Abstract
Purpose
Chemotherapy-induced nausea and vomiting (CINV), common adverse events of chemotherapy, may be associated with considerable healthcare resource utilization. This study was conducted to describe CINV-associated healthcare visits and costs following a first cycle of highly or moderately emetogenic chemotherapy (HEC or MEC).
Methods
This retrospective cohort study used the Premier Perspective™ Database to identify adult patients who received their first HEC or MEC and at least one antiemetic agent from 2003 to 2007 at US hospital-based outpatient facilities. Hospital visits with a CINV-related ICD-9 diagnosis were included from the chemotherapy administration date to 30 days later or 1 day before the second chemotherapy, whichever was first. CINV costs were hospital-reported costs.
Results
Of 19,139 patients (HEC, 16%; MEC, 84%), mean (SD) age was 59 (14) years; 59% were female; 66% were white. CINV prophylaxis included 5-HT3 antagonists (85%), dexamethasone (76%), and NK-1 antagonists (2%). Overall, 13.8% of patients had a CINV-associated visit (HEC, 18%; MEC, 13%): 0.2% for acute CINV (day of chemotherapy, excluding chemotherapy administration visit) and 13.7% for delayed CINV. CINV-associated visits included inpatient (IP, 64%), outpatient (OP, 26%), and emergency room (ER, 10%) visits. Mean (SD) costs of CINV visits were $5,299 ($6,639); for IP, $7,448 ($7,271); OP, $1,494 ($2,172); and ER, $918 ($1,071). Mean per-patient CINV-associated costs across all patients were $731 ($3,069). Sensitivity analysis excluding visits where CINV was a secondary diagnosis code resulted in a CINV incidence of 4.4%, a mean CINV visit cost of $4,043, and a mean per-patient CINV-associated cost across all patients of $176.
Conclusions
CINV visits in the first HEC or MEC cycle were common and costly, especially inpatient hospitalizations in the delayed phase. Strategies to reduce CINV in the delayed phase could reduce healthcare utilization and costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Substantial progress has been made over the past two decades in the pharmacologic prophylaxis and treatment of nausea and vomiting occurring secondary to chemotherapy. National and international clinical practice guidelines for preventing chemotherapy-induced nausea and vomiting (CINV) are frequently updated to reflect advances in antiemetic therapy, including the 5-hydroxytryptamine-3 (5-HT3) receptor antagonists in the early 1990s and the long-acting 5-HT3 antagonist palonosetron and the first neurokinin-1 (NK-1) receptor antagonist, aprepitant, in 2003 [1–3]. Nonetheless, results of recent studies indicate that antiemetic control is incomplete, and CINV continues to be an important clinical problem, particularly nausea and delayed CINV, which is that occurring more than 24 h after chemotherapy [4–10].
In the absence of antiemetic therapy, highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC) cause acute emesis (occurring up to 24 h after chemotherapy) in >90% and 30% to 90% of patients, respectively [2, 3]. Up to 40% or more of patients experience anticipatory CINV, a conditioned response occurring because of prior poor control of CINV [1, 2]. Prevention of CINV on the first cycle of chemotherapy remains an important goal of antiemesis therapy because it may help to minimize CINV during subsequent cycles [11, 12]. Moreover, prevention of acute CINV may decrease the incidence of delayed CINV [13].
Described by patients as one of the most distressing adverse effects of chemotherapy, CINV results in reduced quality of life and substantial adverse functional impact [7, 8, 14]. Moreover, CINV has potential economic consequences, as patients may require additional medical care to treat symptoms and consequences of nausea and vomiting, such as dehydration. Few studies have assessed the impact of CINV and use of antiemetics on subsequent healthcare resource use [4, 15, 16]. In one study, the most frequently used resources were the need for rescue medication, additional office physician and outpatient hospital visits, and hospital admissions [4].
The objectives of this study were to describe the percentage of patients who have a hospital visit (inpatient, outpatient, and emergency room) associated with CINV and to report hospital costs associated with these visits. The study population was patients receiving their first HEC or MEC as outpatients from a hospital in the United States and who received at least one antiemetic agent.
