV, all patients were prescribed with IV granisetron + dexamethasone + IV metoclopramide prior to chemotherapy which was in accordance with the local protocol [23]. Aprepitant which was recommended in all international guidelines was not routinely prescribed (91.2%) in IKN for the prevention of CINV, and 48.5% of studied population were not prescribed with aprepitant following CINV as suggested by the local protocol (Table 1). For the prevention of delayed CINV, all studied patients were prescribed with dexamethasone and metoclopramide, which was inconsistent with local protocol and most international guidelines.
Binomial regression
The outcome of binary logistic regression is shown in Table 2. From multivariate analysis, ECOG performance status and type of chemotherapy used were found to be significant predictors for CINV. The incidence of CINV was three times more likely with ECOG 1 and 5.5 times higher with ECOG 2 onwards compared to those ECOG 0 (OR = 3.071, CI = 1.515–6.223, p = 0.002; OR = 5.451, CI = 1.605–18.515, p = 0.007, respectively). Cisplatin-based chemotherapy was 4.6 times more likely to cause CINV compared to carboplatin-based chemotherapy (OR = 4.587, CI = 1.739–12.099, p = 0.02).
Discussion
Previous studies reported various percentages of CINV occurrence in cancer patients with the use of prophylaxis antiemetics [11,12,13, 24, 25]. A baseline patient characteristic of HEC/MEC-treated patients and CINV incidence were investigated in six Asia Pacific countries, with CINV occurrence reported in the first cycle of chemotherapy ranging from 22 to 45% [11]. Similarly, the current study found that one-third of the patients experienced CINV in the first cycle and half of the studied patients experienced some degree of CINV throughout all cycles. Additionally, this current study further analyzed CINV occurrences within cycles and found CINV to occur in one-fifth of the total cycles received. This is the first study to the authors’ knowledge that reports the event of CINV within cycles. This was carried out because patients may not have a similar emetic risk of CINV in every cycle; thus, they may not experience CINV in every cycle.
All 419 patients were prescribed with at least a dual regimen antiemetic consisting of granisetron and dexamethasone regardless of highly or moderately emetogenic chemotherapy (HEC/MEC) for the prevention of acute CINV as per local protocol [23]. The protocol-consistent in prescribing is because the electronic prescribing system was implemented in this tertiary oncology referral center. This electronic-enhanced prescribing system automatically includes the dual regime antiemetic in the electronic prescription whenever a HEC/MEC is prescribed. This technology prevents the lack of familiarity with the protocol among prescribers and physicians’ prescribing preferences.
For the prevention of delayed CINV, the local protocol recommended for HEC/MEC to be prescribed with dexamethasone single-agent ± aprepitant as part of the continuity of day 1 aprepitant, whereas single-agent dexamethasone or metoclopramide for low emetogenic chemotherapy [23]. However, all studied patients were prescribed dexamethasone and metoclopramide. The practice is inconsistent with local protocol where metoclopramide was not recommended for delayed CINV, and this contributed to the overutilization of metoclopramide. Among international guidelines, MASCC/ESMO suggested the use of either dexamethasone and aprepitant (if aprepitant is used acute-phase) or dexamethasone and metoclopramide for day 2 to day 4 for only cisplatin-treated patients [7, 26].
The local protocol recommended triple regimen therapy, which includes aprepitant in the following cycle when breakthrough emesis or CINV was uncontrolled with a dual regimen. However, in this study, aprepitant was added in the dual regimen in less than 10% of the studied population, and nearly half of the patients were not prescribed with aprepitant following CINV in the previous cycle. This finding is due mainly to the restricted use of NK1 receptor antagonists in this country, which is only prescribed depending on institutional drug budget reimbursement and physicians’ discretion due to its high cost.
There have been conflicting results on CINV incidence in relation to the prescribing of antiemetic according to the guidelines, where there were studies that reported improved outcomes for CINV [13, 25], while other studies showed the opposite [12, 27]. Low adherence to triple regimen antiemetic prophylaxis therapy is not uncommon in other countries. Aapro et al. (2021) recently published data for the year 2018 of 45,324 highly emetogenic chemotherapy-treated patients in five European countries. The real-world study found low adherence to triple regimen antiemetic prophylaxis [24]. Post-marketing surveillance data of CINV prescribing by Yang et al. [28] showed relatively moderate adherence to triple regimen prophylaxis (455/990, 46%) in 21 centers across China. This current study showed a relatively moderate incidence (57%) of overall CINV. However, considering that the current CINV incidence was based on patients treated with dual regimen antiemetic prophylaxis only, this indicates that addition of aprepitant into the regimen in the future may further reduce the CINV incidence. This has been proven in previous studies, whereby no emesis was twice as high with the addition of aprepitant in the dual regimen [18, 29].