Methods
Data source
This was a retrospective observational cohort study using the Premier Perspective™ Database (http://www.premierinc.com/quality-safety/tools-services/prs/index.jsp), a hospital service database that includes detailed patient-level data, differentiated by inpatient stays vs. visits to outpatient facilities of these hospitals. The database includes data from over 600 US hospitals with information on patient demographics (age, sex, race, and marital status), hospital characteristics, principal and secondary diagnoses, payer, cost of care, medication utilization (name, strength, quantity dispensed, and day of administration), department cost and charge detail, length of stay, and physician specialty. A unique patient identification number allows linkage of visits within the same hospital inpatient and outpatient facilities for a given patient. Drugs are not linked to their specific indication for use within the database. The data are de-identified in accordance with the Health Insurance Portability and Accountability Act.
Study cohort
The study population comprised patients aged 18 years or older who received a first HEC or MEC, as well as one or more drugs commonly used for antiemetic prophylaxis, at an outpatient hospital facility during the 5-year period between January 1, 2003, and December 31, 2007. HEC and MEC regimens were defined using the Multinational Association of Supportive Care in Cancer (MASCC) guidelines, which classify emetogenic risk according to single intravenous and oral chemotherapies [17]. Similarly, the single chemotherapy with the highest emetogenic risk was used to classify the emetogenic risk of the regimen in this study. MASCC guidelines require determination of cyclophosphamide dose as mg/m2 because doses ≥1,500 mg/m2 are classified as HEC and doses <1,500 mg/m2, as MEC. Since the Premier Perspective™ Database did not contain information on body surface area (BSA) or patient height and weight needed to estimate BSA [18], values of 1.6 m2 for adult women and 1.9 m2 for adult men were assumed. Antiemetic prophylaxis was defined as any of the following drug classes: 5-HT3 antagonists, corticosteroids, benzodiazepines, antihistamines, NK-1 receptor antagonists, butyrophenones, phenothiazines, and cannabinoids. Drug use in the Premier Perspective™ Database is date- but not time-stamped; thus, any antiemetic drugs given on the same day as chemotherapy were considered as representing CINV prophylaxis rather than treatment.
CINV healthcare resource utilization and costs
Healthcare resource use was assessed starting on the patient’s first chemotherapy administration date and ending at the first of the following: 30 days after the first chemotherapy administration date or 1 day before the second chemotherapy administration.
We defined CINV-associated healthcare resource use as any hospital visit with a primary or secondary ICD-9 code of 787.0 (nausea and vomiting), 787.01 (nausea with vomiting), 787.02 (nausea alone), 787.03 (vomiting alone), 276.5 (volume depletion), or 276.50 (volume depletion, unspecified). This definition was the same as that used in a recent study by Shih and colleagues [16]. In October 2005, the volume depletion codes were expanded to 276.5 (volume depletion disorder), 276.50 (volume depletion, unspecified), 276.51 (dehydration), and 276.52 (hypovolemia); thereafter, codes 276.51 and 276.52 were added to the definition of CINV-associated healthcare resource use.
The costs of healthcare resource use were the hospital costs as reported in the database. Costs for CINV-associated hospitalizations included those categorized as emergency room or outpatient visit; and costs for inpatient CINV visits included total costs for the inpatient hospitalization as reported by the hospital.
Sample characteristics
The following were described for study patients: demographic characteristics (age, sex, marital status, and race), payer type, primary cancer site (based on ICD-9 codes recorded on outpatient or inpatient hospital claims 60 days before or after the index chemotherapy administration visit), and chemotherapy and antiemetic therapies administered from the hospital pharmacy during the chemotherapy administration visit. The primary cancer site was identified using a published hierarchy of 18 anatomical cancer groupings using ICD 9 codes based on the likelihood of each cancer grouping being a primary vs. secondary tumor. Patients with ICD 9 codes from more than one cancer site grouping were classified into a single primary cancer site using this hierarchy (e.g., a patient with ICD 9 codes for colon and lung cancer was be classified as having colon cancer, metastatic to the lung) [19]. Cancers with a prevalence of >2% were reported separately. Chemotherapy regimens pre-defined to be described at baseline included cyclophosphamide plus anthracycline, any cisplatin-containing regimen, carboplatin plus paclitaxel, oxaliplatin plus 5-fluorouracil, irinotecan plus 5-fluorouracil, CHOP (cyclophosphamide plus doxorubicin plus vincristine plus prednisone), and ABVD (doxorubicin plus belomycin plus vinblastine plus dacarbazine). Chemotherapies and antiemetic therapies were identified by searching drug name text field. Additional sample characteristics described included geographic region and type of hospital (teaching hospital and urban hospital) and physician specialty for outpatient visits.
Statistical analysis
Analyses of CINV-related healthcare resource use were descriptive.
Hospital visits were classified according to the number of days since chemotherapy. Acute CINV hospital visits were those on the day of chemotherapy administration, excluding the CINV coded on the chemotherapy visit. Delayed CINV hospital visits were those on days following chemotherapy administration. This approach was designed to follow that used in treatment guidelines [2, 17] and CINV reviews [20], in which acute and delayed CINV are events occurring within 24 and >24 h, respectively, after receipt of chemotherapy. In addition, hospital visits were classified as resulting in inpatient admission, emergency room visits, and outpatient hospital visits (non-emergency room). Patients with multiple visits for CINV were classified into a single mutually exclusive healthcare resource use category according to the following hierarchy: inpatient > emergency room > outpatient hospital visit based on the anticipated cost of each category of visit. This classification was applied overall and separately for the acute and delayed phases. For patients with multiple CINV visits, costs associated with CINV were summed across visits for each patient overall (acute and delayed), within the acute phase, and within the delayed phase.
Mean cost (SD) per CINV visit was calculated for patients with at least one event, as well as across all patients with and without CINV visits. In line with the database used, costs were from the hospital perspective. A tree diagram was used to describe the patient flow over the acute and delayed phases of chemotherapy.
One-way sensitivity analyses were conducted using alternative definitions of CINV-related healthcare resource utilization to explore the impact of (1) excluding CINV costs by using a more narrow CINV ICD-9 definition that excluded volume depletion disorders (codes 276.5×), (2) excluding CINV costs when CINV was the secondary diagnosis code on the visit claim, and (3) restricting CINV healthcare resource use to a shorter period after the chemotherapy administration visit (ranging from 6 to 14 days). A Pearson’s chi-square test was used to evaluate the association between CINV visit location (inpatient, ER, or outpatient) and CINV event type (primary vs. secondary ICD-9 diagnosis code). Analyses were conducted using SAS version 9.1.
Results
A total of 19,139 patients treated at 257 outpatient hospital facilities met the inclusion criteria. Sixteen percent of patients received HEC and 84% MEC. The baseline demographic and clinical characteristics of patients are summarized in Table 1. Overall, the mean age of patients was 59 years, and approximately 60% were women. Patients receiving MEC tended to be older, and a higher percentage were female than those receiving HEC (Table 1). As per the inclusion criteria, all patients received at least one antiemetic agent on the chemotherapy administration visit; the most common antiemetic therapies, listed in Table 1, were 5-HT3 antagonists and corticosteroids, including dexamethasone. Lung cancer was the most common single form of cancer, affecting almost one quarter of patients (Table 1). The most common single and combination chemotherapies administered are listed in Table 2.
A total of 2,641 patients (13.8%) experienced one or more CINV-associated hospital visit after a first cycle of HEC or MEC. The median time to CINV was 7 days (interquartile range, 3–14 days). CINV was the primary ICD-9 code for 31.5% of CINV visits. The corresponding percentages of visits where CINV was the primary ICD-9 code by site of care were inpatient (31.2%), emergency room (62.5%), and outpatient (20.8%), p < 0.0001. Among those patients with a secondary ICD-9 code for CINV, the mean number of additional secondary codes beyond the CINV code was 1.8 (range 0 to 16).