The relationship between CINV incidence and several potential predictors has been studied. Performance status was a significant predictor in the studied population where higher CINV incidence was observed with increasing ECOG value. Few previous studies also reported poor performance status as a significant CINV risk factor [30, 31]. Chan A et al. (2015) reported that patients with ECOG values > 1 were 2.4 times more likely to experience vomiting than patients with an ECOG value of 0 [32]. This information may help to effectively manage CINV where an additional antiemetic may be given depending on the patient’s performance status prior to chemotherapy administration in each cycle. Except for cisplatin-based chemotherapy, the incidence of CINV was similar across the type of chemotherapy agents given when compared to carboplatin-based chemotherapy. Special attention should be given to CINV management when cisplatin is planned to be administered because cisplatin-based chemotherapy is known to be highly emetogenic and may be administered over multiple-day schedules, where prolonged CINV may severely impact the patient’s quality of life and the continuity of the treatment [33]. Split-dose cisplatin is probably an option in preventing CINV as compared to an undivided dose [32].
In this study, close to three-quarter of patients who experienced CINV were not given CINV treatment. Rescue therapy was mostly given to patients experiencing acute CINV. Most patients who did not receive treatment were those experiencing delayed CINV with the severity of Grade 1 according to CTCAE scoring, where interventions were not indicated. The most common rescue given for breakthrough CINV was metoclopramide with regular dosing. Our finding is consistent with a nationwide survey on HEC/MEC recipients in Japan, where it showed that rescue antiemetics were used in less than half of the individuals with breakthrough CINV, and most of them received metoclopramide or domperidone [34].
The limitation of this study is the nature of retrospective-run data collection. Delayed CINV events, which prevented patients to promptly report, were collected during the subsequent cycle of physicians’ clerking. Thus, the physicians’ notes may have varied, and exact reasons may not be recorded. This may lead to underreporting of CINV events by patients as well as inconsistent clerking among physicians. Adherence to the prescribed antiemetics was also not investigated in this study due to inconsistent reporting of adherence data. Non-pharmacological measures for CINV were also not collected and investigated in this study as this was not available from the patients’ EMR. Furthermore, this study was conducted retrospectively without direct patients’ involvement. This study also only established association but not cause-effect between risk factors and CINV outcomes. Interpretation has to be made with caution regarding the study findings.
Conclusion
In conclusion, most patients in this study were prescribed with dual regimen antiemetic prophylaxis. CINV incidence was rather high per patient but relatively low per cycle. The control of delayed-phase CINV was not better managed than acute CINV. Poor performance status and the use of cisplatin-based chemotherapy were significantly associated with higher CINV incidence. This study provides evidence of suboptimal use of recommended agents for CINV. There is a clear need for further research to investigate reasons for such deviations from CINV management guidelines, which is necessary for the improvement of CINV management.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer. 1997;76(8):1055–61.
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW et al (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13(4):219–227
Ballatori E, Roila F, Ruggeri B, Betti M, Sarti S, Soru G et al (2007) The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer 15(2):179–185
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15(5):497–503
Burke TA, Wisniewski T, Ernst FR (2011) Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer 19(1):131–140
Tina Shih YC, Xu Y, Elting LS (2007) Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer 110(3):678–685
Razvi Y, Chan S, McFarlane T, McKenzie E, Zaki P, DeAngelis C et al (2019) ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support Care Cancer 27(1):87–95
Grunberg SM, Warr D, Gralla RJ, Rapoport BL, Hesketh PJ, Jordan K et al (2011) Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity–state of the art. Support Care Cancer 19(Suppl 1):S43–S47
Rapoport BL (2017) Delayed chemotherapy-induced nausea and vomiting: pathogenesis, incidence, and current management. Front Pharmacol 8:19
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G et al (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15(1):103–109
Hsieh RK, Chan A, Kim HK, Yu S, Kim JG, Lee MA et al (2015) Baseline patient characteristics, incidence of CINV, and physician perception of CINV incidence following moderately and highly emetogenic chemotherapy in Asia Pacific countries. Support Care Cancer 23(1):263–272
Hernandez Torres C, Mazzarello S, Ng T, Dranitsaris G, Hutton B, Smith S et al (2015) Defining optimal control of chemotherapy-induced nausea and vomiting-based on patients’ experience. Support Care Cancer 23(11):3341–3359
Aapro M, Molassiotis A, Dicato M, Peláez I, Rodríguez-Lescure Á, Pastorelli D et al (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol 23(8):1986–1992
Hassan BA, Yusoff ZB (2010) Negative impact of chemotherapy on breast cancer patients QOL - utility of antiemetic treatment guidelines and the role of race. Asian Pac J Cancer Prev 11(6):1523–1527
Salihah N, Mazlan N, Lua PL (2016) Chemotherapy-induced nausea and vomiting: exploring patients’ subjective experience. J Multidiscip Healthc 9:145–151
Chanthawong S, Lim YH, Subongkot S, Chan A, Andalusia R, Ahmad Bustamam RS et al (2019) Cost-effectiveness analysis of olanzapine-containing antiemetic therapy for managing highly emetogenic chemotherapy in Southeast Asia: a multinational study. Support Care Cancer 27(3):1109–1119
Mahidin EIBM, Ishak WZBW (2019) P1–218 - Chemotherapy induced nausea and vomiting and quality of life of patients on moderate and highly emetogenic chemotherapy. Ann Oncol 30:vi128
Yu S, Burke TA, Chan A, Kim HK, Hsieh RK, Hu X et al (2015) Antiemetic therapy in Asia Pacific countries for patients receiving moderately and highly emetogenic chemotherapy–a descriptive analysis of practice patterns, antiemetic quality of care, and use of antiemetic guidelines. Support Care Cancer 23(1):273–282
Aapro M (2018) CINV: still troubling patients after all these years. Support Care Cancer 26(Suppl 1):5–9
US Department of Health and Human Services NIoH, National Cancer Institute, editor. Common terminology criteriafor adverse events (CTCAE) Version 5. 2017.
Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S et al (2019) Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol 12(3):728–736
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Malaysia Ministry of Health Systemic Protocol for Chemotherapy. 3 ed2016.
Aapro M, Scotté F, Escobar Y, Celio L, Berman R, Franceschetti A et al (2021) Practice patterns for prevention of chemotherapy-induced nausea and vomiting and antiemetic guideline adherence based on real-world prescribing data. Oncologist 26(6):e1073–e1082
Tamura K, Aiba K, Saeki T, Nakanishi Y, Kamura T, Baba H et al (2015) Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol 20(5):855–865
Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ et al (2017) NCCN guidelines insights: antiemesis, Version 2.2017. J Natl Compr Canc Netw 15(7):883–93
Baburaj G, Abraham AM, George L, Shetty V, Thempalangad RM, Rajesh KS et al (2017) A study on utilization and evaluation of antiemetics in chemotherapy-induced nausea and vomiting. Indian J Med Paediatr Oncol 38(3):334–339
Yang Y, Yang N, Wu L, Ouyang Q, Fang J, Li J et al (2020) Safety and efficacy of aprepitant as mono and combination therapy for the prevention of emetogenic chemotherapy-induced nausea and vomiting: post-marketing surveillance in China. Chin Clin Oncol 9(5):68
Yuan DM, Li Q, Zhang Q, Xiao XW, Yao YW, Zhang Y et al (2016) Efficacy and safety of neurokinin-1 receptor antagonists for prevention of chemotherapy-induced nausea and vomiting: systematic review and meta-analysis of randomized controlled trials. Asian Pac J Cancer Prev 17(4):1661–1675
Uchida M, Mori Y, Nakamura T, Kato K, Kamezaki K, Takenaka K et al (2017) Comparison between antiemetic effects of palonosetron and granisetron on chemotherapy-induced nausea and vomiting in Japanese patients treated with R-CHOP. Biol Pharm Bull 40(9):1499–1505
Hayashi T, Shimokawa M, Miyoshi T, Toriyama Y, Yokota C, Taniguchi J et al (2017) A prospective, observational, multicenter study on risk factors and prophylaxis for low emetic risk chemotherapy-induced nausea and vomiting. Support Care Cancer 25(9):2707–2714
Chan A, Shwe M, Gan Y, Yap K, Chew L, Lim WT (2015) Trajectory and risk factors for chemotherapy-induced nausea and vomiting in Asian patients with head and neck cancer. Head Neck 37(9):1349–1357
Zong X, Zhang J, Ji X, Gao J, Ji J (2016) Patterns of antiemetic prophylaxis for chemotherapy-induced nausea and vomiting in China. Chin J Cancer Res 28(2):168–179
Tamura K, Aiba K, Saeki T, Nakanishi Y, Kamura T, Baba H et al (2017) Breakthrough chemotherapy-induced nausea and vomiting: report of a nationwide survey by the CINV Study Group of Japan. Int J Clin Oncol 22(2):405–412
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Funding
This study is self-funded research, and investigators initiate research. No financial arrangement/compensation by the manufacturer of any products was made upon initiation of this study.
Author information
Authors and Affiliations
Contributions
Badarudin NS carried out the data collection and data analysis and worked on the write-up of the article. Mohamed Shah N contributed to the research design and final write-up of the article. Ismail F assisted in the research design and data analysis of the research. Islahudin F assisted in the research design of the research. Mohd Tahir NA assisted in the discussion of the write-up. Mohd Kassim KNB assisted in research design and data collection at the study site. Yusak S assisted in research design at the study site. All authors have read, approved the article, and meet the criteria for authorship as established by the International Committee of Medical Journal Editors (ICJME).
Corresponding author
Ethics declarations
Ethics approval
Ethical approval for this study was obtained from the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia (NMRR-20–2988-57474), and the study center permission was obtained from the Director of the National Cancer Institute, Ministry of Health Malaysia.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Badarudin, N.S., Mohamed Shah, N., Mohd Kassim, K.N.B. et al. A retrospective study on chemotherapy-induced nausea and vomiting in highly/moderately emetogenic chemotherapy: incidence and prescribing practice. Support Care Cancer 30, 5339–5349 (2022). https://doi.org/10.1007/s00520-022-06956-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06956-0