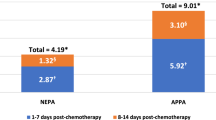
Tables 3 and 4 depict the incidence of CINV-related healthcare resource use and cost of hospital visits for acute and delayed CINV following HEC and MEC and according to type of visit (inpatient, emergency room, or outpatient visit). Visits for acute CINV were less common than those for delayed CINV (0.2% vs. 13.7% of patients affected); and CINV visits were more likely after HEC than MEC (18.0% vs. 13.0% of patients). Inpatient admissions (64%) were the most common type of hospital visit for CINV; 26% were outpatient visits, and 10% were emergency room visits.
Inpatient admissions were also the most costly type of hospital visit for patients with CINV, averaging approximately $7,500 per patient (see Table 4). Per-patient hospital costs ranged from $891 for an emergency room visit for CINV after MEC to $7,678 for an inpatient admission for CINV after HEC. The mean cost of a hospital visit for CINV after first-time HEC or MEC, averaged over all patients in the study, was $731. Figure 1 is a schematic depiction of the incidence and costs of hospital visits associated with CINV after HEC or MEC.
Sensitivity analyses
Results of the sensitivity analyses are summarized in Table 5 as incidence and mean CINV-related costs per patient. When CINV was restricted to visits with a primary ICD-9 code for CINV, the incidence of CINV-related visits decreased from 13.8% to 4.4%, and the mean per-patient cost decreased from $731 to $176. If the time for CINV visits was restricted to within 10 days after chemotherapy, the percentage of patients decreased from 13.8% to 7.8%, and per-patient costs decreased to $304.
Discussion
Over one in eight patients in this retrospective observational study had a follow-up hospital visit associated with CINV after a first cycle of HEC or MEC. Inpatient hospitalizations were the most common type of visit, as well as the most costly on a per-event basis and overall. Almost all visits occurred during the delayed phase, on days following receipt of chemotherapy. The cost of a CINV-associated hospital visit for the first cycle was high, amounting to $5,300 per visit or, averaged across all patients with and without a visit, $730. Costs were most pronounced for patients receiving HEC. These results suggest there is still substantial room for improvement in reducing CINV healthcare resource utilization following HEC or MEC. This is also of importance with regard to the negative health-related quality of life impact associated with CINV, following HEC or MEC.
Patients in this study received chemotherapy on an outpatient basis; therefore, their return visits to hospital could be easily counted, with attribution of CINV for visits coded with a diagnosis for nausea, vomiting, or dehydration. Moreover, the patient population in this study was large (>19,000), was ethnically diverse, and attended outpatient hospitals for chemotherapy throughout the USA, although with some concentration in the eastern and south Atlantic regions. Study data are from 2003 to 2007 and thus represent contemporary treatment of CINV, taking into account the availability of recent advances in antiemetic therapy (NK-1 antagonists and palonosetron) and the generic availability of 5-HT3 antagonist therapy.
By excluding CINV coded on the chemotherapy administration visit claim, rescue medication and nursing time associated with acute CINV were excluded. Moreover, data collection was restricted to resource use in hospitals included in the Premier Perspective™ Database, which could have led to an underestimation of healthcare resource use since follow-up visits to facilities outside of the hospital where the chemotherapy was administered were not captured. The study did not also capture the costs of prescriptions filled at non-hospital pharmacies for CINV rescue (orally administered drugs) and costs borne by patients, such as for complementary care [21]. Lack of information on CINV rescue medications from non-hospital (i.e., retail) pharmacies led to an underestimation in CINV costs. In addition, we did not assess indirect costs, such as for lost work time for patients or caregivers [16].
Costs reported in this study are the actual hospital-reported costs and are likely to approximate costs from the third-party payer perspective. Mean costs are unlikely to reflect the costs of a typical patient because of the relatively few patients who experience expensive events. Nonetheless, mean costs are informative for policy makers or payers because the mean allows a determination of the total cost burden for a population.
The most important study limitation was the lack of a control group to provide a background rate of visits and costs associated with nausea, vomiting, and dehydration secondary to cancer. The difference in CINV-associated healthcare resource use between patients in this study and a control group of patients with cancer but not receiving HEC or MEC may have provided stronger attribution of CINV-associated healthcare resource use to HEC and MEC. The follow-up period, until the day before the next chemotherapy visit or 30 days (whichever came first), may have led to overestimation of healthcare resource use. This time period was selected to capture adverse consequences of CINV, such as dehydration, that could require a hospital visit or inpatient hospitalization. While controlled trials and prior observational studies have assessed delayed CINV for up to 5 days after chemotherapy [8, 9, 16, 22], in reality, patients can experience CINV for longer periods [1–3]. For example, Molassiotis and coworkers [5] report delayed vomiting up to 10 days after MEC among patients in their prospective observational study. Finally, the database does not include patients treated in non-hospital, free-standing chemotherapy clinics. The extent to which these results generalize to this setting is unknown.
The sensitivity analyses were designed to examine the impact of changing the assumptions used in the base case analysis for identifying healthcare costs attributable to CINV. These sensitivity analyses included (1) excluding dehydration from the definition of CINV, since dehydration following chemotherapy may be due to causes other than nausea and/or vomiting; (2) reducing the number of days following chemotherapy where nausea, vomiting, and dehydration were captured, by reducing the number from up to 30 days to between 6 days (the timeframe used in clinical trials to assess patient reported nausea and vomiting) and 14 days (arbitrarily selected); and (3) excluding CINV coded as a secondary diagnosis on the visit claim since, it can be argued, that these costs should be attributed to healthcare conditions other than CINV. The sensitivity analysis with the most significant impact in reducing the percentage of patients and costs associated with CINV was the exclusion of CINV as secondary diagnosis. This was particularly true for outpatient visits and inpatient stays, where 21% and 31% of visits had nausea, vomiting, or dehydration as the primary diagnosis code on the visit claim.
A recent US study examining the cost of CINV provides complementary information to the current study and is the most relevant study for comparison [16]. Shih et al. [16] investigated the costs of CINV associated with HEC or MEC among working-age adults included in an employment-based healthcare claims and work loss database. CINV costs, gathered over up to 6 months after chemotherapy, were reported as total direct medical costs for patients with at least one ICD-9 diagnosis code for CINV minus these total costs for patients without a CINV diagnosis code. The authors used an econometric model to control for confounding factors between the two groups. Adjusted direct medical costs of CINV were $1,280 per month over a 6-month follow-up period, with 28% of patients having CINV resource use [16]. In this study, mean CINV event costs were $5,299 for the first chemotherapy cycle (a period up to 30 days) with a 13.8% CINV incidence within this period ($731 overall per-patient CINV cost). When the current study restricted to CINV with a primary diagnosis, mean CINV event costs were $4,043 for the first chemotherapy cycle with a 4.4% CINV incidence ($176 overall per-patient CINV cost). This comparison suggests a possible overestimation of the current study of including any visits with a primary and secondary CINV diagnoses, but an underestimation when counting visits only with a primary CINV diagnosis as a sensitivity analysis.
Other prior studies have taken somewhat different approaches to examining healthcare resource utilization secondary to CINV or are non-US studies and thus are less useful as comparators to the present study [4, 15, 22, 23].
The use of a hospital service database has enabled us to capture CINV-associated healthcare resource use in a hospital setting. While this study did not collect information specifically on when CINV occurred, it did collect information on when CINV-associated healthcare resource use occurred. In agreement with a prior study [4], our findings indicate that the majority of healthcare resource use and cost burden for CINV is during the delayed phase. An increased use of newer therapies that reduce CINV in the delayed phase may help to reduce the cost burden of CINV. For example, results of a recent modeling study indicate that routine use of the NK-1 antagonist aprepitant for HEC is most cost effective when the likelihood of delayed CINV or the cost of rescue medications is high [24].
Future studies should evaluate the impact of newer antiemetic therapies on healthcare resource use secondary to CINV. We were unable to answer this question in the current study because the hospital database is limited to capturing antiemetic therapy filled by the hospital pharmacy and excludes oral antiemetic prescriptions filled at pharmacies outside the hospital.
References
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
National Comprehensive Cancer Network (2009) Clinical practice guidelines in oncology. Antiemesis. V.1. http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf. Accessed 15 July 2009
Multinational Association of Supportive Care in Cancer (MASCC). Antiemetic guidelines. http://www.mascc.org/mc/page.do?sitePageId=88041&orgId=mascc. Accessed 15 July 2009
Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, Deuson R, Thodtmann J, Lordick F (2004) The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol 15:526–536
Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, Levine E, Cowan R, Loncaster J, Rittenberg C (2008) A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 16:201–208
Glaus A, Knipping C, Morant R, Bohme C, Lebert B, Beldermann F, Glawogger B, Ortega PF, Husler A, Deuson R (2004) Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 12:708–715
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268
Ballatori E, Roila F, Ruggeri B, Betti M, Sarti S, Soru G, Cruciani G, Di Maio M, Andrea B, Deuson RR (2007) The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer 15:179–185
Morrow GR, Roscoe JA, Hickok JT, Stern RM, Pierce HI, King DB, Banerjee TK, Weiden P (1998) Initial control of chemotherapy-induced nausea and vomiting in patient quality of life. Oncology (Williston Park) 12:32–37
Kris MG (2003) Why do we need another antiemetic? Just ask. J Clin Oncol 21:4077–4080
Liau CT, Chu NM, Liu HE, Deuson R, Lien J, Chen JS (2005) Incidence of chemotherapy-induced nausea and vomiting in Taiwan: physicians’ and nurses’ estimation vs. patients’ reported outcomes. Support Care Cancer 13:277–286
Ballatori E, Roila F (2003) Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. Health Qual Life Outcomes 1:46
Ballatori E, Roila F, Ruggeri B, Porrozzi S, Iannopollo M, Soru G, Cruciani G, Daniele B, Locatelli MC, Pellissier J, Deuson R (2007) The cost of chemotherapy-induced nausea and vomiting in Italy. Support Care Cancer 15:31–38
Tina Shih YC, Xu Y, Elting LS (2007) Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer 110:678–685
Roila F, Hesketh PJ, Herrstedt J, Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (2006) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17:20–28
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098
Weiner MG, Livshits A, Carozzoni C, McMenamin E, Gibson G, Loren A, Hennessy S (2003) Derivation of malignacy status from ICD-9 codes. American Medical Informatics Association 2003 Symposium Proceedings: 1050
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358:2482–2494
Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, Margulies A, Browall M, Magri M, Selvekerova S, Madsen E, Milovics L, Bruyns I, Gudmundsdottir G, Hummerston S, Ahmad AM, Platin N, Kearney N, Patiraki E (2005) Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol 16:655–663
Lachaine J, Yelle L, Kaizer L, Dufour A, Hopkins S, Deuson R (2005) Chemotherapy-induced emesis: quality of life and economic impact in the context of current practice in Canada. Support Cancer Ther 2:181–187
Stewart DJ, Dahrouge S, Coyle D, Evans WK (1999) Costs of treating and preventing nausea and vomiting in patients receiving chemotherapy. J Clin Oncol 17:344–351
Moore S, Tumeh J, Wojtanowski S, Flowers C (2007) Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health 10:23–31
Acknowledgments
Editorial assistance was provided by Elizabeth V Hillyer with the financial support of Merck & Co., Inc.
Disclosures
Thomas Burke and Tami Wisniewski are employees of Merck & Co., Inc. Frank Ernst is an employee of Premier Research Services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burke, T.A., Wisniewski, T. & Ernst, F.R. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer 19, 131–140 (2011). https://doi.org/10.1007/s00520-009-0797-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0797-x